Abstract
Renal hypoxia contributes to chronic kidney disease (CKD) progression, as validated in experimental and human CKD. In the early stages, increased oxygen consumption causes oxygen demand/supply mismatch, leading to hypoxia. Hence, early targeting of the determinants and regulators of oxygen consumption in CKD may alter the disease course before permanent damage ensues. Here, we focus on hypoxia inducible factor-1α (HIF-1α) and AMP-activated protein kinase (AMPK) and on the mechanisms by which they may facilitate cellular hypoxia adaptation. We found that HIF-1α activation in the subtotal nephrectomy (STN) model of CKD limits protein synthesis, inhibits apoptosis, and activates autophagy, presumably for improved cell survival. AMPK activation was diminished in the STN kidney and was remarkably restored by HIF-1α activation, demonstrating a novel role for HIF-1α in the regulation of AMPK activity. We also investigated the independent and combined effects of HIF-1α and AMPK on cell survival and death pathways by utilizing pharmacological and knockdown approaches in cell culture models. We found that the effect of HIF-1α activation on autophagy is independent of AMPK, but on apoptosis it is partially AMPK dependent. The effects of HIF-1α and AMPK activation on inhibiting protein synthesis via the mTOR pathway appear to be additive. These various effects were also observed under hypoxic conditions. In conclusion, HIF-1α and AMPK appear to be linked at a molecular level and may act as components of a concerted cellular response to hypoxic stress in the pathophysiology of CKD.
Keywords: hypoxia inducible factor-1α, AMP-activated protein kinase
chronic kidney disease (CKD) is highly prevalent, afflicting more than 10 million Americans. Its impact extends well beyond being a precursor to end-stage renal disease, given the associated morbidity, mortality, and high risk of cardiovascular disease. Existing therapies targeting known mechanisms of CKD progression, such as angiotensin inhibition, have been inadequate in halting or slowing the course of disease. Recent data suggest that oxygen demand/supply mismatch leading to renal tissue hypoxia, which promotes extracellular matrix production, collagen deposition, and fibrosis, may be the common final pathway of progression in CKD (24).
This chronic hypoxia hypothesis proposed by Norman and Fine (28) has gained significant traction and has been validated by several investigators in experimental and human CKD (8, 11, 15, 16, 24, 28). The major focus of research in this area has been on the compromised oxygen supply due to structural changes that impair blood flow. However, tubular hypoxia precedes any structural changes that occur late in the disease (22). In the early stages, transport and metabolic adaptations increase oxygen demand, leading to early hypoxia, which is a fibrogenic stimulus and can instigate subsequent structural changes, further impairing local oxygenation. Hence, interventions that target the determinants and regulators of oxygen consumption at the early stages offer the benefit of potential therapeutics to alter the course of disease prior to the development of significant fibrosis. We and others have shown increased oxygen consumption factored for tubular sodium (Na) reabsorption in the early subtotal nephrectomy (STN) model of CKD in rats despite a significant reduction in glomerular filtration rate (GFR) (7, 14, 26). Lowering nephron oxygen consumption by various treatments improved kidney function (6, 7), but the underlying mechanisms for the reduction in oxygen consumption are not clear.
Hypoxia-inducible factor-1α (HIF-1α) is a primary oxygen sensor and regulator of oxygen delivery and consumption (11) and is expressed in the kidney under hypoxic conditions (29). We have demonstrated previously that pharmacological HIF-1α activation in the STN kidney lowers nephron oxygen consumption and improves kidney function and morphology (6). In this study, we investigated the possible underlying cellular mechanisms for the improvement in kidney oxygenation and function with HIF-1α activation. AMPK is a ubiquitously expressed, highly conserved, key energy sensor and regulator of cellular metabolic activity (13). It is activated by a variety of cellular stresses that increase the cellular AMP/ATP ratio, such as nutrient or glucose deprivation, exercise, hypoxia, or ischemia. AMPK is activated by phosphorylation of its α-catalytic subunit on threonine 172 (Thr172) by upstream kinases. There is abundant AMPK expression in the kidney, but the understanding of its impact on energy metabolism in renal (patho)physiology is limited. There appears to be a significant overlap between the actions and targets of HIF-1α and AMPK and the stimuli that activate them. Hence, we also investigated the interaction between HIF-1α and AMPK in the regulation of cellular hypoxia adaptation in STN.
METHODS
All experimentation was conducted according to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and our experiments were approved by the Veterans Affairs (VA) San Diego Healthcare System Institutional Animal Care and Use Committee. All animal experiments were conducted in male Wistar rats 300–350 g body wt (Harlan). All rats received free access to tap water and standard rat chow.
Subtotal nephrectomy.
This procedure was performed with sterile technique on a temperature-controlled surgical table and under anesthesia with pentobarbital sodium (50 mg/kg ip) (7). A small right flank incision (1.5-cm long) was made. The muscle was clamped for 1 min before cutting to prevent bleeding. Adrenal gland was carefully separated from the right kidney. The exposed right kidney was ligated with a 4-0 silk suture tied tightly around the renal artery and vein. The right kidney was then cut from the vasculature and removed. The adrenal gland and attached vascular tissues were returned to the retroperitoneum. The fascia was closed with a silk suture and the skin with steel wound clips. Then, a left flank incision was made and the left kidney maneuvered to expose the renal artery. Two branches of left renal artery were ligated with 4-0 silk suture. The kidney was replaced back into the body, and the incision was closed as described above. Rats were kept warm with a heating pad until ambulatory and administered a dose of buprenorphine analgesic.
Clearance and renal blood flow experiments.
Under inactin anesthesia (100 mg/kg body wt ip), a tracheostomy tube (PE250 tubing) was placed, and the left internal jugular (PE50), left femoral artery (PE50), and urinary bladder (PE50) were cannulated (7). Body temperature was maintained at 37°C. At the end of the surgical preparation, rats underwent a 60-min equilibration period, during which time [3H]inulin in NaCl-NaHCO3 at a rate of 1 ml/h and NaCl-NaHCO3 solution at 1.4 ml/h were infused. Mean arterial pressure was monitored by connecting the femoral artery catheter to a transducer and a desktop computer loaded with the WinDaq software (DATAQ Instruments, Akron, OH). Blood samples for hematocrit and [3H]inulin were obtained from the femoral artery catheter at the beginning and end of each urine collection. The left kidney blood flow [renal blood flow (RBF), ml/min] was monitored with a perivascular ultrasonic transit time flow probe (Transonics T420, Ithaca, NY) connected to a computer for continuous recording.
Reagents and chemicals.
All chemical reagents used in the studies presented here were purchased from Sigma unless otherwise stated. AICAR (5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside) was purchased from Toronto Research Chemicals. YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole] was obtained from Enzo Life Sciences. Dimethyloxallyl glycine (DMOG) was obtained from Cayman Chemical. For in vivo HIF induction, DMOG (10 mg·kg−1·day−1) was administered subcutaneously from the day of STN surgery until the day of tissue harvesting. We have demonstrated HIF-1α activation with this agent (6). Kidneys were harvested 7–8 days after STN surgery for molecular analyses.
Antibodies.
Anti-AMPK-α, anti-AMPKα-p-Thr172, anti-cleaved caspase-3, anti-LC3I/II, and anti-p-ACC (acetyl-CoA carboxylase) antibodies were all obtained from Cell Signaling Technology. Anti-p-P70 S6 kinase-α (Thr389) was obtained from Santa Cruz Biotechnology. Purified mouse anti-human HIF-1α was obtained from BD Biosciences. Anti-β-actin antibody was obtained from Sigma. Horseradish peroxidase-conjugated secondary IgGs were purchased from Amersham Biosciences.
Cell culture.
Human proximal tubule HK-2 (human kidney-2) cells (a generous gift from Dr. Ora Weisz) were passaged every 3–4 days in 75-mm flasks containing combined Dulbecco's modified Eagle's and Ham's F-12 medium (Life Technologies) supplemented with 5 μg/ml insulin, 0.02 μg/ml dexamethasone, 0.01 μg/ml selenium, 0.05 μg/ml transferrin, 2 mM l-glutamine (all from Sigma), 10% fetal bovine serum (Atlanta Biologicals), 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma). The cells were then incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C. One day before the experiment, cells were plated onto 12-well plates at ∼50–60% confluence. Cells were then treated either with the HIF-1α activators DMOG (1 mM) and/or l-mimosine (250 μM) or the HIF-1α inhibitor YC-1 (10 μM) for 16 h. Control cells were treated with vehicle (DMSO).
Stably transduced Madin-Darby canine kidney (MDCK) type II cells (34), either vector control or stable AMPKα1 knockdown (KD) cells (kindly provided by Dr. Michael Caplan), were cultured in α-MEM (GIBCO), with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified incubator with 5% CO2.
Immunoblotting.
Kidneys were harvested and homogenized in cold lysis buffer [1% Triton X-100, 0.5% deoxycholic acid, 1 mM EDTA, 0.1% SDS, 4 mM NaF, complete protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany), and 1 mM NaVO4 in PBS]. Lysates at 50 μg/lane were resolved on NuPAGE gels in MOPS buffer (Invitrogen, Carlsbad, CA). Gel proteins were transferred to nitrocellulose membranes and immunoblotted with the appropriate primary antibody as indicated. The secondary antibody was horseradish peroxidase-conjugated (Cell Signaling Technology, Boston, MA) for autoradiographic detection by ECL Plus (Amersham Pharmacia, Piscataway, NJ), with densitometric analysis by ImageJ Software (National Institutes of Health, Bethesda, MD). All figures were normalized to a housekeeping protein that was run on the same membrane.
HK-2 and MDCK cells were cultured as described above. After treatment, cells were washed once with ice-cold PBS and lysed in lysis buffer containing 1% Triton X-100, 50 mM NaCl, 50 mM NaF, 5 mM NaPPi, 20 mM Tris·HCl (pH 7.4), 1 mM EDTA, 250 mM sucrose, 1 mM DTT, 1 mM PMSF, and complete protease inhibitor cocktail (Roche). After incubation on ice for 15 min, lysates were centrifuged at 17,000 g for 15 min at 4°C, and the supernatants were supplemented with 4× SDS Laemmli sample buffer. After the samples were heated at 65°C for 10 min, 30 μg of total lysate was separated by SDS-PAGE on a 4–12% gradient gel (Nu-PAGE; Invitrogen), transferred to a nitrocellulose membrane, and then immunoblotted with primary antibodies overnight at 4°C, followed by goat anti-mouse or anti-rabbit secondary antibodies for 1 h at room temperature. Densitometric quantitation of the relevant bands was performed using the Versa-Doc imaging system and Quantity One software (Bio-Rad).
Hypoxia studies.
Cells were seeded in a 12-well plate, and when the experiment was performed they were at ∼50–60% confluence; then, they were exposed to normoxic and hypoxic [Hypoxia Incubator Chamber (Stemcell) filled with 1% O2, 94% N2, and 5% CO2 for 16 h] conditions. Cells were also treated with the AMPK activator AICAR (2 mM) for 16 h. HIF-1α activation and inhibition were induced with DMOG and YC-1, respectively.
Click-it TUNEL Alexa Fluor 488 imaging assay.
Apoptosis was detected by terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP)-alkyne nick-end labeling (TUNEL) using a commercial kit (Click-iT TUNEL Alexa Fluor 488 Imaging Assay Kit; Life Technologies, Eugene, OR). Briefly, MDCK vector or AMPK KD cells were seeded on 12-mm microscope glass coverslips and incubated overnight. Cells were treated with the AMPK activator AICAR (2 mM), the HIF-1α activator DMOG (1 mM), or the HIF-1α inhibitor YC-1 (10 μM) for 16 h. Cells on coverslips were then washed with PBS and fixed with 4% paraformaldehyde for 15 min before being permeabilized with 0.25% Triton X-100 in PBS for 20 min. The permeabilized cells were then washed twice in deionized water. The coverslips were then incubated in TdT reaction buffer for 10 min, followed by TdT and E-dUTP in TdT buffer at 37°C for 1 h. Cells were then washed with 3% BSA in PBS for 5 min. Nuclear staining was carried out using 100 μl 1× Hoechst 33342 solution for 15 min. ProlongGold (Life Technologies) was used as the mounting media. The cells were visualized using Leica DM6000B microscopy (Leica, Solms, Germany). The images were imported into Volocity 4-D software (Perkin-Elmer, Waltham, MA) and, after image reconstruction and contrast correction, exported as TIFF files. Composite images were prepared in Illustrator CS2 (Adobe, San Jose, CA).
AMPKα1 activity.
The level of activated AMPKα1 in renal cortex, medulla, and whole kidney homogenates was determined by a sandwich ELISA to measure AMPKα1 phosphorylated at Thr172 (R & D Systems, Minneapolis, MN) (5).
Immunohistochemistry.
LC3 immunoperoxidase staining was performed using the method described previously (23). As a primary antibody, rabbit polyclonal antibody against LC3A/B (1:200; Cell signaling Technology) was applied for 12 h at 4°C, and biotin-XX goat anti-rabbit IgG (H + L) (1:200; Invitrogen Molecular Probes) secondary antibody was applied for 1 h at room temperature.
Statistical analysis.
Data were analyzed by one- or two-way analysis of variance as appropriate, using commercial software (SigmaPlot) with appropriate post hoc tests. Unless stated otherwise, results are presented as group means ± SE.
RESULTS
Physiological measurements.
Clearance experiments were performed in male Wistar rats at 1 wk after STN surgery. Some STN rats were treated with the prolyl hydroxylase inhibitor DMOG to activate HIF-1α for 1 wk. Systemic characteristics during clearance experiments are presented in Table 1. Mean arterial pressure was modestly elevated in the STN rats compared with controls. RBF and GFR were significantly lower in the STN rats. Treatment with DMOG significantly improved both RBF and GFR in STN rats. Urine flow rates were significantly higher in the STN rats compared with controls. Urinary sodium excretion was lower in the STN rats compared with controls. There was a trend toward increased urinary sodium excretion in the STN rats treated with DMOG. This finding is consistent with prior reports demonstrating a natriuretic effect of HIF-1α activation (21, 36).
Table 1.
Systemic characteristics
| Groups | MAP, mmHg | RBF (Single Kidney), ml/min | GFR, ml/min | Urine Flow Rate, μl/min | Urinary Na Excretion, nEq/min |
|---|---|---|---|---|---|
| Control (n = 6) | 99 ± 3 | 6.3 ± 0.6 | 2.75 ± 0.26 | 3.5 ± 0.5 | 909.98 ± 91 |
| STN (n = 9) | 117 ± 6 | 4.0 ± 0.6 | 0.78 ± 0.08 | 9.3 ± 1.4 | 455.63 ± 41 |
| STN + DMOG (n = 9) | 119 ± 7 | 6.2 ± 0.5 | 1.24 ± 0.06 | 10.5 ± 1.5 | 698.02 ± 115 |
| Analyses | CON vs. STN, P = 0.03 | CON vs. STN, P = 0.02; STN vs. STN + DMOG, P = 0.01 | CON vs. STN, P = 0.0001; STN vs. STN + DMOG, P = 0.0003 | CON vs. STN, P = 0.006 | CON vs. STN, P = 0.0002; STN vs. STN + DMOG, P = 0.05 |
Values are means ± SE.
MAP, mean arterial pressure; RBF, renal blood flow; GFR, glomerular filtration rate; STN, subtotal nephrectomy; DMOG, dimethyloxallyl glycine; CON, control.
Cellular effects of HIF-1α activation in STN.
We have shown previously that HIF-1α activation in the STN kidney lowers nephron oxygen consumption factored for Na reabsorption and improves kidney function and morphology (6). To investigate the possible underlying cellular mechanisms for the improvement in kidney oxygen consumption, function, and morphology with HIF-1α activation, we examined molecular markers of cellular survival and death in the STN kidney and the response to HIF-1α activation.
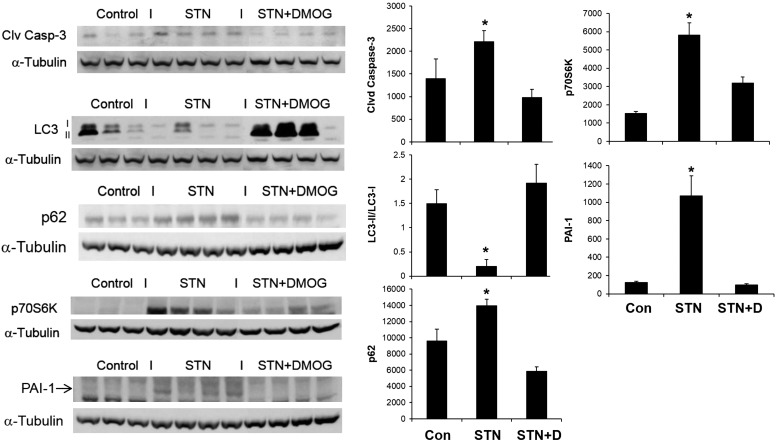
At 1 wk after STN, we examined the kidneys for expression of the cleaved (active) form of caspase 3, which is an important marker of the cell's entry into the apoptotic pathway and commonly used as a readout in an apoptosis assay (27). The STN kidneys showed increased expression of cleaved caspase 3 by immunoblotting, demonstrating evidence of increased apoptosis (Fig. 1).
Fig. 1.
Cellular effects of hypoxia-inducible factor-1α (HIF-1α) activation in subtotal nephrectomy (STN). Western blot densitometry normalized to α-tubulin. Increased apoptosis [cleaved (clvd) caspase 3] in STN was reduced by dimethyloxallyl glycine (DMOG). Autophagy (LC3II/LC3I, p62) was low in STN and increased by DMOG. Increased protein synthesis in STN was reduced by DMOG treatment. Increased plasminogen activator inhibitor-1 (PAI-1) expression in STN kidneys reduced by DMOG treatment. *P < 0.05, STN vs. control (CON) and STN + DMOG (STN + D) in all figures; n = 3–4 each.
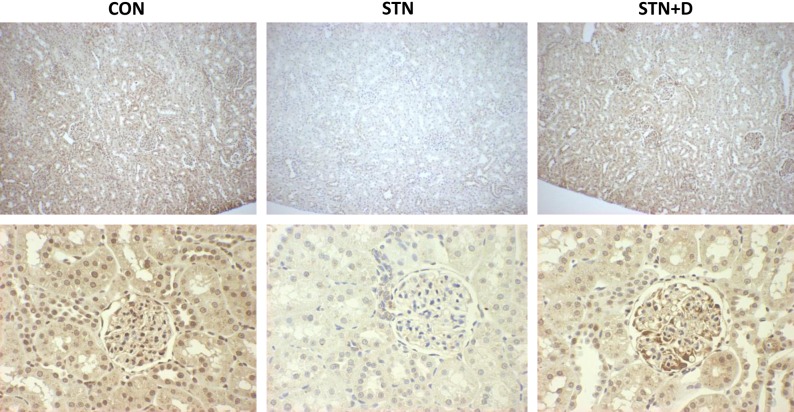
Next, we assessed autophagy by examining the expression of the autophagy protein microtubule-associated protein light chain 3 (LC3). LC3 is processed to cytosolic, preautophagic LC3-I and subsequently proceeds to an autophagosome membrane-incorporated form (LC3-II) upon activation of autophagy. An increase in the ratio of autophagosomal membrane-bound LC3-II to cytosolic LC3-I is an established indicator of autophagic activity and specifically for increased presence of autophagosomes in cells (2, 17). STN kidneys demonstrated decreased autophagy, as demonstrated by a decrease in the ratio of LC3-II to LC3-I (Fig. 1). We also performed immunohistochemical staining of LC3 in kidneys from control, STN, and STN treated with DMOG rats. As demonstrated in Fig. 2, the STN kidneys demonstrated reduced staining compared with controls, and DMOG treatment increased LC3 staining, consistent with the above protein expression results.
Fig. 2.
Reduced LC3 staining in STN. Immunohistochemical staining for light chain 3 (LC3). Control kidneys demonstrate uniform staining of glomeruli and tubules. STN kidneys demonstrated reduced staining, whereas in DMOG-treated STN kidneys, LC3 staining is increased similarly to control kidney. Top: ×100 magnification; bottom: ×400 magnification (n = 3–4/group). Multiple sections were examined. Representative slides are shown.
Sequesterome-1, or p62, is a linker protein that binds to ubiquitinated proteins destined for removal. It also binds to LC3 and serves as a link between LC3 and ubiquitinated substrates by binding LC3 and anchoring its cargo proteins into the forming autophagosome (17). The accumulation of p62 is a marker that autophagy is low or deficient. We observed increased p62 accumulation in the STN kidneys, also indicating a low level of autophagy (Fig. 1). Remarkably, treatment of STN animals with DMOG to activate HIF-1α for 7 days (6), referred to in Fig. 1 as STN + D, rescinded both of these processes by decreasing apoptosis and increasing autophagy in the STN kidneys.
STN kidneys also demonstrated evidence of increased protein synthesis, as assessed by the expression of phosphorylated p70S6K-serine/threonine kinase downstream of mTOR, reflecting the compensatory hypertrophy that is characteristic of remnant nephrons. Interestingly, the increased phosphorylated p70S6K was also reversed by HIF-1α induction. All results were significant (P < 0.05) for control vs. STN and STN vs. STN + DMOG (Fig. 1). Finally, we examined the expression of the fibrosis marker plasminogen activator inhibitor-1 (PAI-1), which has been implicated in renal fibrosis in experimental and human CKD (9, 10). PAI-1 suppresses fibrinolysis and extracellular matrix degradation by plasminogen activators, eventually promoting fibrosis and extracellular matrix accumulation. We observed an early increase in PAI-1 expression in the STN kidney. Along with the other favorable effects of HIF-1α activation by DMOG described above, PAI-1 expression was also reduced with this treatment.
These results suggest that HIF-1α activation in the STN kidney facilitates hypoxic adaptation by limiting the energy-consuming process of protein synthesis, inhibiting apoptosis, and activating autophagy as a defense mechanism for improved cell survival. These effects on protein synthesis, apoptosis, and autophagy are consistent with known effects of HIF-1α induction in other tissues but have not been described in the kidney.
AMPK pathway in STN.
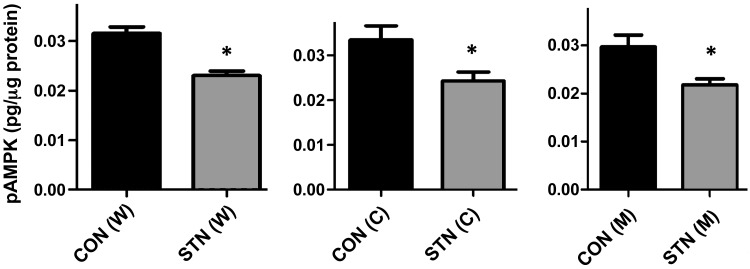
There has been increasing interest in the AMPK pathway in the regulation of metabolism and transport in the kidney, and its role in diabetic nephropathy has been described (20). The early STN kidney shares several features with the early diabetic kidney, hyperfiltration, hypertrophy, and increased oxygen consumption factored for Na reabsorption, which are exaggerated in the STN kidney. We have reported recently that whereas total AMPK expression was elevated in STN, consistent with the cellular environment of hypoxic/metabolic stress in STN, the expression of activated AMPK (phosphorylated at Thr172, p-AMPK) was decreased along with a low p-AMPK/AMPK ratio (30). Here, we examined specifically the regional levels of activated AMPKα1 phosphorylated at Thr172 separately in cortical and medullary samples. Levels of activated p-AMPKα1 were lower in the STN kidney in both cortical and medullary regions (Fig. 3), demonstrating a global diminished expression of activated AMPK in the STN kidney.
Fig. 3.
Reduced AMP-activated protein kinase (AMPK)α1 activation in STN. Reduced AMPKα1 activation (p-AMPK) pg/μg protein in whole kidney (W) STN, cortex (C), and medulla (M) by ELISA. *P < 0.05, STN vs. CON; n = 3–4 in each group.
Interaction between HIF-1α and AMPK.
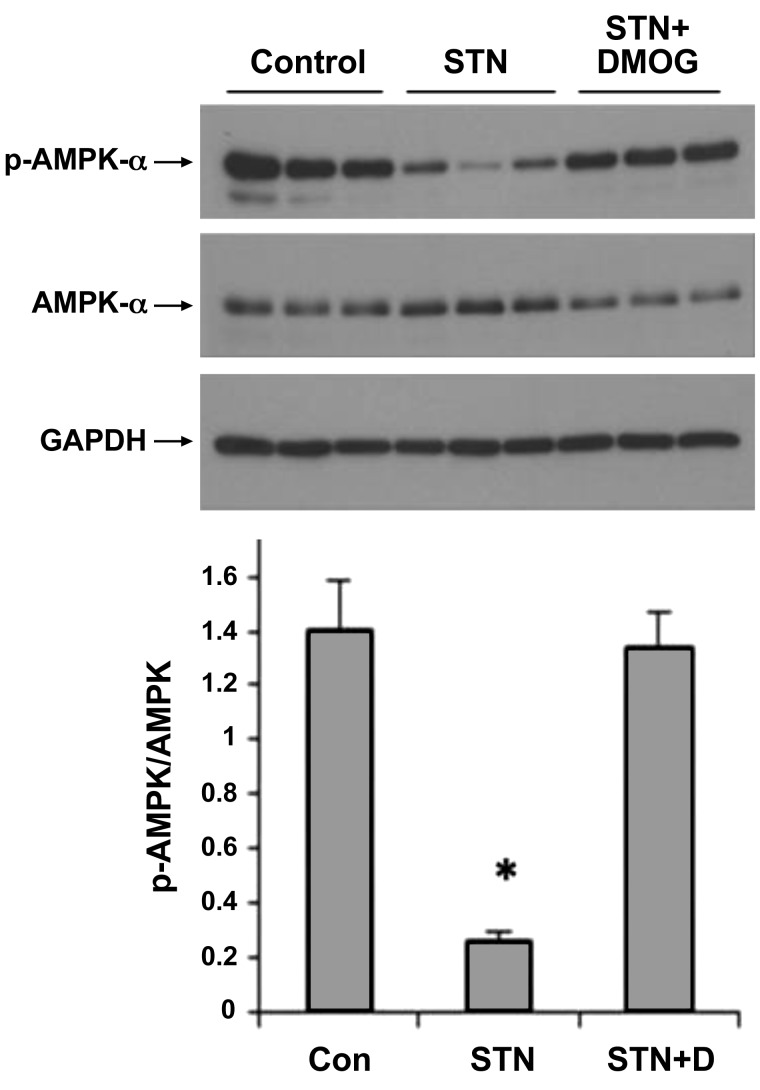
We have demonstrated previously that lowering nephron oxygen consumption by HIF-1α or AMPK activation independently improves kidney function and morphology (6, 30). Given the overlap between the actions and targets of HIF-1α and AMPK, including protein synthesis (via mTOR), apoptosis and autophagy pathways, and the stimuli (cellular hypoxic and metabolic stress) that activate them, we examined the response to HIF-1α induction on AMPK status in the kidney. STN rats were treated with DMOG to activate HIF-1α. Remarkably, HIF-1α induction increased levels of activated p-AMPK expression and lowered total AMPK expression to control levels, thus increasing p-AMPK/AMPK ratio to control values (P < 0.05 for STN vs. STN + DMOG; Fig. 4). These results indicate a previously unrecognized role of HIF-1α in the regulation of AMPK activation.
Fig. 4.
Suppressed AMPK activation in STN restored by HIF-1α activation. Increase in levels of p-AMPK in STN kidneys treated with DMOG for HIF-1α activation. Western blot and densitometry normalized to GAPDH. *P < 0.05, STN vs. CON and STN + D; n = 3 in each group.
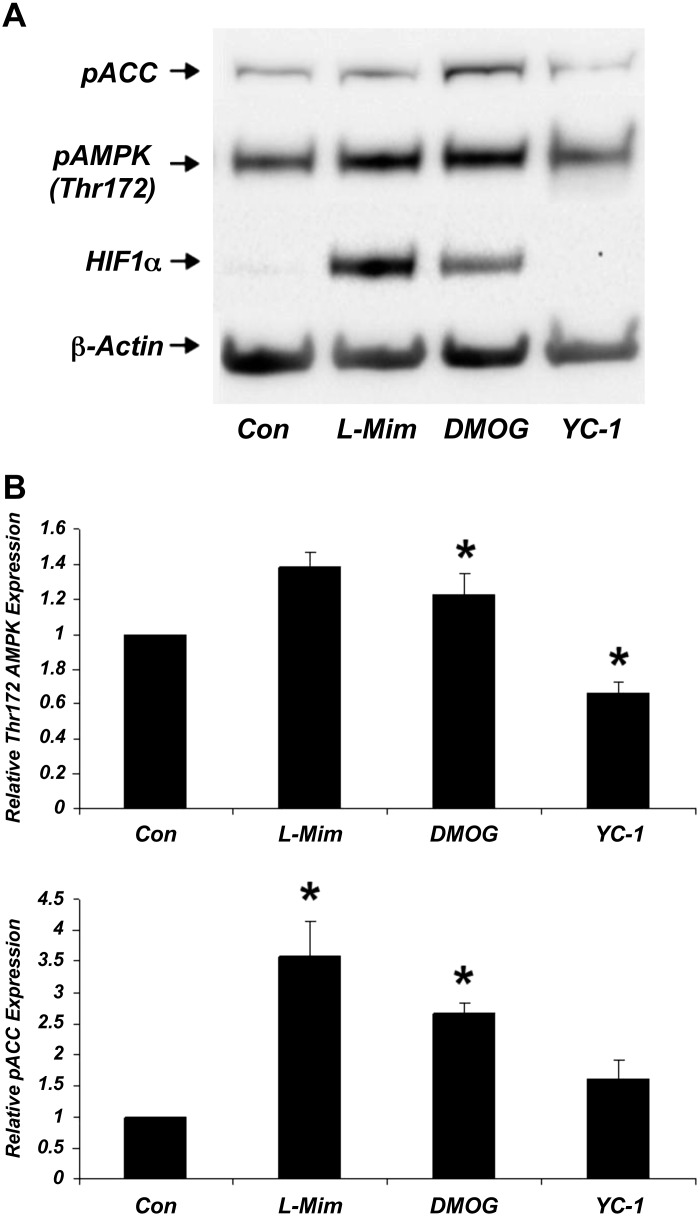
To further explore the interaction between HIF-1α and AMPK in a simpler in vitro system, we next performed cell culture experiments. HK-2 cells, an immortalized human proximal tubular epithelial cell line, were treated with HIF-1α activators DMOG (1 mM) and l-mimosine (250 μM) and the HIF-1α inhibitor YC-1 (10 μM), for 16 h, and the expression of p-AMPK (Thr172) and its downstream target phospho-acetyl-CoA carboxylase (p-ACC) were assessed (Fig. 5). We confirmed HIF-1α activation and inhibition with HIF inducers and inhibitors, respectively. We also observed an increase in p-AMPK and p-ACC expression with HIF inducers and a decrease with HIF inhibition, endorsing the in vivo results.
Fig. 5.
Regulation of AMPK by HIF-1α in human kidney-2 (HK-2) cells. Increase in p-AMPK (Thr172) and its downstream target p-ACC (acetyl-CoA carboxylase) by both HIF inducers 250 μM l-mimosine (l-mim) and 1 mM DMOG and decrease with HIF inhibitor 10 μM 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) is demonstrated in HK-2 cells. Cells were exposed to all treatments for 16 h. A: representative immunoblot. B: summary densitometry results from 3–6 replicate measurements of p-AMPK and p-ACC normalized to β-actin under the 4 treatment conditions (*P < 0.05 relative to CON).
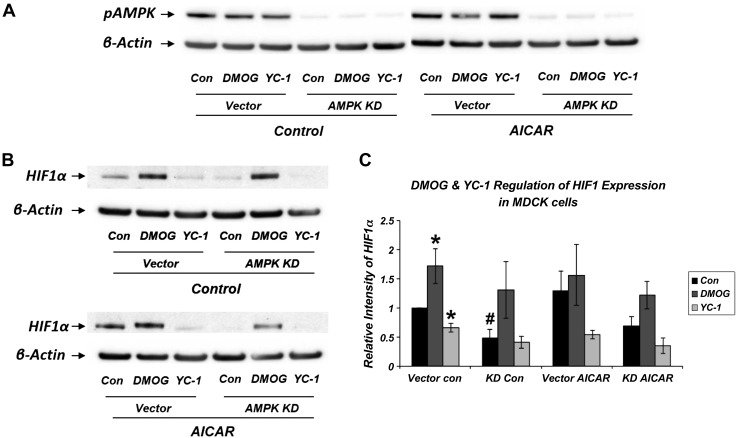
To investigate whether the interaction between HIF-1α and AMPK is bidirectional, we examined the impact of modifying AMPK activity on HIF-1α expression. Stably transduced MDCK epithelial cells with or without stable AMPK KD (34) were treated in the presence or absence of the AMPK activator AICAR (2 mM) for 16 h and the HIF-1α modulators DMOG or YC-1 (Fig. 6). As measured by p-Thr172 band intensity, AMPK was substantially inhibited in the AMPK KD cells (Fig. 6A). As expected, DMOG and YC-1 activated and inhibited HIF-1α expression, respectively, under control conditions (Fig. 6, B and C). Although HIF activity did not appear to be affected by the AMPK activator AICAR, there was a modest but significant decrease in HIF-1α expression with AMPK KD. This suggests that a degree of tonic AMPK activity may be needed for optimal HIF-1α expression.
Fig. 6.
HIF-1α activity regulation by DMOG and YC-1 with AMPK modulation in Madin-Darby canine kidney (MDCK) cells. A: representative immunoblot demonstrating modulation of AMPK activity (p-Thr172 signal) by treatment with the AMPK activator AICAR (2 mM for 16 h) vs. AMPKα1 knockdown (KD) (34). B: representative immunoblot demonstrating modulation of HIF-1α expression under these conditions with HIF-1α modulators. C: Western blot densitometry normalized to β-actin. Increase in HIF-1α expression with DMOG and decrease with YC-1 is shown under control conditions (*P < 0.05 vs. vector control; n = 4–7). Presence of AICAR (2 mM for 16 h) had no significant effect on HIF-1α expression, but AMPK KD inhibited HIF-1α expression (#P < 0.05 for AMPK KD CON vs. vector CON; n = 4–7).
Cellular effects of HIF-1α and AMPK interaction.
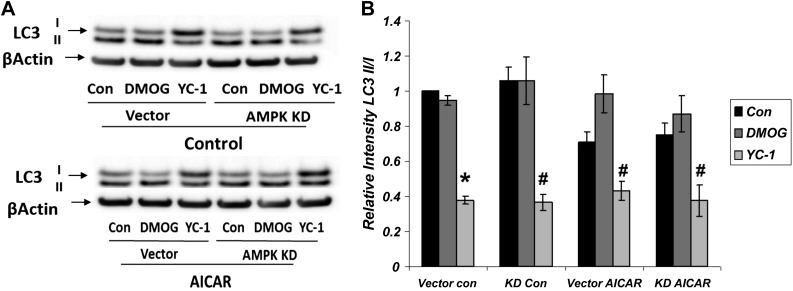
We also examined the independent and combined effects of HIF-1α and AMPK on cell survival and death pathways. Autophagy was assessed by the increased ratio of LC3-II to LC3-I, as described above, in the presence of HIF-1α and AMPK activation and inhibition (Fig. 7). LC3-II/LC3-I ratio was lowered significantly in the presence of HIF-1α inhibition by YC-1, indicating lowered autophagy with HIF-1α inhibition. The effects of YC-1 were significant in all groups compared with controls and DMOG treatment. Modifying AMPK activity with AICAR or KD did not significantly alter the effects of YC-1. These results demonstrate that the effect of HIF-1α activation on the levels of autophagy proteins LC3-II and LC3-I is independent of AMPK.
Fig. 7.
HIF-1α inhibition lowers autophagy independently of AMPK activity. Western blot densitometry normalized to β-actin. Significant decrease in LC3 II/I is seen with HIF-1α inhibition by YC-1 (*P < 0.002, vector YC-1 vs. vector control and vector YC-1 vs. vector DMOG; n = 3). Effects of YC-1 are significant in all groups compared with controls and DMOG treatment (#P < 0.05, YC-1 vs. control and DMOG; n = 3). AMPK modulation (KD or AICAR) does not significantly alter effects of YC-1. AICAR treatment in the absence of HIF-1α modulators significantly reduced LC3 II/I (P < 0.05).
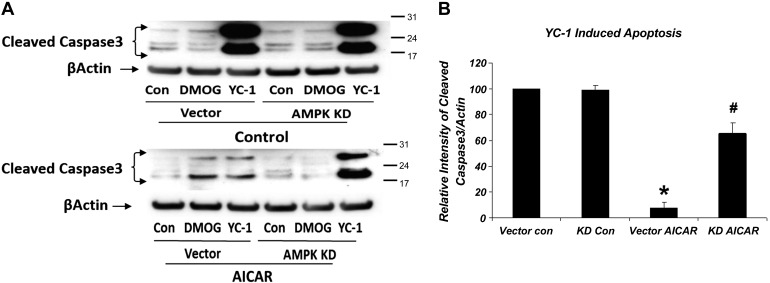
Next, we examined cellular apoptosis by studying cleaved caspase 3 expression, as described above (Fig. 8). Expression of cleaved caspase 3 was increased dramatically, indicating increased apoptosis with HIF-1α inhibition by YC-1, and stayed elevated in AMPK KD cells treated with YC-1. Figure 8B shows the data only in the presence of YC-1 due to the large increase in expression of cleaved caspase 3 in its presence. Interestingly, the levels of cleaved caspase 3 expression were significantly and dramatically lowered by AMPK activation with AICAR even in the presence of YC-1. This effect of AICAR was attenuated in AMPK KD cells treated with AICAR, but there was still a significant difference when compared with untreated AMPK KD in the presence of YC-1. These results indicate that the AICAR-dependent inhibition of apoptosis is mediated by AMPK activation and also demonstrate that HIF-1α inhibition increases apoptosis dramatically, an effect that appears to be partially AMPK dependent.
Fig. 8.
HIF-1α inhibition-induced apoptosis is attenuated by AMPK activation. Western blot densitometry normalized to β-actin. Dramatic increase in expression of cleaved caspase 3 with HIF-1α inhibition by YC-1. This increase is significantly lowered in the presence of AMPK activation by AICAR (*P = 0.002, vector CON vs. vector AICAR). AMPK KD attenuated the effects of AICAR treatment (#P = 0.008, vector AICAR vs. KD AICAR; n = 3).
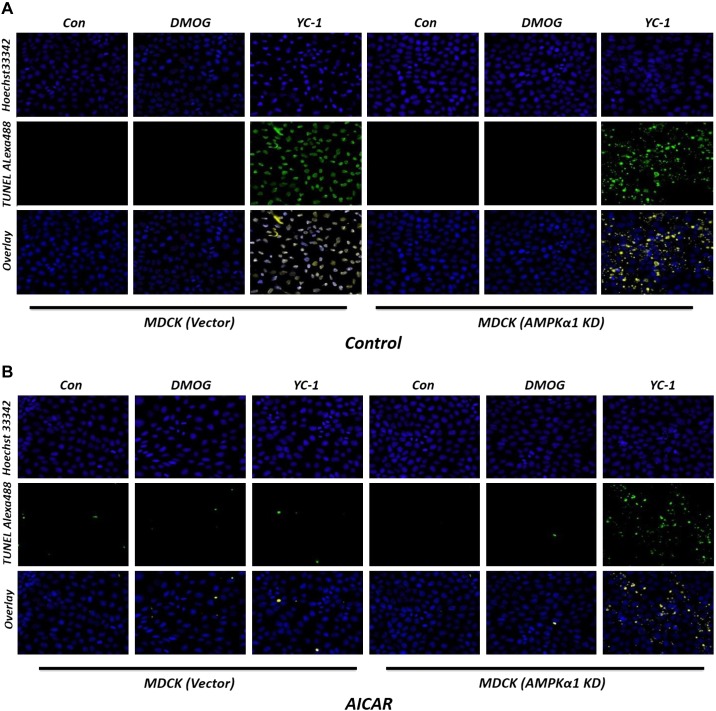
To confirm these effects of HIF-1α and AMPK modulation on apoptosis, we also performed TUNEL staining on vector control and AMPKα1-KD MDCK cells treated under the same conditions (Fig. 9). Again, the HIF-1α inhibitor YC-1 dramatically promoted apoptosis in the vast majority of both vector control and AMPKα1 KD MDCK cells in the absence of AICAR treatment, as indicated by positive TUNEL staining (green; Fig. 9A). YC-1-induced apoptosis (TUNEL positivity) was inhibited dramatically following treatment with AICAR in the vector control MDCK cells, but this effect of AICAR was attenuated in AMPK KD cells (Fig. 9B), which is consistent with the results shown in Fig. 8 and suggests that the AICAR effect is mediated in large part through AMPK activation.
Fig. 9.
Apoptosis induced by HIF-1α inhibition is attenuated by AMPK activation in MDCK cells, as detected by TUNEL staining. MDCK vector control cells (left) or AMPK KD cells (right) were treated with vehicle (CON), HIF-1α activator (1 mM DMOG), or HIF-1α inhibitor (10 μM YC-1) in the absence (A) or presence (B) of AMPK activator (2 mM AICAR) for 16 h, as indicated. Cells were then fixed and detected by terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP)-alkyne nick-end labeling (TUNEL) and fluorescence microscopy (×63 objective). A and B, top: Hoechst 33342 nuclear staining. A and B, middle: dUTP-labeled DNA breaks. A and B, bottom: merged images. YC-1-induced apoptosis was inhibited by treatment with the AMPK activator AICAR, but this effect was reversed in AMPK KD cells. Results shown are representative of n = 3 replicate experiments.
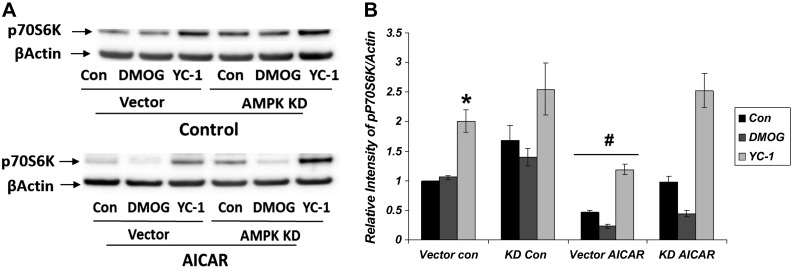
Finally, we examined the effects of interaction between HIF-1α and AMPK on protein synthesis and cellular proliferation via the mTOR pathway (Fig. 10). HIF-1α inhibition by YC-1 increased the expression of p70S6K significantly, indicating mTOR activation and increase in protein synthesis. In the presence of AICAR, the effects of YC-1 on p70S6K expression were attenuated in vector-treated cells but not in AMPK KD cells. DMOG treatment significantly lowered p70S6K only in the presence of AICAR. These results suggest that there are additive effects of HIF-1α and AMPK activation on inhibiting protein synthesis via the mTOR pathway.
Fig. 10.
Additive effects of HIF-1α and AMPK on inhibition of protein synthesis. Western blot densitometry normalized to β-actin. Increased expression of p70S6K with HIF-1α inhibition by YC-1 (*P = 0.01, vector YC-1 vs. vector control and vector DMOG; n = 4). This increase is significantly attenuated in all groups in the presence of AICAR in vector-treated (#P < 0.05 for all vs. vector CON; n = 4) but not in AMPK KD cells. DMOG significantly lowered p70S6K only in the presence of AICAR.
Effects of hypoxia on HIF-1α and AMPK interaction.
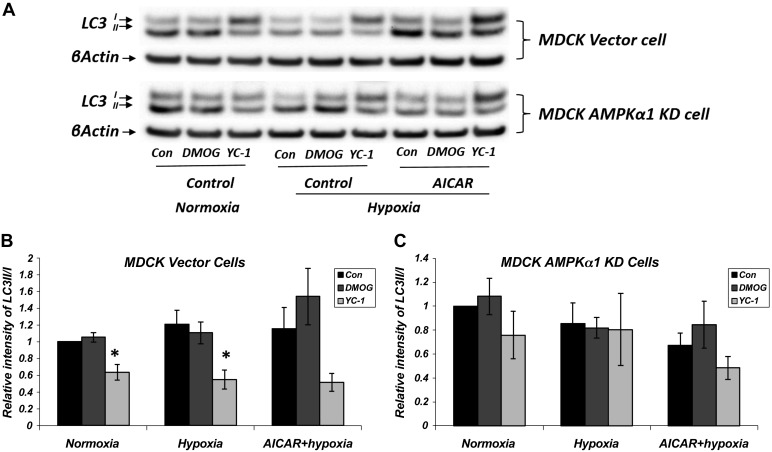
To explore the effect of hypoxia on HIF-1α and AMPK activation in vitro, we again used stably transduced MDCK cells, either vector control or AMPKα1-KD cells exposed to normoxic or hypoxic conditions induced by using a hypoxia incubator chamber. Cells were treated with either DMOG, YC-1, or AICAR as described above, and we examined the effects of HIF-1α and AMPK on cellular pathways in the presence of hypoxia. Similar to normoxic conditions, YC-1 treatment decreased the LC3-II/LC3-I ratio in hypoxia significantly. AMPK activation by AICAR treatment did not change the YC-1 effect under hypoxic conditions (Fig. 11). These results demonstrate that the effects of HIF-1α on the autophagy proteins LC3-II and LC3-I are independent of AMPK under both normoxic and hypoxic conditions.
Fig. 11.
HIF-1α inhibitor YC-1 compound inhibits autophagy under both normoxic and hypoxic conditions. A: MDCK vector and AMPKα1 KD cells were treated with normoxia, hypoxia, or hypoxia + AICAR (2 mM) along with HIF-1α modulators for 16 h. Cell lysates were immunoblotted using LC3 antibody; an increase in LC3 II/I ratio indicates induction of autophagy. B: Western blot densitometry normalized to β-actin. A significant decrease in LC3 II/I ratio was observed with HIF-1α inhibitor (YC-1; 10 μM) treatment in the MDCK vector group (*P < 0.01, vector YC-1 vs. vector CON in normoxia and hypoxia); n = 7 for normoxia and hypoxia and n = 5 for AICAR + hypoxia. AMPK activation by AICAR treatment did not significantly alter the effects of YC-1 on autophagy under either normoxic or hypoxic conditions.
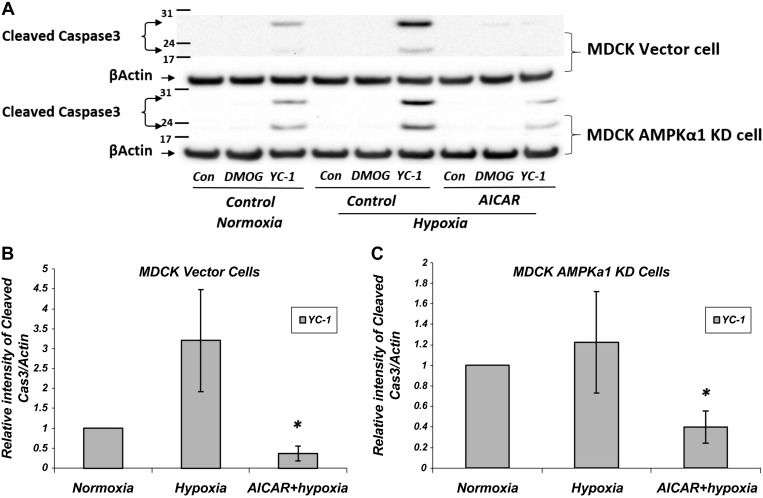
Above, we showed that the HIF-1α inhibitor YC-1 significantly increases cleaved caspase 3 expression, and the AMPK activator AICAR attenuates this under normoxic conditions. To investigate whether this AMPK effect also occurs under hypoxic conditions, we exposed MDCK cells to hypoxic conditions overnight before lysis to detect cellular apoptosis by immunoblotting for cleaved caspase 3 expression (Fig. 12). Expression of cleaved caspase 3 was increased dramatically with HIF-1α inhibition by YC-1 in hypoxia and stayed elevated in AMPK KD cells under both normoxic and hypoxic conditions. Moreover, the levels of cleaved caspase 3 expression were decreased significantly and dramatically by AMPK activation with AICAR in the presence of YC-1. This effect of AICAR on the expression of cleaved caspase 3 was attenuated in AMPK KD cells treated with AICAR. However, there was still a significant difference compared with untreated AMPK KD cells in the presence of YC-1. Taken together, these results indicate that the AICAR-dependent inhibition of apoptosis induced by the HIF-1α inhibitor YC-1 is mediated largely via AMPK activation under both normoxic and hypoxic conditions.
Fig. 12.
Apoptosis induced by the HIF-1α inhibitor YC-1 is attenuated by AMPK activation under hypoxic conditions in MDCK cells. A: MDCK vector control cells and AMPKα1 KD cells were treated under either normoxic or hypoxic conditions in the presence or absence of 2 mM AICAR along with the HIF-1α activator DMOG (1 mM) or HIF-1α inhibitor YC-1 (10 μM) for 16 h; n = 7 for normoxia and hypoxia and n = 5 for hypoxia + AICAR. Cell lysates were probed with the apoptosis marker caspase 3 (Cas3) and β-actin antibodies as indicated. B and C: Western blot densitometry normalized to β-actin. As seen under normoxic conditions, only YC-1-treated cells had a significant cleaved Cas3 signal. Hypoxia increased cleaved Cas3 levels, but cleaved Cas3 levels were substantially and significantly decreased following treatment with the AMPK activator AICAR (*P < 0.05, hypoxia vs. hypoxia vs. AICAR). AMPK KD attenuated the effects of AICAR treatment on the expression of cleaved Cas3 in MDCK cells, but there was still a significant difference compared with untreated AMPK KD cells in the presence of YC-1.
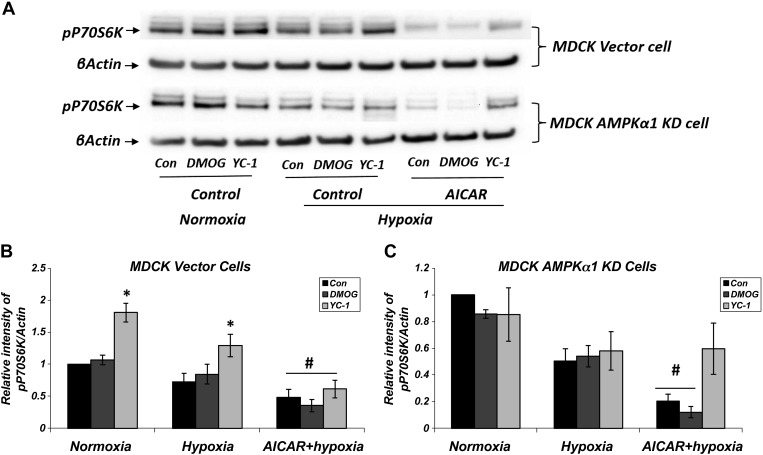
Finally, we examined the effects of interaction between hypoxia and AMPK on protein synthesis and cellular proliferation via the mTOR signal pathway. The expression of pP70S6K was decreased under hypoxic conditions in both MDCK vector and AMPK KD cells (Fig. 13). This decrease was further exaggerated in the presence of AICAR in both cells. YC-1 treatment significantly increased the expression of p-P70S6k in both normoxia and hypoxia. These results support the additive effects of HIF-1α and AMPK activation on inhibiting protein synthesis in hypoxia similarly to normoxia.
Fig. 13.
Additive effects of hypoxia and AMPK activation on the inhibition of protein synthesis. A: MDCK vector and AMPKα1 KD cells were treated with normoxia, hypoxia, and hypoxia + AICAR (2 mM) along with HIF-1α modulators for 16 h; n = 7 for normoxia and hypoxia and n = 5 for hypoxia + AICAR. The cell lysates were immunoblotted with p-p70S6K antibody, as indicated the protein synthesis. B: Western blot densitometry normalized to β-actin. Decreased expression of p-p70S6K with hypoxia conditions was observed in both MDCK vector and AMPKα1 KD cells. This decrease was further exaggerated in the presence of AICAR (#P < 0.05; n = 5). YC-1 increased the expression of p-p70S6K in both normoxia and hypoxia (*P < 0.05, YC-1 vs. CON).
DISCUSSION
We have reported previously on the early hemodynamic and metabolic adaptations that occur in the aftermath of nephron loss 1 wk following STN (7, 32, 33). At this stage, tubulointerstitial and glomerular injury is not evident despite significant hemodynamic, transport, and metabolic alterations. A major adaptation to nephron loss in early STN is significant hyperfiltration in the remaining nephrons. Hyperfiltration begets hyperreabsorption, which likely occurs at an increased metabolic cost, as tubular transport necessitates the majority of renal oxygen consumption. Indeed, we and others have reported elevated oxygen consumption factored for Na reabsorption in the STN kidney (7, 14, 26).
Tubular transport-driven oxygen consumption is the major determinant of intrarenal oxygenation (3, 4). Hence, increased oxygen consumption in STN driven by tubular transport likely leads to cortical and medullary tubular hypoxia in the early stages of STN (4, 7, and 14 days) accompanied by expression of HIF-1α and its target proteins (22). Tissue hypoxia appears to precede any other pathological changes in the kidney and persists into the late stage of STN (22, 35, 38). HIF-1α, the master regulator of cell response to hypoxia, is expressed in the kidney under hypoxic conditions (29). It induces several target genes involved in adaptation to decreased oxygen availability that impact O2 delivery via vasomotor regulation and cellular O2 consumption via several pathways (31). In this study, we observed that HIF-1α activation in the STN kidney had significant effects consistent with cellular hypoxia adaptation, including inhibition of protein synthesis and apoptosis and activation of autophagy as a defense mechanism for improved cell survival (Fig. 1). These may explain the significant improvement in renal function (Table 1), fibrosis marker PAI-1 (Fig. 1), and morphology observed with HIF-1α activation in STN (6).
In the STN kidney, there is evidence of inherent HIF-1α activation in the early and late stages, starting at week 1 and peaking between weeks 4 and 6, after which it returns to basal levels and eventually lower levels than controls (32, 38). However, this expression appears to be submaximal, as treatment with HIF-1α activators further increased the levels of HIF-1α and its target proteins, leading to improved outcomes (reviewed in Ref. 25). The underlying reasons for suboptimal HIF-1α expression are not clear and may be multifactorial. Yu et al. (38) demonstrated that the upregulation of HIF-1α expression was accompanied by an increased expression of prolyl hydroxylase domain 2 and 3, which are key enzymes that induce posttranslational degradation of HIF-1α via the ubiquitin-proteasome pathway. This finding suggests that the expression of HIF-1α in this setting was kept in check with the concomitant upregulation of its regulator. There may be other factors in the pathophysiology of STN, such as inflammatory cytokines, growth factors, and oxidative stresses that may overwhelm or inhibit maximal HIF expression or activity, but these remain to be clearly defined. Finally, there is also evidence of AMPK affecting HIF expression and activity. Interestingly, AMPK activity was shown to be important for HIF-1α transcriptional activity under hypoxic conditions (19). Our findings here also demonstrate that tonic AMPK activity may be required for robust HIF expression (Fig. 6).
There is significant overlap between the actions and targets of HIF-1α and AMPK and the stimuli that activate them. AMPK activity is induced by hypoxia even when cellular ATP levels are not significantly depleted (12, 18). Moreover, there is some emerging evidence of interaction between these two signaling pathways in other organs. For instance, AMPK-mediated autophagy in chondrocytes was shown to be HIF dependent, which is itself a stimulator of autophagy (1). Also, inhibition of protein synthesis by suppression of mTOR during hypoxia occurs via AMPK in the short term and via HIF in the long term (13, 37). AMPK activity was shown to be important for HIF-1α transcriptional activity under hypoxic conditions (19). Together, these studies and our results presented here suggest that AMPK and HIF may closely interact to mount a coordinated cellular response to hypoxic stress by regulation of both supply and demand of ATP. A schematic depiction of our model in STN is shown in Fig. 14 and is discussed below.
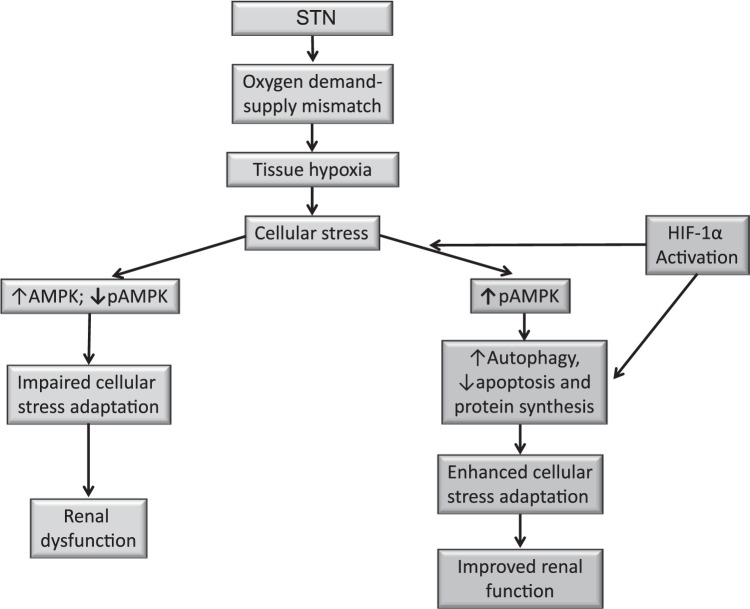
Fig. 14.
Proposed model for HIF-1α and AMPK interactions in cellular hypoxia adaptation in STN. In the early STN kidney, oxygen demand/supply mismatch leads to tissue hypoxia and cellular stress. Despite elevated total AMPK levels, the activation of AMPK is impaired, thus compromising the cellular stress adaptation and leading to subsequent renal dysfunction. HIF-1α activation restores AMPK activation and restores adaptive responses to hypoxic stress via independent and AMPK-dependent effects on cell survival and death pathways, leading to improved renal function.
There has been increasing interest in the role of the AMPK pathway in renal (patho)physiology. In this study, we found that activation of AMPK in the early STN kidney was impaired despite elevated total AMPK levels. The primary signal that induces AMPK activation in the early STN kidney is not obvious, but a similar decrease in p-AMPK has been observed in the kidney in experimental models of diabetes and high-fat diet (5, 20). Both of these models are characterized by hypertrophy and hyperfiltration in the kidney, as observed in the STN kidney. Indeed, suppression of p-AMPK by stimulation of the phosphatidylinositol 3-kinase/Akt/mTOR signaling pathway involved in protein synthesis and diabetic hypertrophy has been invoked to explain this phenomenon in the diabetic kidney (20). We have observed increased activation of the mTOR pathway in STN shown by increased p70S6K expression, and this may inhibit AMPK expression.
Of note is that optimal HIF expression may be an important determinant of AMPK expression and activity. Our results indicate that AMPK activation appears to be regulated on some level by HIF-1α. There are no reported data regarding specific mechanistic pathways underlying this regulation. We speculate that this regulation may be mediated via the effects of HIF-1α on kinases upstream to AMPK (i.e., liver kinase B1 or calcium/calmodulin dependent kinase kinase-β), as total AMPK levels were elevated in STN, but the phosphorylation/activation was reduced. Further exploration of this potential mechanism is an important goal for future studies.
We also investigated the independent and combined effects of HIF-1α and AMPK on cell survival and death pathways by utilizing cell culture as a more tractable approach. Our results demonstrate that the effect of HIF-1α activation on autophagy is independent of AMPK but on apoptosis appears to be partially AMPK dependent. Finally, the effects of HIF-1α and AMPK activation on inhibiting protein synthesis via the mTOR pathway appear to be additive. We further investigated the interaction between HIF-1α and AMPK in the presence of hypoxia in vitro. Overall, the results in the presence of hypoxia were qualitatively similar to those in normoxia, but there was more variability in the presence of hypoxia, especially in the AMPK KD cells. It is important to acknowledge that the major limitation of our studies is the use of pharmacological agents especially for HIF-1α activation and inhibition, which may have off-target effects. Additional studies with genetic manipulation of these pathways are needed to further investigate their synergistic and independent effects.
In conclusion, we have demonstrated that HIF-1α and AMPK, independent pathways that regulate cellular stress adaptation, may act as components of a concerted cellular response to energy deprivation in pathophysiology. The importance of the interaction between these key regulators of cellular metabolism has not been studied carefully in kidney disease, and thus additional studies are needed to understand the mechanisms of the coordinated actions of HIF and AMPK in the regulation of energy metabolism.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K08-DK-084305 (P. Singh), Veterans Affairs (VA) Merit Award BX002175 (P. Singh), the University of Alabama at Birmingham-University of California San Diego O'Brien Center (P30-DK-079337), R01-DK-075048 (K. R. Hallows), R01-DK-84184 (N. M. Pastor-Soler), the Pittsburgh Center for Kidney Research (P30-DK-079307), and the VA Research Service. S. Miyamoto and K. Sharma were supported with a VA Merit Award (5I01 BX000277 to K. Sharma).
DISCLOSURES
There are no competing financial interests declared by the authors.
AUTHOR CONTRIBUTIONS
H.L., J.S., J.L.T., S.M., and P.S. performed experiments; H.L., J.S., J.L.T., S.M., N.M.P.-S., K.R.H., and P.S. analyzed data; H.L., J.S., J.L.T., K.S., N.M.P.-S., K.R.H., and P.S. interpreted results of experiments; H.L., J.S., J.L.T., S.M., and P.S. prepared figures; H.L., J.S., K.S., N.M.P.-S., K.R.H., and P.S. edited and revised manuscript; K.R.H. and P.S. conception and design of research; K.R.H. and P.S. approved final version of manuscript; P.S. drafted manuscript.
ACKNOWLEDGMENTS
MDCK and HK-2 cells were kindly provided by Drs. Michael Caplan and Ora Weisz, respectively. Some of the results have been presented as abstracts at American Society of Nephrology and Experimental Biology Annual Meetings.
REFERENCES
- 1.Bohensky J, Leshinsky S, Srinivas V, Shapiro IM. Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mTOR suppression. Pediatr Nephrol 25: 633–642, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1059–F1062, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1063–F1068, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 22: 1846–1855, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng A, Arndt MA, Satriano J, Singh P, Rieg T, Thomson S, Tang T, Blantz RC. Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade. Am J Physiol Renal Physiol 299: F1365–F1373, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng A, Tang T, Singh P, Wang C, Satriano J, Thomson SC, Blantz RC. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int 75: 197–204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckardt KU, Rosenberger C, Jürgensen JS, Wiesener MS. Role of hypoxia in the pathogenesis of renal disease. Blood Purif 21: 253–257, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol 17: 2999–3012, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Fogo AB. Renal fibrosis: not just PAI-1 in the sky. J Clin Invest 112: 326–328, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunaratnam L, Bonventre JV. HIF in kidney disease and development. J Am Soc Nephrol 20: 1877–1887, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol 29: 3455–3464, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallows KR, Mount PF, Pastor-Soler NM, Power DA. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am J Physiol Renal Physiol 298: F1067–F1077, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris DC, Chan L, Schrier RW. Remnant kidney hypermetabolism and progression of chronic renal failure. Am J Physiol Renal Fluid Electrolyte Physiol 254: F267–F276, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol 28: 998–1006, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, Akematsu T, Akira S, Al-Younes HM, Al-Zeer MA, Albert ML, Albin RL, Alegre-Abarrategui J, Aleo MF, Alirezaei M, Almasan A, Almonte-Becerril M, Amano A, Amaravadi R, Amarnath S, Amer AO, Andrieu-Abadie N, Anantharam V, Ann DK, Anoopkumar-Dukie S, Aoki H, Apostolova N, Arancia G, Aris JP, Asanuma K, Asare NY, Ashida H, Askanas V, Askew DS, Auberger P, Baba M, Backues SK, Baehrecke EH, Bahr BA, Bai XY, Bailly Y, Baiocchi R, Baldini G, Balduini W, Ballabio A, Bamber BA, Bampton ET, Bánhegyi G, Bartholomew CR, Bassham DC, Bast RC Jr, Batoko H, Bay BH, Beau I, Béchet DM, Begley TJ, Behl C, Behrends C, Bekri S, Bellaire B, Bendall LJ, Benetti L, Berliocchi L, Bernardi H, Bernassola F, Besteiro S, Bhatia-Kissova I, Bi X, Biard-Piechaczyk M, Blum JS, Boise LH, Bonaldo P, Boone DL, Bornhauser BC, Bortoluci KR, Bossis I, Bost F, Bourquin JP, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady NR, Brancolini C, Brech A, Brenman JE, Brennand A, Bresnick EH, Brest P, Bridges D, Bristol ML, Brookes PS, Brown EJ, Brumell JH, Brunetti-Pierri N, Brunk UT, Bulman DE, Bultman SJ, Bultynck G, Burbulla LF, Bursch W, Butchar JP, Buzgariu W, Bydlowski SP, Cadwell K, Cahová M, Cai D, Cai J, Cai Q, Calabretta B, Calvo-Garrido J, Camougrand N, Campanella M, Campos-Salinas J, Candi E, Cao L, Caplan AB, Carding SR, Cardoso SM, Carew JS, Carlin CR, Carmignac V, Carneiro LA, Carra S, Caruso RA, Casari G, Casas C, Castino R, Cebollero E, Cecconi F, Celli J, Chaachouay H, Chae HJ, Chai CY, Chan DC, Chan EY, Chang RC, Che CM, Chen CC, Chen GC, Chen GQ, Chen M, Chen Q, Chen SS, Chen W, Chen X, Chen X, Chen X, Chen YG, Chen Y, Chen Y, Chen YJ, Chen Z, Cheng A, Cheng CH, Cheng Y, Cheong H, Cheong JH, Cherry S, Chess-Williams R, Cheung ZH, Chevet E, Chiang HL, Chiarelli R, Chiba T, Chin LS, Chiou SH, Chisari FV, Cho CH, Cho DH, Choi AM, Choi D, Choi KS, Choi ME, Chouaib S, Choubey D, Choubey V, Chu CT, Chuang TH, Chueh SH, Chun T, Chwae YJ, Chye ML, Ciarcia R, Ciriolo MR, Clague MJ, Clark RS, Clarke PG, Clarke R, Codogno P, Coller HA, Colombo MI, Comincini S, Condello M, Condorelli F, Cookson MR, Coombs GH, Coppens I, Corbalan R, Cossart P, Costelli P, Costes S, Coto-Montes A, Couve E, Coxon FP, Cregg JM, Crespo JL, Cronjé MJ, Cuervo AM, Cullen JJ, Czaja MJ, D'Amelio M, Darfeuille-Michaud A, Davids LM, Davies FE, De Felici M, de Groot JF, de Haan CA, De Martino L, De Milito A, De Tata V, Debnath J, Degterev A, Dehay B, Delbridge LM, Demarchi F, Deng YZ, Dengjel J, Dent P, Denton D, Deretic V, Desai SD, Devenish RJ, Di Gioacchino M, Di Paolo G, Di Pietro C, Diaz-Araya G, Diaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dikic I, Dinesh-Kumar SP, Ding WX, Distelhorst CW, Diwan A, Djavaheri-Mergny M, Dokudovskaya S, Dong Z, Dorsey FC, Dosenko V, Dowling JJ, Doxsey S, Dreux M, Drew ME, Duan Q, Duchosal MA, Duff K, Dugail I, Durbeej M, Duszenko M, Edelstein CL, Edinger AL, Egea G, Eichinger L, Eissa NT, Ekmekcioglu S, El-Deiry WS, Elazar Z, Elgendy M, Ellerby LM, Eng KE, Engelbrecht AM, Engelender S, Erenpreisa J, Escalante R, Esclatine A, Eskelinen EL, Espert L, Espina V, Fan H, Fan J, Fan QW, Fan Z, Fang S, Fang Y, Fanto M, Fanzani A, Farkas T, Farré JC, Faure M, Fechheimer M, Feng CG, Feng J, Feng Q, Feng Y, Fésüs L, Feuer R, Figueiredo-Pereira ME, Fimia GM, Fingar DC, Finkbeiner S, Finkel T, Finley KD, Fiorito F, Fisher EA, Fisher PB, Flajolet M, Florez-McClure ML, Florio S, Fon EA, Fornai F, Fortunato F, Fotedar R, Fowler DH, Fox HS, Franco R, Frankel LB, Fransen M, Fuentes JM, Fueyo J, Fujii J, Fujisaki K, Fujita E, Fukuda M, Furukawa RH, Gaestel M, Gailly P, Gajewska M, Galliot B, Galy V, Ganesh S, Ganetzky B, Ganley IG, Gao FB, Gao GF, Gao J, Garcia L, Garcia-Manero G, Garcia-Marcos M, Garmyn M, Gartel AL, Gatti E, Gautel M, Gawriluk TR, Gegg ME, Geng J, Germain M, Gestwicki JE, Gewirtz DA, Ghavami S, Ghosh P, Giammarioli AM, Giatromanolaki AN, Gibson SB, Gilkerson RW, Ginger ML, Ginsberg HN, Golab J, Goligorsky MS, Golstein P, Gomez-Manzano C, Goncu E, Gongora C, Gonzalez CD, Gonzalez R, González-Estévez C, González-Polo RA, Gonzalez-Rey E, Gorbunov NV, Gorski S, Goruppi S, Gottlieb RA, Gozuacik D, Granato GE, Grant GD, Green KN, Gregorc A, Gros F, Grose C, Grunt TW, Gual P, Guan JL, Guan KL, Guichard SM, Gukovskaya AS, Gukovsky I, Gunst J, Gustafsson AB, Halayko AJ, Hale AN, Halonen SK, Hamasaki M, Han F, Han T, Hancock MK, Hansen M, Harada H, Harada M, Hardt SE, Harper JW, Harris AL, Harris J, Harris SD, Hashimoto M, Haspel JA, Hayashi S, Hazelhurst LA, He C, He YW, Hébert MJ, Heidenreich KA, Helfrich MH, Helgason GV, Henske EP, Herman B, Herman PK, Hetz C, Hilfiker S, Hill JA, Hocking LJ, Hofman P, Hofmann TG, Höhfeld J, Holyoake TL, Hong MH, Hood DA, Hotamisligil GS, Houwerzijl EJ, Høyer-Hansen M, Hu B, Hu CA, Hu HM, Hua Y, Huang C, Huang J, Huang S, Huang WP, Huber TB, Huh WK, Hung TH, Hupp TR, Hur GM, Hurley JB, Hussain SN, Hussey PJ, Hwang JJ, Hwang S, Ichihara A, Ilkhanizadeh S, Inoki K, Into T, Iovane V, Iovanna JL, Ip NY, Isaka Y, Ishida H, Isidoro C, Isobe K, Iwasaki A, Izquierdo M, Izumi Y, Jaakkola PM, Jäättelä M, Jackson GR, Jackson WT, Janji B, Jendrach M, Jeon JH, Jeung EB, Jiang H, Jiang H, Jiang JX, Jiang M, Jiang Q, Jiang X, Jiang X, Jiménez A, Jin M, Jin S, Joe CO, Johansen T, Johnson DE, Johnson GV, Jones NL, Joseph B, Joseph SK, Joubert AM, Juhász G, Juillerat-Jeanneret L, Jung CH, Jung YK, Kaarniranta K, Kaasik A, Kabuta T, Kadowaki M, Kagedal K, Kamada Y, Kaminskyy VO, Kampinga HH, Kanamori H, Kang C, Kang KB, Kang KI, Kang R, Kang YA, Kanki T, Kanneganti TD, Kanno H, Kanthasamy AG, Kanthasamy A, Karantza V, Kaushal GP, Kaushik S, Kawazoe Y, Ke PY, Kehrl JH, Kelekar A, Kerkhoff C, Kessel DH, Khalil H, Kiel JA, Kiger AA, Kihara A, Kim DR, Kim DH, Kim DH, Kim EK, Kim HR, Kim JS, Kim JH, Kim JC, Kim JK, Kim PK, Kim SW, Kim YS, Kim Y, Kimchi A, Kimmelman AC, King JS, Kinsella TJ, Kirkin V, Kirshenbaum LA, Kitamoto K, Kitazato K, Klein L, Klimecki WT, Klucken J, Knecht E, Ko BC, Koch JC, Koga H, Koh JY, Koh YH, Koike M, Komatsu M, Kominami E, Kong HJ, Kong WJ, Korolchuk VI, Kotake Y, Koukourakis MI, Kouri Flores JB, Kovács AL, Kraft C, Krainc D, Krämer H, Kretz-Remy C, Krichevsky AM, Kroemer G, Krüger R, Krut O, Ktistakis NT, Kuan CY, Kucharczyk R, Kumar A, Kumar R, Kumar S, Kundu M, Kung HJ, Kurz T, Kwon HJ, La Spada AR, Lafont F, Lamark T, Landry J, Lane JD, Lapaquette P, Laporte JF, László L, Lavandero S, Lavoie JN, Layfield R, Lazo PA, Le W, Le Cam L, Ledbetter DJ, Lee AJ, Lee BW, Lee GM, Lee J, Lee JH, Lee M, Lee MS, Lee SH, Leeuwenburgh C, Legembre P, Legouis R, Lehmann M, Lei HY, Lei QY, Leib DA, Leiro J, Lemasters JJ, Lemoine A, Lesniak MS, Lev D, Levenson VV, Levine B, Levy E, Li F, Li JL, Li L, Li S, Li W, Li XJ, Li YB, Li YP, Liang C, Liang Q, Liao YF, Liberski PP, Lieberman A, Lim HJ, Lim KL, Lim K, Lin CF, Lin FC, Lin J, Lin JD, Lin K, Lin WW, Lin WC, Lin YL, Linden R, Lingor P, Lippincott-Schwartz J, Lisanti MP, Liton PB, Liu B, Liu CF, Liu K, Liu L, Liu QA, Liu W, Liu YC, Liu Y, Lockshin RA, Lok CN, Lonial S, Loos B, Lopez-Berestein G, López-Otín C, Lossi L, Lotze MT, Lõw P, Lu B, Lu B, Lu B, Lu Z, Luciano F, Lukacs NW, Lund AH, Lynch-Day MA, Ma Y, Macian F, MacKeigan JP, Macleod KF, Madeo F, Maiuri L, Maiuri MC, Malagoli D, Malicdan MC, Malorni W, Man N, Mandelkow EM, Manon S, Manov I, Mao K, Mao X, Mao Z, Marambaud P, Marazziti D, Marcel YL, Marchbank K, Marchetti P, Marciniak SJ, Marcondes M, Mardi M, Marfe G, Mariño G, Markaki M, Marten MR, Martin SJ, Martinand-Mari C, Martinet W, Martinez-Vicente M, Masini M, Matarrese P, Matsuo S, Matteoni R, Mayer A, Mazure NM, McConkey DJ, McConnell MJ, McDermott C, McDonald C, McInerney GM, McKenna SL, McLaughlin B, McLean PJ, McMaster CR, McQuibban GA, Meijer AJ, Meisler MH, Meléndez A, Melia TJ, Melino G, Mena MA, Menendez JA, Menna-Barreto RF, Menon MB, Menzies FM, Mercer CA, Merighi A, Merry DE, Meschini S, Meyer CG, Meyer TF, Miao CY, Miao JY, Michels PA, Michiels C, Mijaljica D, Milojkovic A, Minucci S, Miracco C, Miranti CK, Mitroulis I, Miyazawa K, Mizushima N, Mograbi B, Mohseni S, Molero X, Mollereau B, Mollinedo F, Momoi T, Monastyrska I, Monick MM, Monteiro MJ, Moore MN, Mora R, Moreau K, Moreira PI, Moriyasu Y, Moscat J, Mostowy S, Mottram JC, Motyl T, Moussa CE, Muller S, Müller S, Münger K, Münz C, Murphy LO, Murphy ME, Musaro A, Mysorekar I, Nagata E, Nagata K, Nahimana A, Nair U, Nakagawa T, Nakahira K, Nakano H, Nakatogawa H, Nanjundan M, Naqvi NI, Narendra DP, Narita M, Navarro M, Nawrocki ST, Nazarko TY, Nemchenko A, Netea MG, Neufeld TP, Ney PA, Nezis IP, Nguyen HP, Nie D, Nishino I, Nislow C, Nixon RA, Noda T, Noegel AA, Nogalska A, Noguchi S, Notterpek L, Novak I, Nozaki T, Nukina N, Nürnberger T, Nyfeler B, Obara K, Oberley TD, Oddo S, Ogawa M, Ohashi T, Okamoto K, Oleinick NL, Oliver FJ, Olsen LJ, Olsson S, Opota O, Osborne TF, Ostrander GK, Otsu K, Ou JH, Ouimet M, Overholtzer M, Ozpolat B, Paganetti P, Pagnini U, Pallet N, Palmer GE, Palumbo C, Pan T, Panaretakis T, Pandey UB, Papackova Z, Papassideri I, Paris I, Park J, Park OK, Parys JB, Parzych KR, Patschan S, Patterson C, Pattingre S, Pawelek JM, Peng J, Perlmutter DH, Perrotta I, Perry G, Pervaiz S, Peter M, Peters GJ, Petersen M, Petrovski G, Phang JM, Piacentini M, Pierre P, Pierrefite-Carle V, Pierron G, Pinkas-Kramarski R, Piras A, Piri N, Platanias LC, Poggeler S, Poirot M, Poletti A, Poüs C, Pozuelo-Rubio M, Praetorius-Ibba M, Prasad A, Prescott M, Priault M, Produit-Zengaffinen N, Progulske-Fox A, Proikas-Cezanne T, Przedborski S, Przyklenk K, Puertollano R, Puyal J, Qian SB, Qin L, Qin ZH, Quaggin SE, Raben N, Rabinowich H, Rabkin SW, Rahman I, Rami A, Ramm G, Randall G, Randow F, Rao VA, Rathmell JC, Ravikumar B, Ray SK, Reed BH, Reed JC, Reggiori F, Régnier-Vigouroux A, Reichert AS, Reiners JJ Jr, Reiter RJ, Ren J, Revuelta JL, Rhodes CJ, Ritis K, Rizzo E, Robbins J, Roberge M, Roca H, Roccheri MC, Rocchi S, Rodemann HP, Rodríguez de Córdoba S, Rohrer B, Roninson IB, Rosen K, Rost-Roszkowska MM, Rouis M, Rouschop KM, Rovetta F, Rubin BP, Rubinsztein DC, Ruckdeschel KM, Rucker EB 3rd, Rudich A, Rudolf E, Ruiz-Opazo N, Russo R, Rusten TE, Ryan KM, Ryter SW, Sabatini DM, Sadoshima J, Saha T, Saitoh T, Sakagami H, Sakai Y, Salekdeh GH, Salomoni P, Salvaterra PM, Salvesen G, Salvioli R, Sanchez AM, Sánchez-Alcázar JA, Sánchez-Prieto R, Sandri M, Sankar U, Sansanwal P, Santambrogio L, Saran S, Sarkar S, Sarwal M, Sasakawa C, Sasnauskiene A, Sass M, Sato K, Sato M, Schapira AH, Scharl M, Schätzl HM, Scheper W, Schiaffino S, Schneider C, Schneider ME, Schneider-Stock R, Schoenlein PV, Schorderet DF, Schuller C, Schwartz GK, Scorrano L, Sealy L, Seglen PO, Segura-Aguilar J, Seiliez I, Seleverstov O, Sell C, Seo JB, Separovic D, Setaluri V, Setoguchi T, Settembre C, Shacka JJ, Shanmugam M, Shapiro IM, Shaulian E, Shaw RJ, Shelhamer JH, Shen HM, Shen WC, Sheng ZH, Shi Y, Shibuya K, Shidoji Y, Shieh JJ, Shih CM, Shimada Y, Shimizu S, Shintani T, Shirihai OS, Shore GC, Sibirny AA, Sidhu SB, Sikorska B, Silva-Zacarin EC, Simmons A, Simon AK, Simon HU, Simone C, Simonsen A, Sinclair DA, Singh R, Sinha D, Sinicrope FA, Sirko A, Siu PM, Sivridis E, Skop V, Skulachev VP, Slack RS, Smaili SS, Smith DR, Soengas MS, Soldati T, Song X, Sood AK, Soong TW, Sotgia F, Spector SA, Spies CD, Springer W, Srinivasula SM, Stefanis L, Steffan JS, Stendel R, Stenmark H, Stephanou A, Stern ST, Sternberg C, Stork B, Strålfors P, Subauste CS, Sui X, Sulzer D, Sun J, Sun SY, Sun ZJ, Sung JJ, Suzuki K, Suzuki T, Swanson MS, Swanton C, Sweeney ST, Sy LK, Szabadkai G, Tabas I, Taegtmeyer H, Tafani M, Takács-Vellai K, Takano Y, Takegawa K, Takemura G, Takeshita F, Talbot NJ, Tan KS, Tanaka K, Tanaka K, Tang D, Tang D, Tanida I, Tannous BA, Tavernarakis N, Taylor GS, Taylor GA, Taylor JP, Terada LS, Terman A, Tettamanti G, Thevissen K, Thompson CB, Thorburn A, Thumm M, Tian F, Tian Y, Tocchini-Valentini G, Tolkovsky AM, Tomino Y, Tonges L, Tooze SA, Tournier C, Tower J, Towns R, Trajkovic V, Travassos LH, Tsai TF, Tschan MP, Tsubata T, Tsung A, Turk B, Turner LS, Tyagi SC, Uchiyama Y, Ueno T, Umekawa M, Umemiya-Shirafuji R, Unni VK, Vaccaro MI, Valente EM, Van den Berghe G, van der Klei IJ, van Doorn W, van Dyk LF, van Egmond M, van Grunsven LA, Vandenabeele P, Vandenberghe WP, Vanhorebeek I, Vaquero EC, Velasco G, Vellai T, Vicencio JM, Vierstra RD, Vila M, Vindis C, Viola G, Viscomi MT, Voitsekhovskaja OV, von Haefen C, Votruba M, Wada K, Wade-Martins R, Walker CL, Walsh CM, Walter J, Wan XB, Wang A, Wang C, Wang D, Wang F, Wang F, Wang G, Wang H, Wang HG, Wang HD, Wang J, Wang K, Wang M, Wang RC, Wang X, Wang X, Wang YJ, Wang Y, Wang Z, Wang ZC, Wang Z, Wansink DG, Ward DM, Watada H, Waters SL, Webster P, Wei L, Weihl CC, Weiss WA, Welford SM, Wen LP, Whitehouse CA, Whitton JL, Whitworth AJ, Wileman T, Wiley JW, Wilkinson S, Willbold D, Williams RL, Williamson PR, Wouters BG, Wu C, Wu DC, Wu WK, Wyttenbach A, Xavier RJ, Xi Z, Xia P, Xiao G, Xie Z, Xie Z, Xu DZ, Xu J, Xu L, Xu X, Yamamoto A, Yamamoto A, Yamashina S, Yamashita M, Yan X, Yanagida M, Yang DS, Yang E, Yang JM, Yang SY, Yang W, Yang WY, Yang Z, Yao MC, Yao TP, Yeganeh B, Yen WL, Yin JJ, Yin XM, Yoo OJ, Yoon G, Yoon SY, Yorimitsu T, Yoshikawa Y, Yoshimori T, Yoshimoto K, You HJ, Youle RJ, Younes A, Yu L, Yu L, Yu SW, Yu WH, Yuan ZM, Yue Z, Yun CH, Yuzaki M, Zabirnyk O, Silva-Zacarin E, Zacks D, Zacksenhaus E, Zaffaroni N, Zakeri Z, Zeh HJ 3rd, Zeitlin SO, Zhang H, Zhang HL, Zhang J, Zhang JP, Zhang L, Zhang L, Zhang MY, Zhang XD, Zhao M, Zhao YF, Zhao Y, Zhao ZJ, Zheng X, Zhivotovsky B, Zhong Q, Zhou CZ, Zhu C, Zhu WG, Zhu XF, Zhu X, Zhu Y, Zoladek T, Zong WX, Zorzano A, Zschocke J, Zuckerbraun B. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8: 445–544, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol 26: 5336–5347, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem 278: 39653–39661, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292: F617–F627, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Chen L, Yi F, Xia M, Li PL. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1alpha in the renal medulla. Circ Res 102: 1101–1108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 15: 1277–1288, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Shikata K, Miyasaka K, Okada S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka HU, Nishishita S, Sato C, Funakoshi A, Nishimori H, Uchida HA, Ogawa D, Makino H. Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage: anti-inflammatory effect of cholecystokinin. Diabetes 61: 897–907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol 110: e1–7, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Nath KA, Croatt AJ, Hostetter TH. Oxygen consumption and oxidant stress in surviving nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1354–F1362, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin TT, Yu VL, Miller DK. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Norman JT, Fine LG. Intrarenal oxygenation in chronic renal failure. Clin Exp Pharmacol Physiol 33: 989–996, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Satriano J, Sharma K, Blantz RC, Deng A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Renal Physiol 305: F727–F733, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24: 97–106, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Singh P, Blantz RC, Rosenberger C, Gabbai FB, Schoeb TR, Thomson SC. Aberrant tubuloglomerular feedback and HIF-1alpha confer resistance to ischemia after subtotal nephrectomy. J Am Soc Nephrol 23: 483–493, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh P, Deng A, Blantz RC, Thomson SC. Unexpected effect of angiotensin AT1 receptor blockade on tubuloglomerular feedback in early subtotal nephrectomy. Am J Physiol Renal Physiol 296: F1158–F1165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 108: 2462–2467, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 85: 1292–1307, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension 55: 1129–1136, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 300: C385–C393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, Fang Y, Ding X, Liu H, Zhu J, Zou J, Xu X, Zhong Y. Transient hypoxia-inducible factor activation in rat renal ablation and reduced fibrosis with l-mimosine. Nephrology 17: 58–67, 2012. [DOI] [PubMed] [Google Scholar]