Abstract
Leptin and its neuronal targets, which produce proopiomelanocortin (POMC) and agouti-related protein (AgRP), regulate energy balance. This study characterized leptin, POMC, and AgRP in the cerebrospinal fluid (CSF) of 47 healthy human subjects, 23 lean and 24 overweight/obese (OW/OB), as related to BMI, adiposity, plasma leptin, soluble leptin receptor (s-OB-R), and insulin. POMC was measured since the POMC prohormone is the predominant POMC peptide in CSF and correlates with hypothalamic POMC in rodents. Plasma AgRP was similarly characterized. CSF leptin was 83-fold lower than in plasma and correlated strongly with BMI, body fat, and insulin. The relative amount of leptin transported into CSF declined with increasing BMI, ranging from 4.5 to 0.52%, consistent with a saturable transport mechanism. CSF sOB-R was 78-fold lower than in plasma and correlated negatively with plasma and CSF leptin. CSF POMC was higher in lean vs. OW/OB subjects (P < 0.001) and correlated negatively with CSF leptin (r = −0.60, P < 0.001) and with plasma leptin, insulin, BMI, and adiposity. CSF AgRP was not different in lean vs. OW/OB; however, plasma AgRP was higher in lean subjects (P = 0.001) and correlated negatively with BMI, adiposity, leptin, insulin, and HOMA (P < 0.005). Thus, CSF measurements may provide useful biomarkers for brain leptin and POMC activity. The striking negative correlation between CSF leptin and POMC could be secondary to leptin resistance and/or neuronal changes associated with obesity but may also indicate that POMC plays a primary role in regulating body weight and adiposity. The role of plasma AgRP as a neuroendocrine biomarker deserves further study.
Keywords: proopiomelanocortin, agouti-related protein, leptin, cerebrospinal fluid
the adipocyte-derived hormone leptin communicates levels of energy stores to key brain regions and elicits a host of neuronal responses that regulate energy balance (6, 14). Leptin enters the brain by a saturable transport mechanism that involves, at least in part, a short isoform (Ob-Ra) of the leptin receptor found in the choroid plexus and cerebral microvessels (1, 9). Another circulating leptin receptor isoform, the soluble leptin receptor (sOB-R or Ob-Re), can also impact leptin transport into the brain. sOB-R may be derived from ectodomain shedding of the long form of the leptin receptor OB-Rb that is essential for leptin signaling (7). The physiological role of sOB-R is not yet completely understood. There is evidence that sOB-R functions as a leptin-binding protein that can inhibit leptin transport into brain but can also delay leptin clearance from the circulation (11, 33). High levels of sOB-R can block leptin's actions (26), but sOB-R overexpression results in a lean phenotype in mice (16). In humans, plasma sOB-R levels correlate negatively with BMI and increase with fasting(4, 34).
The hypothalamic melanocortin system plays a critical role in responding to leptin, and disruption of this system at multiple levels causes obesity in humans and animals. This system consists of proopiomelanocortin (POMC) and agouti-related protein (AgRP) neurons and the brain melanocortin receptors (MC-Rs). The POMC-derived peptide α-MSH inhibits food intake and stimulates energy expenditure, whereas AgRP is an MC-R antagonist that stimulates food intake and decreases energy expenditure. Leptin stimulates POMC gene expression and peptide release and inhibits AgRP gene expression and peptide release (13). The physiology of this system has been well studied in rodents, but such studies are not possible in humans unless reliable biomarkers for brain leptin, POMC, and AgRP can be found. We have previously shown during pregnancy, despite hyperleptinemia, that elevated sOB-R may serve to decrease leptin transport into brain and that cerebrospinal fluid (CSF) levels of target neuropeptides are consistent with leptin resistance (21). However, little is known about CSF leptin and its relationship to sOB-R and to appropriate target neuropeptides in normal human subjects as a function of body weight and adiposity. The purpose of this study was to examine concentrations of leptin, POMC, and AgRP in the CSF of normal lean and obese subjects, as related to BMI and adiposity and plasma hormone levels, to determine whether CSF measurements can provide useful biomarkers of brain leptin and melanocortin activity. The relationships between CSF leptin and plasma leptin and sOB-R were studied, and sOB-R was measured for the first time in CSF. POMC was measured by specific ELISA that detects the POMC prohormone, which is the predominant POMC peptide in human CSF, with levels up to 50-fold higher than its processed peptide products (32, 37). Although it is the POMC-derived peptide α-MSH that engages brain MC-Rs, CSF levels of α-MSH are quite low. Furthermore, previous studies in the rodent have shown that CSF POMC levels, rather then CSF α-MSH levels, reflect hypothalamic POMC activity under a variety of conditions (24). AgRP was measured in CSF by ELISA and RIA with relative specificities for full-length AgRP and AgRP83–132, both of which are detected in human CSF. Finally, AgRP was also measured in plasma as a potential indicator of hypothalamic AgRP activity. Although it is clear that circulating POMC is of pituitary origin and does not reflect hypothalamic POMC activity, the origin of circulating AgRP is at present unknown. Our results support the use of CSF measurements as biomarkers of brain leptin and melanocortin activity that could be useful in studying the physiology and pathophysiology of human energy balance.
MATERIALS AND METHODS
Subjects.
Subjects were 47 healthy males and females (23 males, 24 females) aged 19–50 yr; 23 were lean and 24 were overweight/obese (OW/OB). Female subjects were studied in the early follicular phase of the menstrual cycle. Subjects were screened by medical history and all subjects were nonsmokers, were taking no medications other than vitamins, and had no history of major psychiatric disorder, alcoholism, or neurological disease. Additionally, subjects were excluded for any other clinically significant medical condition or eating disorder, for recent weight change ± 5%, and for use of weight loss products or dieting during the 6 mo prior to the study. Details on BMI, body fat percentage, and leptin and insulin levels are provided in Table 1. This study was approved by the Columbia University Institutional Review Board, and written informed consent was obtained from all subjects prior to their participation.
Table 1.
Subject characteristics
| Lean |
OW/OB |
|||
|---|---|---|---|---|
| Characteristic | Males (n = 12) | Females (n = 11) | Males (n = 11) | Females (n = 13) |
| BMI, kg/m2 | 23 ± 0.3 | 22 ± 0.6 | 34 ± 1.4 | 34 ± 2.5 |
| Body fat, % | 22 ± 1.7 | 29 ± 1.7 | 32 ± 3.5 | 51 ± 1.6 |
| Plasma leptin, ng/ml | 1.7 ± 0.4 | 7.5 ± 1.1 | 17 ± 3.2 | 46 ± 8.1 |
| CSF leptin, pg/ml | 50 ± 11 | 185 ± 28 | 225 ± 33 | 422 ± 21 |
| Insulin, μIU/ml | 7.3 ± 0.9 | 8.6 ± 1.5 | 22 ± 5.1 | 26 ± 3.5 |
Values are means ± SE. OW/OB, overweight/obese; BMI, body mass index; CSF, cerebrospinal fluid.
Protocol.
Subjects were studied in the outpatient clinical research center between 800 and 1000 after an overnight fast (from 2100). A lumbar puncture (LP) was performed using a 25-G Whitacre needle; the first 0.5 ml of CSF was discarded before collection of the 10-ml study sample. CSF samples were immediately transferred to polyethylene tubes and placed on ice. Peripheral venous EDTA plasma and serum samples were obtained immediately after the LP. CSF and blood samples were centrifuged and stored at −80°C until assays were performed. Body composition was determined by dual-energy X-ray absorptiometry (QDR 4500 A & Delphi W model) in 31 subjects; pregnancy was ruled out by β-human chorionic gonadotropin test.
Assays.
CSF leptin was measured by sensitive ELISA (R & D Systems, Minneapolis, MN) with a sensitivity of 7 pg/ml (21). Plasma leptin was measured using the same assay with appropriate dilution. Plasma and CSF sOB-R were measured by ELISA (R & D Systems). POMC was measured by two-site ELISA; the capture monoclonal antibody is directed at ACTH (10–18), and detection antibody is directed against γ-MSH (21, 30). There is 100% crossreactivity with 22K pro-ACTH but none with ACTH, α-MSH, γ-MSH, or β-endorphin. Affinity-purified human 31K POMC was used for standards. Assay sensitivity was 8 fmol/ml. ACTH was measured by RIA with an antiserum directed against ACTH (7–18) (IgG, Nashville, TN) (23). AgRP was measured by both ELISA and RIA with relative sensitivities for full-length AgRP and AgRP83–132, respectively. The ELISA (R & D Systems) uses recombinant full-length human AgRP standard. There is 17% cross-reactivity with AgRP83–132 (21). Assay sensitivity is 7 pg/ml. The RIA was performed as described previously using an antibody provided by Dr. Gregory Barsh and human AgRP83–132 for the standard (Phoenix Pharmaceuticals, Burlingame, CA) (2). There is 20% cross-reactivity with full-length AgRP. Assay sensitivity is 25 pg/ml. Serum insulin was measured by the solid-phase enzyme-labeled chemiluminescent immunometric assay Immulite1000 (Siemens Healthcare Diagnostics). Ghrelin was measured by ELISA (Millipore, Billerica, MA). Serum glucose was measured by the hexokinase method.
Characterization of the POMC and AgRP immunoactivity in CSF.
POMC immunoactivity was characterized by gel filtration of extracted CSF pooled from 12 subjects. CSF (12 ml) was extracted using a Sep-Pak C-18 cartridge (Waters Associates, Milford, MA) as described previously, except that peptides were eluted with acetonitrile-0.1% trifluoroacetic acid (60:40) (38). The extract was chromatographed on a Sephadex G-75 column, and fractions were assayed for POMC by ELISA and ACTH by RIA (21). The column was calibrated with affinity-purified 31K human POMC and ACTH-(1–39). AgRP immunoactivity was characterized in a 10-ml CSF pool from a male subject that was concentrated using an Amicon Ultra Centrifugal Filter (Millipore) and subjected to reverse phase HPLC. Samples were eluted with a gradient of 80% acetronitrile containing 0.1% TFA (39). Fractions were assayed for AgRP by RIA. The column was calibrated with 5 ng of human AgRP83–132 (Phoenix Pharmaceuticals) and with 5 ng of human full-length AgRP (R & D Systems).
Statistical analysis.
Data are expressed as means ± SE. Differences in parameters between the normal-weight lean subjects and OW/OB subjects were determined by unpaired t-test; significance was set at P < 0.05. Correlations were determined by linear regression analysis with Pearson's correlation. Analyses were performed with GraphPad Prism version 6.0b for Mac (GraphPad Software, San Diego, CA). Multiple regression analyses were performed using Statistical Analysis Systems version 9.4 (SAS Institute, Cary, NC). Insulin resistance was calculated in 37 subjects who had glucose measurements using the homeostasis model assessment (HOMA) (17).
RESULTS
Plasma and CSF leptin and sOB-R levels.
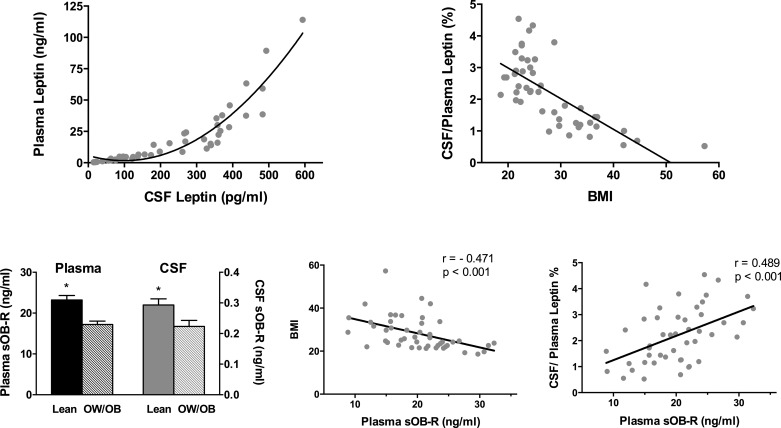
As expected, plasma leptin correlated strongly with BMI (r = 0.850) and body fat percentage (r = 0.847); (P < 0.0001). The mean CSF leptin concentration was 225 ± 23.4 (SE) pg/ml, 83-fold lower then the mean plasma leptin concentration of 18.7 ± 3.5 ng/ml. CSF leptin also correlated strongly with BMI (r = 0.709) and body fat percentage (r = 0.943, P < 0.0001). Plasma and CSF leptin concentrations in male and female lean and OW/OB subjects are depicted in Table 1. The relationship between plasma and CSF leptin is depicted in Fig. 1. There was a strong positive linear correlation between plasma and CSF leptin (r = 0.847, P < 0.0001), but this relationship was best described by a nonlinear second-order polynomial quadratic fit (r = 0.939, P < 0.0001). The relative amount of leptin measured in CSF, expressed as a percentage of plasma leptin, declined with increasing BMI, ranging from 4.5 to 0.52%, consistent with a saturable transport mechanism (Fig. 1).
Fig. 1.
Correlation between cerebrospinal fluid (CSF) and plasma leptin (top left) and between body mass index (BMI) and the CSF/plasma leptin ratio (expressed as %; top right). Mean (± SE) plasma and CSF soluble leptin receptor (sOB-R) levels were significantly higher in lean vs. overweight/obese (OW/OB) subjects (bottom left). Plasma sOB-R correlated negatively with BMI and positively with the %CSF/plasma leptin (bottom middle and bottom right).
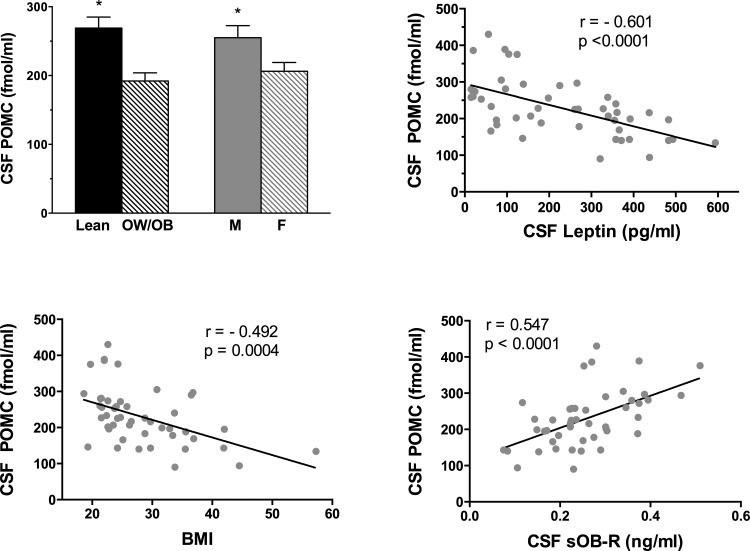
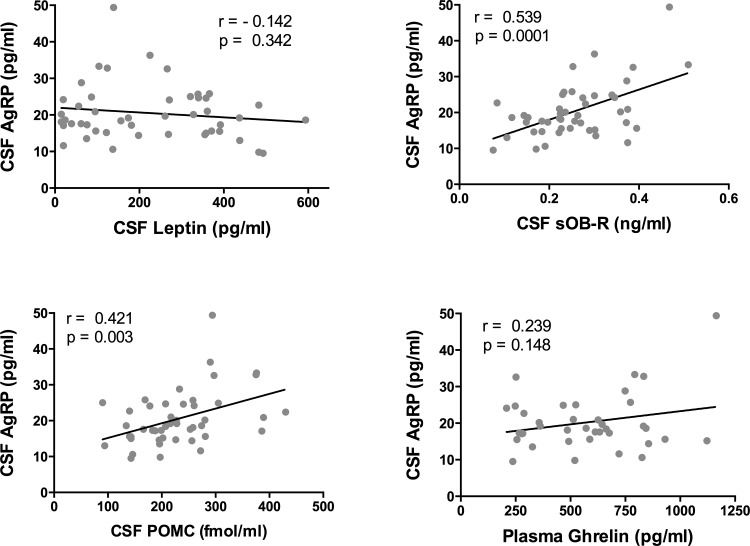
Plasma and CSF sOB-R levels are depicted in Fig. 1. Plasma sOB-R was higher in lean vs. OW/OB subjects (P < 0.0001). Mean CSF sOB-R was 78-fold lower than the mean plasma level and was also higher in lean vs. OW/OB subjects (P = 0.012). Plasma sOB-R correlated negatively with BMI (r = −0.471) and with leptin in plasma (r = −0.438) and CSF (r = −0.422, P < 0.01). Plasma sOB-R correlated positively with the CSF/plasma leptin percentage (r = 0.489, P < 0.001; Fig. 1); this correlation was not significant when the lean and OW/OB groups were analyzed separately. CSF sOB-R correlated negatively with CSF leptin (r = −0.504, P = 0.0004), plasma leptin (r = −0.526, P = 0.0002), and BMI (r = −0.411, P = 0.004). In contrast to the strong correlation between plasma and CSF leptin, plasma and CSF sOB-R were only weakly correlated (r = 0.312, P = 0.03). A sex difference was noted for CSF sOB-R, with significantly higher levels in males vs. females (0.287 ± 0.02 vs. 0.229 ± 0.02 ng/ml, P = 0.038); no sex difference was noted for plasma sOB-R. Positive correlations for CSF sOB-R (but not plasma sOB-R) were noted with CSF POMC (r = 0.547, P < 0.0001; Fig. 2) and with CSF AgRP (r = 0.539, P = 0.0001; Fig. 3).
Fig. 2.
Mean (± SE) CSF proopiomelanocortin (POMC) levels were significantly higher in lean vs. OW/OB subjects and in males vs. females (top left). There were strong negative correlations between CSF POMC and CSF leptin (top right) and between CSF POMC and BMI (bottom left). There was a strong positive correlation between CSF POMC and CSF sOB-R (bottom right).
Fig. 3.
Correlations of CSF agouti-related protein (AgRP) with other variables. CSF AgRP did not correlate significantly with CSF leptin (top left) or plasma ghrelin (bottom right). Significant positive correlations were noted between CSF AgRP and CSF sOB-R (top right) and between CSF AgRP and CSF POMC (bottom left).
CSF POMC levels.
High levels of POMC were measured in CSF. Levels were 14-fold higher than in plasma; CSF and plasma POMC were not correlated. CSF POMC levels correlated with CSF ACTH levels (r = 0.676, P < 0.0001), but POMC was 20-fold higher than ACTH on a molar basis. CSF POMC was higher in lean (269 ± 16.0) vs. OW/OB (192 ± 12) subjects (P = 0.0003) (Fig. 2). There was a strong negative correlation between CSF POMC and CSF leptin (r = −0.601, P < 0.0001; Fig. 2). Significant negative correlations were also noted between CSF POMC and plasma leptin (r = −0.531, P < 0.0001), insulin (r = −0.328, P = 0.03), and BMI (r = −0.492, P = 0.0004). When adjusted for BMI, there was no longer a significant correlation between CSF POMC and insulin. Correlations between CSF POMC and other measures are provided in Table 2. CSF POMC was higher in males than in females (P = 0.01; Fig. 2); however, the negative correlations between CSF POMC and leptin remained when the sexes were analyzed separately: r = −0.478, P = 0.02 for males and r = −0.634, P = 0.0009 for females. The negative correlations between CSF POMC and leptin also remained when the lean (r = −0.437) and OW/OB (r = −0.421) groups were analyzed separately (P = 0.04). However, this correlation was no longer significant when the lean female group was analyzed separately (r = −0.446, P = 0.16) and not seen at all when the lean male group was analyzed separately (r = 0.063, P = 0.85).
Table 2.
Pearson correlations between CSF POMC and other measurements
| Measurement | r Value | P Value |
|---|---|---|
| BMI, kg/m2 | −0.49 | 0.0004 |
| Body fat, % | −0.50 | 0.004 |
| Plasma leptin | −0.53 | 0.0001 |
| CSF leptin | −0.60 | <0.0001 |
| Plasma insulin | −0.33 | 0.03 |
| HOMA | −0.20 | 0.23 |
| CSF sOB-R | 0.55 | <0.0001 |
| Plasma sOB-R | 0.23 | 0.11 |
| Plasma POMC | −0.12 | 0.52 |
| CSF ACTH | 0.68 | <0.0001 |
| CSF AgRP | 0.42 | 0.003 |
| Plasma AgRP | 0.40 | 0.007 |
POMC, proopiomelanocortin; HOMA, homeostasis model assessment; sOB-R, soluble leptin receptor; AgRP, agouti-related peptide.
CSF and plasma and AgRP levels.
AgRP was measured in CSF by both ELISA and RIA and in plasma by ELISA only. Unless otherwise specified, all values in the text and figures were obtained with the ELISA, as it was more sensitive and had a lower interassay coefficient of variation. CSF AgRP was not significantly different in lean vs. OW/OB subjects or in male vs. female subjects when measured either by ELISA or RIA. Nonsignificant negative correlations were noted between CSF AgRP and leptin and BMI (Table 3). However, significant positive correlations were noted between CSF AgRP and CSF POMC (r = 0.421, P = 0.003) and CSF sOB-R (r = 0.539, P = 0.0001) (Fig. 3).
Table 3.
Pearson correlations between CSF AgRP and other measurements
| Measurement | r Value | P Value |
|---|---|---|
| BMI, kg/m2 | −0.11 | 0.45 |
| Body fat, % | −0.12 | 0.50 |
| Plasma leptin | −0.20 | 0.17 |
| CSF leptin | −0.14 | 0.34 |
| CSF sOB-R | 0.54 | 0.0001 |
| Plasma insulin | 0.01 | 0.95 |
| CSF POMC | 0.42 | 0.003 |
| CSF ACTH | 0.58 | 0.0001 |
| CSF AgRP (RIA) | 0.41 | 0.01 |
| Plasma AgRP | −0.15 | 0.32 |
| Plasma ghrelin | 0.24 | 0.15 |
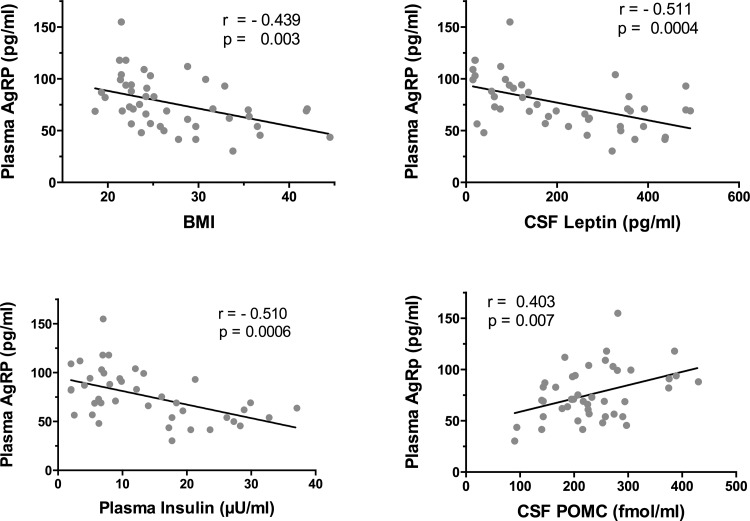
In contrast to CSF, plasma AgRP was significantly higher in lean (87.4 ± 5.0 pg/ml) than in OB/OW (63.8 ± 4.5 pg/ml) subjects (P = 0.001). No significant sex difference was noted. There were significant negative correlations between plasma AgRP and BMI as well as with adiposity, plasma, and CSF leptin and insulin (Fig. 4 and Table 4). After adjustment for BMI, the negative correlation between plasma AgRP and insulin persisted (P = 0.055). Plasma AgRP also correlated negatively with HOMA (r = −0.580, P = 0.0003). A highly significant positive correlation was noted between plasma AgRP and CSF POMC (r = 0.403, P = 0.007; Fig. 4).
Fig. 4.
Correlations of plasma AgRP with other variables. There were significant negative correlations between plasma AgRP and BMI and CSF leptin (top right and top left) and insulin (bottom left). There was a significant positive correlation between plasma AgRP and CSF POMC (bottom right).
Table 4.
Pearson correlations between plasma AgRP and other measurements
| Measurement | r Value | P Value |
|---|---|---|
| BMI, kg/m2 | −0.44 | 0.003 |
| Body fat, % | −0.51 | 0.005 |
| Plasma leptin | −0.40 | 0.006 |
| CSF leptin | −0.51 | 0.0004 |
| Plasma insulin | −0.51 | 0.0006 |
| HOMA | −0.58 | 0.0003 |
| CSF POMC | 0.40 | 0.007 |
| CSF AgRP-ELISA | −0.15 | 0.32 |
| CSF AgRP-RIA | −0.20 | 0.25 |
| CSF sOB-R | 0.29 | 0.05 |
| Ghrelin | 0.01 | 0.96 |
Characterization of the POMC and AgRP immunoactivity in CSF.
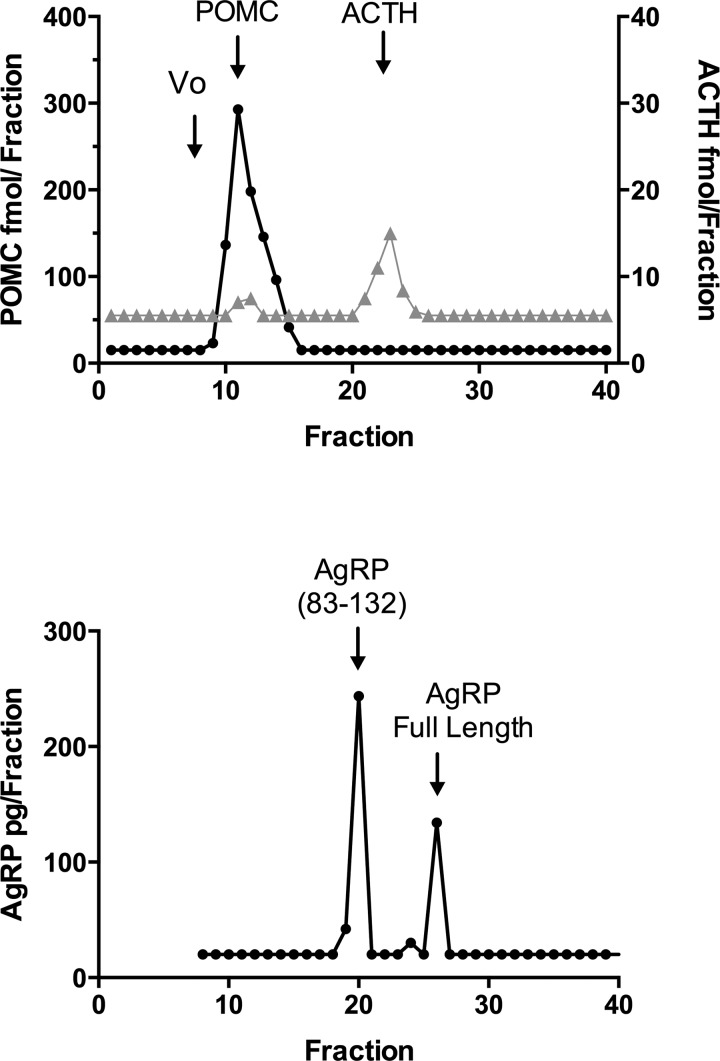
POMC and ACTH immunoactivity were characterized by gel filtration in an extracted 12-ml CSF pool. The majority of the POMC immunoactivity eluted in the same position as affinity-purified 31K human POMC (Fig. 5). A much smaller amount of ACTH immunoactivity was detected with the ACTH RIA, with most of the immunoactivity eluting in the position of ACTH-(1–39). AgRP immunoactivity in the CSF was characterized by HPLC, and fractions were assayed by AgRP RIA. There were two peaks of AgRP immunoactivity corresponding to the COOH-terminal and full-length peptides (Fig. 5).
Fig. 5.
Sephadex G-75 chromatography of pooled CSF extracts (top). Fractions were assayed for POMC (●) by ELISA and ACTH (gray triangles) by RIA. Arrows indicate the void volume (Vo) and the elution positions of POMC and ACTH standards. HPLC of a CSF pool (bottom) with fractions assayed for AgRP by RIA. Arrows indicate the elution positions of full-length AgRP and AgRP83–132 standards.
DISCUSSION
This study provides novel information about levels of the melanocortin neuropeptides POMC and AgRP in human CSF as a function of BMI and adiposity. The results also confirm and extend previous studies that have examined the relationship between plasma and CSF leptin in lean and obese subjects and provide new data about the relationship between leptin and sOB-R in plasma and CSF. A strength of this study is that all subjects were healthy volunteers, in contrast to previous studies of leptin in CSF that included patients having LPs for clinical purposes with various diagnoses and degrees of illness.
CSF leptin was 83-fold lower than in plasma and correlated strongly with plasma leptin in a nonlinear manner over a wide spectrum of BMI. CSF leptin correlated strongly with BMI, adiposity, and plasma insulin. Leptin was measured with a sensitive ELISA that enabled detection of leptin in all CSF samples. CSF leptin, expressed as a percentage of plasma leptin, declined with increasing BMI, ranging from 4.5 to 0.52%, consistent with a saturable transport mechanism. This confirms and extends previous human studies showing that CSF leptin reflects adipocyte-derived plasma leptin levels (3, 28) and is consistent with studies in mice demonstrating that leptin enters the brain by a receptor-mediated saturable transport mechanism (22).
Plasma levels of sOB-R were measured to determine whether sOB-R impacted leptin transport into brain, as reflected by CSF leptin levels. We have shown previously that there is a diurnal rhythm for plasma sOB-R and that levels were lowest after midnight when CSF leptin levels peaked and postulated that this fall in sOB-R may facilitate leptin transport into brain at night (37). We have also shown that during pregnancy elevated plasma leptin levels are accompanied by elevated sOB-R levels without any change in CSF leptin levels, consistent with an inhibitory effect of sOB-R on leptin transport into brain (21). In contrast, in the current study, the relative amount of leptin in CSF was higher in the presence of high circulating sOB-R levels. Plasma sOB-R correlated negatively with BMI and plasma leptin, as reported previously (4, 34). In addition, we show for the first time that plasma sOB-R also correlated negatively with CSF leptin. However, plasma sOB-R correlated positively with the CSF to plasma leptin ratio. This may in part be a consequence of the already saturated transport system in the very obese subjects. Of note is that no significant correlation between sOB-R and CSF to plasma leptin ratio was noted among the lean subjects. Thus under some conditions sOB-R may influence leptin transport into brain, but this was not evident in the current study.
sOB-R was also measured for the first time in CSF, and levels were 78-fold lower than plasma levels. In contrast to leptin, sOB-R is not known to be transported from the blood into the brain. It is possible that CSF sOB-R may be derived from blood, but it may also be derived from brain and may reflect cellular expression of the long form of the leptin receptor in brain. CSF sOB-R and plasma sOB-R were similar in that both were significantly higher in lean vs. OW/OB subjects, but differences were noted when sOB-R levels in CSF and plasma were correlated with other variables, including CSF POMC, which correlated strongly with CSF but not plasma sOB-R. A sex difference was also noted for CSF but not plasma sOB-R levels. These differences suggest that CSF sOB-R may derive from a central rather than a peripheral source.
High levels of POMC were detected in CSF, as we have reported previously, but we now show significant differences related to adiposity and sex difference. Characterization by gel filtration showed that the majority of the POMC immunoactivity in CSF was eluted in the position of the 31K POMC standard. CSF POMC levels correlated with but were 20-fold higher than CSF levels of the POMC-derived peptide ACTH. No change in the ratio of POMC to ACTH was noted in lean vs. OW/OB subjects. Thus there was no indication that high POMC levels resulted from decreased POMC processing, which could affect energy balance (36). CSF POMC was 14-fold higher than plasma POMC, and plasma and CSF POMC were not correlated, consistent with prior studies showing that plasma and CSF POMC derive from the pituitary and brain, respectively, and are regulated differently (27, 32). Although it is the POMC-derived peptide α-MSH that engages brain MC-Rs, it is difficult to reproducibly measure the low levels of α-MSH in CSF. This may in part result from the inactivation of α-MSH by the enzyme prolylcarboxypeptidase (35). An earlier study in human subjects did not find any differences in CSF α-MSH levels related to BMI (19). However, previous studies in the rodent have shown that levels of the intact POMC prohormone in CSF rather than ACTH or α-MSH can serve as a better measure of hypothalamic POMC activity (24). Of note is that diurnal rhythms have been reported for POMC in human CSF and for POMC expression in the rodent hypothalamus (29, 37). In the current study, CSF POMC was higher in lean vs. OW/OB subjects, and there were striking negative correlations with leptin and BMI. CSF POMC was higher in males than in females, but the negative correlation between CSF POMC and leptin and BMI remained when the sexes were analyzed separately. This is similar to our study in female monkeys that showed negative correlations between CSF POMC and leptin and BMI (39). These results suggest that POMC plays a primary role in regulating body weight and adiposity. It is possible that the lower level of POMC in the CSF of obese subjects is secondary to the leptin resistance that is well documented in POMC neurons in obese animals (5). Alternatively, it could be a consequence of the decreased neuronal remodeling that is caused by a high-fat diet in animals or the hypothalamic inflammation and gliosis that have been demonstrated in obese animals and humans. (10, 18, 31). However, in our study, the fact that a negative correlation between CSF POMC and leptin persisted when the lean subjects were analyzed separately supports the interpretation that POMC plays a primary role in regulating body weight and adiposity.
AgRP was measured in CSF and plasma with the expectation that CSF AgRP would reflect hypothalamic AgRP activity, as anatomic studies in the monkey and rat show dense AgRP staining along the third ventricle (8). CSF AgRP was not different in lean vs. OW/OB individuals when measured by either ELISA or RIA. Only nonsignificant negative correlations were noted between CSF AgRP and leptin and BMI. However, CSF AgRP was strongly correlated with CSF POMC and with CSF sOB-R. In contrast to CSF AgRP, there were highly significant negative correlations between plasma AgRP and BMI, adiposity, leptin, insulin, and HOMA. Of note is that the negative correlation with insulin persisted when adjusted for BMI. This is consistent with the known role of AgRP neurons in responding to insulin and regulating glucose metabolism independently of changes in body weight (12, 25). Plasma AgRP also exhibited a strong positive correlation with CSF POMC. The origin of circulating AgRP is not entirely clear, but there is evidence that circulating AgRP may reflect hypothalamic AgRP activity. Anatomic evidence shows heavy AgRP fiber staining in the monkey median eminence, which could be secreted into blood (8). Furthermore, we have found that AgRP increases in human plasma after fasting or diet-induced weight loss, consistent with changes in hypothalamic AgRP expression seen after fasting and weight loss in rodents (20). Thus our measurements of plasma AgRP may in fact reflect brain AgRP activity. If so, the negative correlations with BMI, adiposity, and leptin are consistent with the known inhibitory effects of leptin on AgRP neurons. Although the adrenals express significant amounts of AgRP mRNA and may be another potential source for circulating AgRP, it is notable that plasma AgRP levels did not change significantly in rats after adrenalectomy (15). We have similarly found that AgRP persists in human plasma after bilateral adrenalectomy in two cases (Wardlaw SL and Page-Wilson G, unpublished observations). The differences noted in the current study between CSF and plasma AgRP levels may relate to anatomic differences in AgRP fiber tracks that gain access to CSF and blood, respectively. Both plasma and CSF AgRP are strongly positively correlated with CSF POMC. The explanation for this is at present unclear, but it may be that the activities of both sets of neurons and the entire brain melanocortin circuit are increased in lean vs. obese subjects. Of note is that the reactive gliosis and suppression of neurogenesis in response to high-fat feeding in rodents have been shown to affect both POMC and AgRP neurons (10, 18). More mechanistic studies will be required to elucidate the nature of this interesting relationship between POMC and AgRP in human subjects as related to body weight and adiposity.
In summary, these studies provide evidence that CSF measurements may provide useful biomarkers for brain leptin, leptin receptor activity, and POMC activity and could be useful in studying the physiology and pathophysiology of human energy balance. The results also suggest that plasma AgRP measurements may be a useful biomarker of brain AgRP activity.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant RO1-DK-093920 (to S. L. Wardlaw), the National Center for Advancing Translational Sciences (National Institutes of Health Grants UL1-TR-000040 and UL1-RR-024156), NIDDK Grant RO1-DK-64773 (to R. L. Leibel and M. Rosenbaum), and the Atkins Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.P.-W. and S.L.W. conception and design of research; G.P.-W., K.M., M.R., R.L.L., R.S., and S.L.W. performed experiments; G.P.-W. and S.L.W. interpreted results of experiments; G.P.-W. and S.L.W. drafted manuscript; G.P.-W., A.W., M.R., R.L.L., R.S., and S.L.W. edited and revised manuscript; G.P.-W., K.M., A.W., M.R., R.L.L., R.S., and S.L.W. approved final version of manuscript; K.M. and S.L.W. analyzed data; K.M. prepared figures.
ACKNOWLEDGMENTS
We are grateful to our study subjects for their participation, to Shveta Dighe for help with recruitment and administration, and to Catherine Huang for help with data analysis.
REFERENCES
- 1.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Breen TL, Conwell IM, Wardlaw SL. Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain Res 1032: 141–148, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348: 159–161, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 51: 2105–2112, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest 121: 2087–2093, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge H, Huang L, Pourbahrami T, Li C. Generation of soluble leptin receptor by ectodomain shedding of membrane-spanning receptors in vitro and in vivo. J Biol Chem 277: 45898–45903, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140: 1408–1415, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks WA, Flier JS. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology 143: 775–783, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA 107: 14875–14880, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem 276: 6343–6349, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Korner J, Chua SC Jr, Williams JA, Leibel RL, Wardlaw SL. Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology 70: 377–383, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Wardlaw SL. The central melanocortin system and the regulation of energy balance. Front Biosci 12: 3994–4010, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Li JY, Finniss S, Yang YK, Zeng Q, Qu SY, Barsh G, Dickinson C, Gantz I. Agouti-related protein-like immunoreactivity: characterization of release from hypothalamic tissue and presence in serum. Endocrinology 141: 1942–1950, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Lou PH, Yang G, Huang L, Cui Y, Pourbahrami T, Radda GK, Li C, Han W. Reduced body weight and increased energy expenditure in transgenic mice over-expressing soluble leptin receptor. PLoS One 5: e11669, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 18.McNay DE, Briancon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest 122: 142–152, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam SY, Kratzsch J, Kim KW, Kim KR, Lim SK, Marcus C. Cerebrospinal fluid and plasma concentrations of leptin, NPY, and alpha-MSH in obese women and their relationship to negative energy balance. J Clin Endocrinol Metab 86: 4849–4853, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen KT, Atalayer D, Page-Wilson G, Meece K, Bainbridge HA, Gordon R, White A, Smiley RM, Korner J, Wardlaw SL. Effects of acute and chronic calorie restriction on plasma and CSF leptin and melanocortin neuropeptides in human subjects. 97th Annual Meeting of the Endocrine Society, San Diego, CA, 2015. [Google Scholar]
- 21.Page-Wilson G, Reitman-Ivashkov E, Meece K, White A, Rosenbaum M, Smiley RM, Wardlaw SL. Cerebrospinal fluid levels of leptin, proopiomelanocortin, and agouti-related protein in human pregnancy: evidence for leptin resistance. J Clin Endocrinol Metab 98: 264–271, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci 68: 2705–2714, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos AD, Wardlaw SL. Endogenous alpha-MSH modulates the hypothalamic-pituitary-adrenal response to the cytokine interleukin-1beta. J Neuroendocrinol 11: 315–319, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Pritchard LE, Oliver RL, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, White A. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology 144: 760–766, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Ren H, Orozco IJ, Su Y, Suyama S, Gutierrez-Juarez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, Accili D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell 149: 1314–1326, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaab M, Kausch H, Klammt J, Nowicki M, Anderegg U, Gebhardt R, Rose-John S, Scheller J, Thiery J, Kratzsch J. Novel regulatory mechanisms for generation of the soluble leptin receptor: implications for leptin action. PLoS One 7: e34787, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlachter LB, Wardlaw SL, Tindall GT, Frantz AG. Persistence of beta-endorphin in human cerebrospinal fluid after hypophysectomy. J Clin Endocrinol Metab 57: 221–224, 1983. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2: 589–593, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Steiner RA, Kabigting E, Lent K, Clifton DK. Diurnal rhythm in proopiomelanocortin mRNA in the arcuate nucleus of the male rat. J Neuroendocrinol 6: 603–608, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Stovold R, Meredith SL, Bryant JL, Babur M, Williams KJ, Dean EJ, Dive C, Blackhall FH, White A. Neuroendocrine and epithelial phenotypes in small-cell lung cancer: implications for metastasis and survival in patients. Br J Cancer 108: 1704–1711, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsigos C, Crosby SR, Gibson S, Young RJ, White A. Proopiomelanocortin is the predominant adrenocorticotropin-related peptide in human cerebrospinal fluid. J Clin Endocrinol Metab 76: 620–624, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol 214: 301–305, 2008. [DOI] [PubMed] [Google Scholar]
- 34.van Dielen FM, van 't Veer C, Buurman WA, Greve JW. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. J Clin Endocrinol Metab 87: 1708–1716, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest 119: 2291–2303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol 660: 213–219, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardlaw SL, Burant CF, Klein S, Meece K, White A, Kasten T, Lucey BP, Bateman RJ. Continuous 24-hour leptin, proopiomelanocortin, and amino acid measurements in human cerebrospinal fluid: correlations with plasma leptin, soluble leptin receptor, and amino acid levels. J Clin Endocrinol Metab 99: 2540–2548, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia L, Matera C, Ferin M, Wardlaw SL. Interleukin-1 stimulates the central release of corticotropin-releasing hormone in the primate. Neuroendocrinology 63: 79–84, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Xiao E, Kim AJ, Dutia R, Conwell I, Ferin M, Wardlaw SL. Effects of estradiol on cerebrospinal fluid levels of agouti-related protein in ovariectomized rhesus monkeys. Endocrinology 151: 1002–1009, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]