Abstract
Podocytes (terminally differentiated epithelial cells of the glomeruli) play a key role in the maintenance of glomerular structure and permeability and in the incipiency of various renal abnormalities. Injury to podocytes is considered a major contributor to the development of kidney disease as their loss causes proteinuria and progressive glomerulosclerosis. The physiological function of podocytes is critically dependent on proper intracellular calcium handling; excessive calcium influx in these cells may result in the effacement of foot processes, apoptosis, and subsequent glomeruli damage. One of the key proteins responsible for calcium flux in the podocytes is transient receptor potential cation channel, subfamily C, member 6 (TRPC6); a gain-of-function mutation in TRPC6 has been associated with the onset of the familial forms of focal segmental glomerulosclerosis (FSGS). Recent data also revealed a critical role of this channel in the onset of diabetic nephropathy. Therefore, major efforts of the research community have been recently dedicated to unraveling the TRPC6-dependent effects in the initiation of podocyte injury. This mini-review focuses on the TRPC6 channel in podocytes and colligates recent data in an attempt to shed some light on the mechanisms underlying the pathogenesis of TRPC6-mediated glomeruli damage and its potential role as a therapeutic target for the treatment of chronic kidney diseases.
Keywords: TRPC6, intracellular calcium, glomerulosclerosis, podocyte, glomerulus, diabetic nephropathy, proteinuria
Role of Podocytes in the Development of Kidney Diseases
current discussions of the mechanisms of glomerular barrier injury and subsequent proteinuria (a core component of chronic kidney disease) tend to focus on the role of glomerular epithelial cells: podocytes. Indeed, podocytes are key components of the renal filtration barrier; their impairment has been reported to underlie glomerulopathies in such diseases as hypertension, preeclampsia, and diabetes mellitus (51, 61). Podocyte loss is one of the primary characteristics of focal segmental glomerulosclerosis (FSGS), a histological pattern of renal injury that can arise from a diverse range of causes and mechanisms and is a common cause of nephrotic syndrome (19, 32, 50). Basically, podocytes and their foot processes function as a final frontier which prevents the leak of plasma proteins into primary urine. Podocytes express numerous receptors and respond to various factors and metabolic products; however, these cells have limited proliferative capacity, and when glomerular growth and hemodynamic stresses exceed the ability of podocytes to undergo hypertrophy, they become irreversibly injured and detach. Podocytopenia is contemporaneous with proteinuria, which, if left untreated, can rapidly progress to end-stage renal disease that warrants dialysis and renal transplantation (6). Normal renal filtration is critically dependent on podocyte function, and it is of crucial importance to better understand the mechanisms of podocyte injury, apoptosis, and detachment to develop new therapeutic intervention strategies.
Transient Receptor Potential Canonical Channel 6 as a Determinant of Podocyte Injury
Transient receptor potential canonical channel (TRPC) proteins, which belong to the larger TRP superfamily of channels, form Ca2+-permeable channels that are important players in the pathogenesis of renal and cardiovascular diseases (3, 13, 64). An association between altered TRPC channels function and/or expression with the development of various renal complications occurring due to podocytopenia has garnered the attention of many investigators (2, 15, 21–24, 33, 40–42, 47–49, 58). The TRPC family is composed of seven structurally related channels (TRPC1-7) (11, 64). It was reported that TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 are expressed in podocytes (20, 28, 58). However, the role of the various members of the TRPC family in podocytes in normal and disease conditions is still somewhat controversial. To date, at least TRPC3 (35, 61), TRPC5 (23, 49, 58), and TRPC6 (28, 29, 31) channels have been functionally and pharmacologically shown to be involved in calcium entry in the podocytes. In other types of cells (for instance, smooth muscle cells of the aorta) (12), an interdependent functional profile of TRPC channels was revealed (smooth muscle cells of TRPC6-deficient mice have been shown to have higher basal cation entry, which was abolished by TRPC3-specific siRNA). This observation has not been confirmed in the TRPC6−/− podocytes (29); however, the compensatory pathways existing within the TRPC family of channels should be always kept in mind while knockout models are being studied.
While compelling data indicate the role of TRPC5 in maintaining of calcium flux in podocytes and development of proteinuric kidney disease (49, 58), a large body of data that have emerged recently allowed pointing at TRPC6 as a promising member of the TRPC family, which could play a role in podocyte depletion in disease conditions. First, several independent laboratories have reported the identification of gain-of-function mutations of TRPC6 associated with autosomal dominant FSGS (25, 46, 62). In addition, changes in channel expression may also contribute to the disease (62); furthermore, it was proposed that TRPC6 is involved in the pathogenesis of the nongenetic forms of proteinuric disease: increased TRPC6 expression is found in glomeruli from patients with such renal pathologies as membranous glomerulonephritis and minimal-change disease (41). Furthermore, overexpression of wild-type or mutant (overactive) TRPC6 in podocytes is sufficient to cause a kidney disease consistent with FSGS (36). Nevertheless, both increased TRPC6 channel activity and expression lead to a pathologically high calcium influx in podocytes, which eventually causes their loss either through apoptosis, detachment, or lack of proliferation (24, 37, 53). Therefore, direct inhibition of TRPC6 channels may be of therapeutic benefit in various glomerulopathies (63).
TRPC6 is located on the podocyte membrane, where it is integrated into a signaling complex that interacts with nephrin, podocin, α-actinin-4, and some other proteins critical for podocyte function (11, 27, 40, 41, 46). As one of many examples, it was discovered that a certain mutant of podocin (P118L) fails to activate TRPC6 channels, and this may compromise the function of the slit diaphragm protein complex and aggravate proteinuria, progressive podocyte loss, and glomerulosclerosis (10). It was also reported that podocin acts as a switch which determines the preferred mode of TRPC6 activation; knockdown of podocin markedly increased stretch-evoked activation of TRPC6, but nearly abolished TRPC6 activation initiated by a diacylglycerol analog (4). It should be noted that TRPC6 channels are usually silent in the absence of stimuli; therefore, TRPC6 activation is important under physiological conditions, and normal functionality of the channel contributes to the integrity of the kidney filtration barrier. On the other hand, it should be emphasized that various stimuli in pathological conditions (or genetic liability) can lead to hyperactivity of the channel, which significantly contributes to podocyte depletion. While known gain-of-function mutations in the TRPC6 gene result only in a small fraction of known cases of FSGS, mutations in other genes such as NPHS2, ACTN4, INF2, and APOL1 might also result in calcium overload in podocytes via activation of TRPC6, producing the same pathological effect as gain-of-function mutations in the TRPC6 gene. Importantly, excessive calcium flux in podocytes mediated by TRPC6 channels is deleterious not only in FSGS but also in many other kidney diseases such as diabetic nephropathy (1, 33, 34, 43, 54, 57, 60, 66).
Potential Stimuli Causing Excessive Calcium Influx Through TRPC6 in Disease Conditions
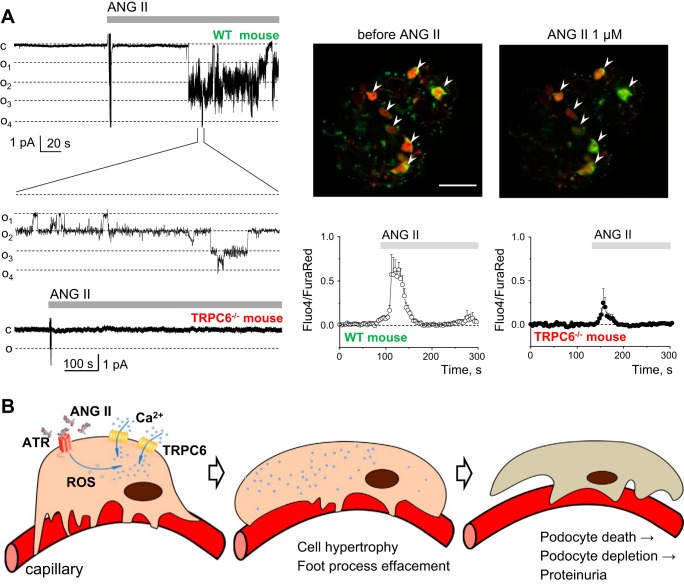
Recent studies suggested several major activators of the TRPC6 channels which are reported to be increased in disease conditions and could mediate enhanced calcium influx in the podocytes. One of the likely triggers of a calcium-dependent pathway of programmed podocyte death is angiotensin II (ANG II); TRPC channels have been associated with ANG II-induced calcium influx in many renal cell types (14, 16–18, 22, 55). ANG II released into the renal interstitium is one of the key mediators of renal inflammation and fibrosis in progressive chronic nephropathies. Studies in models of chronic hypertension and protein-induced renal damages revealed that inhibition of ANG II receptors (ATRs) is effective against proteinuria (8, 9, 59). Angiotensin-converting enzyme (ACE) inhibitors and ATR blockers can attenuate progressive glomerulosclerosis in disease models and slow disease progression in humans (48). It was also shown that ANG II enhances albuminuria by activating TRPC6 channels in podocytes (15). Furthermore, alteration of TRPC6 expression and Ca2+ influx are involved in ANG II-induced apoptosis (65). Also, it was demonstrated that the deleterious effects of ANG II on podocytes and its pathogenic role in glomerular diseases involve enhanced TRPC6 expression (42). Therefore, the association between ANG II and TRPC6 channel is well established (1, 5, 15, 29, 42, 58). Recent data revealed that G protein-coupled receptors (GPCRs) linked to Gq signaling, which causes activation of receptors for ANG II, endothelins, thromboxanes, and some other GPCR agonists, induce glomerular injury by activating TRPC6 (60). Our data also provided evidence that ANG II increases native TRPC channel activity in the podocytes of freshly isolated glomeruli (this effect was lacking in the TRPC6−/− knockout mice) (29) (see Fig. 1A).
Fig. 1.
Effects of ANG II on transient receptor potential cation channel, subfamily C, member 6 (TRPC6) channel activity and calcium influx in the podocytes. A, left: representative current traces demonstrating the effects of ANG II on TRPC6 channel activity recorded in cell-attached patches made on podocytes of the freshly isolated glomeruli obtained from wild-type (WT) and TRPC6−/− mice. Continuous current traces and addition of ANG II (1 μM) to the external bath solution are shown. All patches were held at a −60-mV test potential during the course of the experiment. The c and oi denote closed and open current levels, respectively (adapted from Ref. 29). Right, top row: representative images of WT mouse glomeruli stained with Fluo4 (green pseudocolor) and Fura Red (red) before and after application of 1 μM ANG II; podocytes are marked with arrows. Note an increase in green signal and a decrease in red signal intensities upon addition of ANG II, which reflect an elevation in the intracellular calcium concentration. Bottom row: graphs demonstrate representative calcium transients caused by the application of 1 μM ANG II to the podocytes in WT and TRPC6−/− mouse glomeruli. Scale bar = 30 μm. B: scheme illustrating podocyte retraction as a result of the excessive calcium influx through the TRPC6 channels under a pathological stimulus (elevated ANG II levels) originating in a disease condition. ROS, reactive oxygen species; ATR, ANG II receptor.
Furthermore, ANG II causes an acute release of H2O2 in the kidney (44). This observation is largely in line with the report which showed that ANG II-dependent activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species (ROS) (47). Furthermore, Kim et al. (34) have provided compelling evidence of the fact that in the podocytes ROS-producing NADPH oxidases are part of a complex with TRPC6 channels (when podocin is present) and contribute to the channel's activation. Interestingly, TRPC6 expression and/or calcium influx in the podocytes has been shown to be induced by glucose (38), insulin (33), and ATP (30, 47), and these processes have also been associated with ROS production. Additionally, the role of TRPC6 channels in oxidative stress-induced podocyte ischemic injury has been recently demonstrated (67). ROS are ubiquitous cellular signals, which are closely associated with the development and progression of glomerular sclerosis, and elimination of ROS can be protective against kidney injury (7, 39, 45, 52, 56). These findings are indeed very intriguing and allow us to further speculate that ROS production (caused by various stimuli) is a common mechanism of TRPC6 channel activation in the podocytes (see Fig. 1B). However, by no means should we say that the mechanisms described above are the only signaling pathways critical for TRPC6 (as well as other TRPC channels in podocytes) regulation. For instance, there is some uncertainty whether TRPC6 channels are intrinsically mechanosensitive (4, 61), or the GPCRs and/or phospholipases respond to mechanical stimuli and then activate TRPC6; however, there is no controversy about the fundamental observation that TRPC6 is certainly a component of mechanotransduction cascades, which become overly active during hyperfiltration, undoubtedly an issue in diabetes and chronic kidney disease (26).
Conclusion
Recent joint efforts of many research teams have led to critical advances in our understanding of the podocyte biology and its role in the maintenance of the kidney filtration barriers. To date, it is widely accepted that increased calcium influx through the TRPC6 channels is one of the major determining factors of podocyte injury in various renal pathologies, including FSGS, diabetic nephropathy, and nephrotic syndrome. Various animal models useful for the studies of TRPC6 have been created to date; for instance, global TRPC6 knockout mice (in which TRPC6 is knocked out in all cell types of the whole body, including podocytes) are viable and show no gross phenotype, besides the slightly higher mean arterial blood pressure (12). Furthermore, mice with podocyte-specific overexpression of TRPC6 [B6.Cg-Tg(NPHS2-Trpc6)F419Walz/J] are commercially available. These transgenic mice exhibit albuminuria, podocyte structural injuries, glomerular lesions, tubulointerstitial damage, and other pathological features of FSGS (36) and represent an excellent model for the study of TRPC6 under FSGS conditions. In the light of the recent observations showing the interchangeable functionality of TRPC family members in other cell types, it would be rather intriguing to study calcium entry in the podocytes on the basis of a multiple TRPC channel knockout model.
Major research efforts are currently focused on exploring cellular pathways which transduce the activating signal to the TRPC6 channel. Selective manipulation of these pathways may be an effective means of modulating kidney injury; however, specific mechanisms of these processes and many questions, like whether TRPC6 channels are susceptibility or initiation factors of renal disease progression, remain uncertain. Nevertheless, it is clear that the ability of podocytes to precisely regulate the intracellular Ca2+ level plays a crucial role in glomerular diseases; manipulating Ca2+ levels by inhibiting TRPC channels or targeting their upstream effectors holds strong promise for treating patients with chronic kidney disease and preventing podocyte depletion at early stages of renal diseases, for instance, in diabetes. Efforts to understand the role of ANG II, ROS, ATP, and other stimuli in the regulation of TRPC channels in healthy and pathophysiological states have a strong potential for scientific and medical implications in furthering our understanding of TRPC-mediated diseases.
GRANTS
This research was partially supported by National Heart, Lung, and Blood Institute Grant R01 HL108880 and R01 HL122662, American Diabetes Association Grant 1-15-BS-172 (to A. Staruschenko), and the Ben J. Lipps Research Fellowship from the American Society of Nephrology (to D. V. Ilatovskaya).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.V.I. and A.S. prepared figures; D.V.I. and A.S. drafted manuscript; D.V.I. and A.S. edited and revised manuscript; D.V.I. and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize to the investigators whose relevant publications were inadvertently not directly discussed due to space limitation.
REFERENCES
- 1.Abkhezr M, Dryer SE. Angiotensin II and canonical transient receptor potential-6 activation stimulate release of a signal transducer and activator of transcription 3-activating factor from mouse podocytes. Mol Pharmacol 86: 150–158, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Abkhezr M, Dryer SE. STAT3 regulates steady-state expression of synaptopodin in cultured mouse podocytes. Mol Pharmacol 87: 231–239, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23: 297–328, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson M, Kim EY, Hagmann H, Benzing T, Dryer SE. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am J Physiol Cell Physiol 305: C276–C289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson M, Roshanravan H, Khine J, Dryer SE. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol 229: 434–442, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 5: 151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Meng XF, Zhang C. Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. Biomed Res Int 2013: 839761, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi X, Hu B, Yu SY, Yin L, Meng Y, Wang B, Yang J, Lin J, Huang D, Chen L. Losartan treating podocyte injury induced by Ang II via downregulation of TRPC6 in podocytes. J Renin Angiotensin Aldosterone Syst [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest 119: 943–953, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dattilo M, Penington NJ, Williams K. Inhibition of TRPC5 channels by intracellular ATP. Mol Pharmacol 73: 42–49, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich A, Chubanov V, Gudermann T. Renal TRPathies. J Am Soc Nephrol 21: 736–744, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol 25: 6980–6989, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol 299: F689–F701, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Sours-Brothers S, Coleman R, Ding M, Graham S, Kong DH, Ma R. Canonical transient receptor potential 1 channel is involved in contractile function of glomerular mesangial cells. J Am Soc Nephrol 18: 1437–1445, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, Wu G, Bowling B, Byrd A, Hall G, Sparks M, Zhang ZS, Homstad A, Barisoni L, Birbaumer L, Rosenberg P, Winn MP. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol 22: 526–535, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans JF, Lee JH, Ragolia L. Ang-II-induced Ca2+ influx is mediated by the 1/4/5 subgroup of the transient receptor potential proteins in cultured aortic smooth muscle cells from diabetic Goto-Kakizaki rats. Mol Cell Endocrinol 302: 49–57, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Fellner SK, Arendshorst WJ. Angiotensin II-stimulated Ca2+ entry mechanisms in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am J Physiol Renal Physiol 294: F212–F219, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 289: F1012–F1019, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Fornoni A, Merscher S, Kopp JB. Lipid biology of the podocyte—new perspectives offer new opportunities. Nat Rev Nephrol 10: 379–388, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel M, Sinkins WG, Zuo CD, Estacion M, Schilling WP. Identification and localization of TRPC channels in the rat kidney. Am J Physiol Renal Physiol 290: F1241–F1252, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem 285: 23466–23476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham S, Ding M, Sours-Brothers S, Yorio T, Ma JX, Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Renal Physiol 293: F1381–F1390, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Greka A, Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol 22: 1969–1980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeringa SF, Moller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4: e7771, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Huber TB, Schermer B, Muller RU, Hohne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstadt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA 103: 17079–17086, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilatovskaya DV, Levchenko V, Ryan RP, Cowley AW Jr, Staruschenko A. NSAIDs acutely inhibit TRPC channels in freshly isolated rat glomeruli. Biochem Biophys Res Commun 408: 242–247, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, Staruschenko A. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int 86: 506–514, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Physiol Cell Physiol 305: C1050–C1059, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilatovskaya DV, Staruschenko A. Single-channel analysis of TRPC channels in the podocytes of freshly isolated glomeruli. Methods Mol Biol 998: 355–369, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jefferson JA, Shankland SJ. The pathogenesis of focal segmental glomerulosclerosis. Adv Chronic Kidney Dis 21: 408–416, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol 302: F298–F307, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EY, Anderson M, Wilson C, Hagmann H, Benzing T, Dryer SE. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am J Physiol Cell Physiol 305: C960–C971, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Kim EY, Alvarez-Baron CP, Dryer SE. Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes. Mol Pharmacol 75: 466–477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, Ruiz P, Mezzano SA, Li J, Wei C, Reiser J, Young JI, Walz K. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One 5: e12859, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Liu BC, Song X, Lu XY, Li DT, Eaton DC, Shen BZ, Li XQ, Ma HP. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim Biophys Acta 1833: 1434–1442, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol 34: 946–952, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Moller CC, Flesche J, Reiser J. Sensitizing the slit diaphragm with TRPC6 ion channels. J Am Soc Nephrol 20: 950–953, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Moller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Moller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Ryan RP, Cowley AW, Staruschenko A. Real-time electrochemical detection of ATP and H2O2 release in freshly isolated kidneys. Am J Physiol Renal Physiol 305: F134–F141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putri AY, Thaha M. Role of oxidative stress on chronic kidney disease progression. Acta Med Indones 46: 244–252, 2014. [PubMed] [Google Scholar]
- 46.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, vila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roshanravan H, Dryer SE. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol 23: 1917–1928, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A. Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schena FP. Primary glomerulonephritides with nephrotic syndrome. Limitations of therapy in adult patients. J Nephrol 12, Suppl 2: S125–S130, 1999. [PubMed] [Google Scholar]
- 51.Scott RP, Quaggin SE. The cell biology of renal filtration. J Cell Biol 209: 199–210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol 18: 16–28, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Sonneveld R, van der Vlag J, Baltissen MP, Verkaart SA, Wetzels JF, Berden JH, Hoenderop JG, Nijenhuis T. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol 184: 1715–1726, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Takenaka T, Suzuki H, Okada H, Inoue T, Kanno Y, Ozawa Y, Hayashi K, Saruta T. Transient receptor potential channels in rat renal microcirculation: actions of angiotensin II. Kidney Int 62: 558–565, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Tan RJ, Zhou D, Xiao L, Zhou L, Li Y, Bastacky SI, Oury TD, Liu Y. Extracellular superoxide dismutase protects against proteinuric kidney disease. J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thilo F, Liu Y, Loddenkemper C, Schuelein R, Schmidt A, Yan Z, Zhu Z, Zakrzewicz A, Gollasch M, Tepel M. VEGF regulates TRPC6 channels in podocytes. Nephrol Dial Transplant 27: 921–929, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstadt H, Hsu HH, Schlondorff J, Ramos A, Greka A. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3: ra77, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian ML, Shen Y, Sun ZL, Zha Y. Efficacy and safety of combining pentoxifylline with angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in diabetic nephropathy: a meta-analysis. Int Urol Nephrol 47: 815–822, 2015. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Jirka G, Rosenberg PB, Buckley AF, Gomez JA, Fields TA, Winn MP, Spurney RF. Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest 125: 1913–1926, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson C, Dryer SE. A mutation in TRPC6 channels abolishes their activation by hypoosmotic stretch but does not affect activation by diacylglycerol or G protein signaling cascades. Am J Physiol Renal Physiol 306: F1018–F1025, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Winn MP, Daskalakis N, Spurney RF, Middleton JP. Unexpected role of TRPC6 channel in familial nephrotic syndrome: does it have clinical implications? J Am Soc Nephrol 17: 378–387, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Woudenberg-Vrenken TE, Bindels RJ, Hoenderop JG. The role of transient receptor potential channels in kidney disease. Nature Rev Nephrol 5: 441–449, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Ding J, Fan Q, Liu S. TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp Biol Med 234: 1029–1036, 2009. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Song Z, Guo Y, Zhou M. The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ-induced diabetic rats. Mol Cell Biochem 399: 155–165, 2015. [DOI] [PubMed] [Google Scholar]
- 67.Zhao B, Yang H, Zhang R, Sun H, Liao C, Xu J, Meng K, Jiao J. The role of TRPC6 in oxidative stress-induced podocyte ischemic injury. Biochem Biophys Res Commun 461: 413–420, 2015. [DOI] [PubMed] [Google Scholar]