Abstract
Our previous publication showed that inhibition of arginase prevents the development of diabetic nephropathy (DN). However, identification of targets that retard the progression of established DN–which is more clinically relevant–is lacking. Therefore, we tested the hypothesis that arginase inhibition would prevent the progression of established DN. Effects of arginase inhibition were compared with treatment with the angiotensin-converting enzyme inhibitor captopril, a current standard of care in DN. Experiments were conducted in Ins2Akita mice treated with the arginase inhibitor S-(2-boronoethyl)-l-cysteine (BEC) or captopril starting at 6 wk of age for 12 wk (early treatment) or starting at 12 wk of age for 6 wk (late treatment). Early and late treatment with BEC resulted in protection from DN as indicated by reduced albuminuria, histological changes, kidney macrophage infiltration, urinary thiobarbituric acid-reactive substances, and restored nephrin expression, kidney nitrate/nitrite, kidney endothelial nitric oxide synthase phosphorylation, and renal medullary blood flow compared with vehicle-treated Ins2Akita mice at 18 wk of age. Interestingly, early treatment with captopril reduced albuminuria, histological changes, and kidney macrophage infiltration without affecting the other parameters, but late treatment with captopril was ineffective. These findings highlight the importance of arginase inhibition as a new potential therapeutic intervention in both early and late stages of diabetic renal injury.
Keywords: arginase, established diabetic nephropathy, nitric oxide, ACEi
pathogenesis of diabetic nephropathy (DN) is a multifactorial process and not completely understood. DN has become the leading cause of end-stage renal disease (ESRD) in the United States (13). DN is a chronic disorder initiated early by the development of glomerular hyperfiltration/hypertrophy, followed by thickening of the glomerular basement membrane, increased urinary albumin excretion (UAE) rate, often within 5–10 yr of its detection, and ultimately progression to ESRD. Among the pathogenic factors are hyperglycemia, increased systemic and glomerular pressure, increased activity of the renin-angiotensin-aldosterone system, endothelial dysfunction, and stimulation of several cytokines and growth factors by metabolic and hemodynamic factors (14). Several therapeutic interventions targeting these mechanisms have been developed and implemented with various degrees of success. Current therapies, including blood pressure and glucose control and other life style changes, have been only modestly successful in delaying the progression of renal failure (1). Unfortunately, the majority of current research is focused on drug discovery to reduce the development of DN rather than the progression of DN, which is more clinically relevant. Therefore, more effective approaches to reduce the progression of DN are urgently needed. Angiotensin-converting enzyme (ACE) inhibitors (ACEi) are recognized as the standard of care in DN and have been shown to postpone the development or slow down the progression of DN (1). However, this therapy does not produce a full reversal or even halting of renal function deterioration (23). Thus, it is important to identify pharmacotherapeutics that will halt the development and/or reverse the progression of diabetic kidney disease.
Decreased nitric oxide (NO) bioavailability–due in part to reduced endothelial NO synthase (eNOS) activity or expression–and endothelial dysfunction play key roles in the pathophysiology of diabetes and DN (9, 10, 19, 29, 32, 38). As l-arginine is the substrate for arginases as well as all NOS isozymes, changes in arginase activity can have a significant impact on NO production via substrate competition (39). Arginases are expressed as two distinct isozymes (arginase-1 and arginase-2), and arginase-2 is almost exclusively the isozyme expressed in the kidney (16, 27, 28, 36). In this regard, our previous work demonstrated that pharmacological blockade or genetic deficiency of arginase-2 confers kidney protection in diabetic mouse models (28). We further showed that arginase inhibition mediates renal tissue protection in DN via an eNOS-dependent mechanism (42). As those studies focused only on early changes of nephropathy, the role of arginase inhibition in progressive DN, including glomerulosclerosis and tubulointerstitial disease, was unknown. We therefore proceeded to test the hypothesis that arginase inhibition will also prevent the progression of established DN.
The current study used a pharmacologic approach to evaluate the efficacy of arginase inhibition in a model of established DN compared with treatment with the ACE inhibitor captopril. Overall, our results with delayed treatment of arginase inhibition in established diabetes will address a clinically relevant question. Our results indicate that arginase inhibition may be a promising treatment for late-stage DN.
MATERIALS AND METHODS
Diabetic mouse models.
Experiments were conducted in male D2.B6-Ins2Akita/MatbJ and their wild-type littermate mice (DBA/2J background; Jackson Laboratory, stock number 007562) starting at 6 wk of age (∼3 wk of diabetes). Ins2Akita mice, recommended by the Animal Models of Diabetes Complications Consortium (AMDCC) as a model of DN (6, 7), develop hyperglycemia at 3 wk of age. Mice with blood glucose levels >350 mg/dl were considered diabetic. Mice were provided ad libitum access to food and water. Urine collections were obtained by housing mice in metabolic cages for 18 h with free access to water while food was withheld to prevent any contamination. Animal experiments were approved by the Penn State University College of Medicine Institutional Animal Care and Use Committee.
Drug delivery.
Vehicle (PBS) or S-(2-Boronoethyl)-l-cysteine (BEC; 2.3 mg·kg−1·day−1) was administered by continuous subcutaneous infusion via a mini-osmotic pump (Alzet, Durect, Cupertino, CA) as described previously (28, 42). Captopril (Sigma) was provided daily in drinking water (24 mg/l). Treatments were started at 6 wk of age (early treatment) or at 12 wk of age (late treatment) until 18 wk of age in Ins2Akita mice. At the end of experiments (18 wk of age), mice were euthanized; kidneys were removed and plasma was collected for further studies.
Analytical methodology.
The following methods were employed as described in the indicated references: measurement of urine albumin by ELISA using an Albuwell M kit (Exocell, Philadelphia, PA) (2, 28, 41); urine creatinine was determined using an enzymatic assay (Crystal Chem, Downers Grove, IL); assay of urinary thiobarbituric acid-reactive substances (TBARS) (22, 42); and measurement of renal medullary blood flow by a Laser Doppler Flow Meter (model BLF-21D, Transonic Systems, Ithaca, NY) (28). A Coda blood pressure system (Kent Scientific, Torrington, CT) was used to measure systolic blood pressure (41, 42); mice were allowed to rest quietly for 15 min at room temperature before measurement. All blood pressure measurements were performed at the same time for all groups to prevent any diurnal variations.
Kidney nitrate and nitrite assay.
Kidney tissues were homogenized in 1× PBS, pH 7.4, and centrifuged at 10,000 g for 20 min at 4°C. Supernatants were transferred to a clean tube and were used to determine protein concentration and nitrate/nitrite using a Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI, catalog no. 780001) as we described previously (43).
Western blot.
Kidney tissues were homogenized in 0.1% Triton X-100 supplemented with protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, IN) and sodium orthovanadate (Sigma). Tissue homogenates were centrifuged at 20,000 g for 10 min. Fifteen-microgram kidney lysates were separated on a 4∼12% NuPAGE Bis-Tris gel (Invitrogen), and the separated proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). After blocking in 5% nonfat milk, membranes were incubated with primary antibodies overnight at 4°C, followed by incubation in corresponding secondary antibodies for 1 h at room temperature. Primary antibodies and dilutions were as follows: anti-nephrin antibody (1:500, catalog no. 20R-NP002, Fitzgerald Industries International, Acton, MA), anti-eNOS antibody (1:1,000, catalog no. ab76198, Abcam, Cambridge, MA), anti-phospho-eNOS at S1177 antibody (1:1,000, catalog no. 9570S, Cell Signaling Technology, Danvers, MA), and anti-GAPDH antibody (1:1,000, catalog no. 5174S, Cell Signaling Technology). Enhanced chemiluminescence solution (Thermo Fisher Scientific) was used to develop membranes after final washing, followed by exposure to X-ray films. Densitometry was performed using Image J (National Institutes of Health, http://rsbweb.nih.gov/ij/index.html). Nephrin abundance was expressed relative to the average abundance in nondiabetic mice following normalization to the internal reference GAPDH as described (42, 43), and phospho-eNOS/eNOS was expressed relative to the average ratio in nondiabetic mice.
Histology and immunohistochemistry.
Mouse kidneys were fixed in 10% neutral buffered formalin overnight, followed by 70% ethanol wash and paraffin embedment. Periodic acid Schiff (PAS) staining was performed on 3-μm tissue sections. All glomeruli (between 51–110 glomeruli) in a single transverse section for each mouse were examined under a microscope at ×400 total magnification and scored individually in a blinded manner and then averaged. All images were obtained with an Olympus BX51 microscope and DP71 digital camera using cellSens Standard 1.6 imaging software (Olympus America, Center Valley, PA). Images were taken with ×100 (oil) objective with a total magnification of ×1,000. Semiquantitative scores (0–4+) were assigned based on the masked readings as previously described (28, 41, 42).
Immunohistochemistry for macrophages was performed using anti-mouse Mac-2 antibody (clone M3/38; Cedarlane, Burlington, NC) on paraffin sections. The number of glomerular macrophages was counted in 40 glomeruli per section (number of macrophages in glomeruli divided by the number of glomeruli) in blinded fashion under ×40 magnification and averaged as described previously (28, 42).
Statistical analysis.
Comparisons between groups were examined by using SPSS version 21.0 software for Windows (SPSS, Chicago, IL). Data are expressed as means ± SE. One-way ANOVA was used when more than two groups were compared, and significance of observed differences among the groups was evaluated with a least significant difference post hoc test. Statistical significance was identified at P < 0.05.
RESULTS
Arginase inhibition reduces the progression of established DN in Ins2Akita mice.
To assess the significance of arginase inhibition in DN, we treated Ins2Akitamice with vehicle or BEC via osmotic minipump, or captopril (24 mg/l daily in drinking water), beginning at either 6 wk of age until 18 wk of age (early treatment) or at 12 wk of age until 18 wk of age (late treatment). As shown in Table 1, Ins2Akita vehicle-treated mice had increased blood glucose level, and decreased body weight, compared with nondiabetic mice. Early or late treatment with either BEC or captopril did not reduce blood glucose levels or blood pressure.
Table 1.
Effects of early and late treatment with arginase inhibitor or captopril in diabetic Ins2Akita mice at 18 wk of age
| Nondiabetic | Ins2Akita + Vehicle | Ins2Akita + BEC Early | Ins2Akita + BEC Late | Ins2Akita + Captopril Early | Ins2Akita + Captopril Late | |
|---|---|---|---|---|---|---|
| Mouse number | 11 | 8 | 9 | 10 | 9 | 9 |
| Body wt, g | 31.5 ± 0.8 | 24.6 ± 1* | 26.3 ± 0.5*‡ | 24.7 ± 0.3* | 20.3 ± 1.2* | 24.2 ± 0.7* |
| Blood glucose, mg/dl | 156 ± 3 | 494 ± 4† | 500 ± 1† | 500 ± 1† | 493 ± 5† | 489 ± 9† |
| Systolic BP, mmHg | 120 ± 3 | 113 ± 2 | 124 ± 4 | 119 ± 2 | 119 ± 5 | 115 ± 5 |
Data are means ± SE. BEC, S-(2-boronoethyl)-l-cysteine; BP, blood pressure.
P < 0.001,
P < 0.0001 compared with nondiabetic.
P < 0.01 compared with Ins2Akita + captopril early.
We next measured urine albumin excretion (UAE) as an indicator of diabetic kidney injury (Fig. 1). At 6 wk of age (3 wk after diabetes; before start of treatment), all groups of Ins2Akita mice had a significant increase in UAE compared with nondiabetic mice. At 12 wk of age (6 wk after early treatment), early treatment of captopril–but not early treatment with BEC–significantly reduced albuminuria compared with vehicle-treated mice. At 18 wk of age, early or late treatment of BEC significantly reduced albuminuria (Fig. 1) and urine albumin/creatinine ratio (Fig. 2) compared with vehicle-treated mice. In contrast, only early but not late treatment with captopril reduced albuminuria (Fig. 1) and urine albumin/creatinine ratio (Fig. 2) to a similar extent as early treatment with BEC. Although late treatment with captopril showed a tendency to reduce albuminuria, UAE was not significantly different from that of vehicle-treated Ins2Akita mice.
Fig. 1.
Late treatment with arginase inhibitor attenuates albuminuria (UAE) in Ins2Akita mice at 18 wk of age. Five groups of Ins2Akita mice at 6 wk of age were initially selected to receive the various treatments at the times indicated. Urine was collected from each group of mice at 6, 12, and 18 wk of age for the measurement of UAE. Ins2Akita mice were treated with vehicle, S-(2-boronoethyl)-l-cysteine (BEC; 2.3 mg·kg−1·day−1) via osmotic minipump, or captopril (24 mg/l daily in drinking water) starting at 6 wk (early treatment) or 12 wk (late treatment) of age until 18 wk of age. Results are means ± SE. *P < 0.05, **P < 0.001 compared with corresponding nondiabetic. #P < 0.05, ##P < 0.01 compared with corresponding vehicle-treated Ins2Akita mice.
Fig. 2.

Late treatment with arginase inhibitor attenuates urine albumin/creatinine ratio in Ins2Akita mice at 18 wk of age. Ins2Akita mice were treated with vehicle, BEC (2.3 mg·kg−1·day−1) via osmotic minipump, or captopril (24 mg/l daily in drinking water) starting at 6 wk (early treatment) or 12 wk (late treatment) of age until 18 wk of age. Urine was collected for measurement of urine albumin/creatinine ratio at 18 wk of age. Results are means ± SE. *P < 0.01, **P < 0.0001 compared with nondiabetic. #P < 0.01 compared with vehicle-treated Ins2Akita mice.
Arginase inhibition restores kidney NO production and eNOS phosphorylation in established DN.
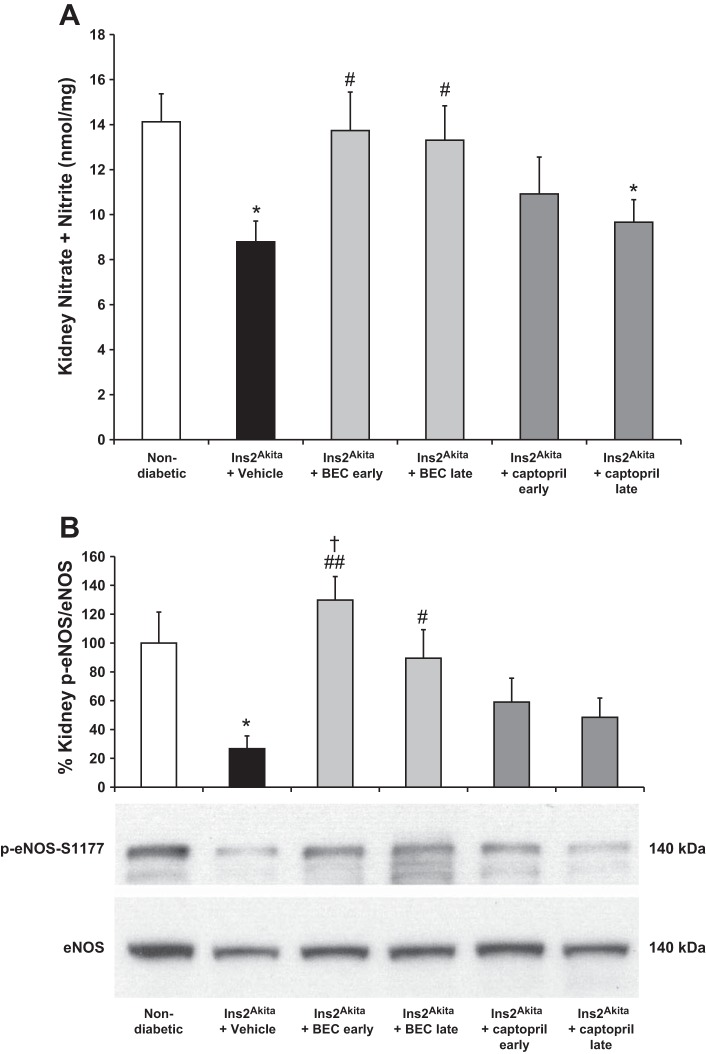
Since endothelial dysfunction, characterized by reduced NO bioavailability, is a central pathophysiological mechanism that contributes to diabetes and DN (9, 10, 19, 29, 32, 38), we first measured kidney nitrate + nitrite as an indicator of NO status at 18 wk of age. Kidney nitrate + nitrite level was significantly reduced in vehicle-treated Ins2Akita mice compared with nondiabetic mice (Fig. 3A). Early or late treatment of BEC significantly restored kidney nitrate + nitrite level at 18 wk of age compared with vehicle-treated mice. In contrast, neither early nor late treatment with captopril affected kidney nitrate + nitrite levels, which were comparable with levels in vehicle-treated Ins2Akita mice.
Fig. 3.
Late treatment with arginase inhibitor restores kidney nitric oxide (NO) production and endothelial NO synthase (eNOS) phosphorylation in Ins2Akita mice at 18 wk of age. A: mouse kidney tissue lysates were used to measure nitrate and nitrite concentration in Ins2Akita mice at 18 wk of age. B: eNOS phosphorylation was measured in kidney tissue lysates using Western blot. Levels of phospho-eNOS and total eNOS protein were determined by densitometry. The mean phospho-eNOS/eNOS value for normal mice was arbitrarily set to 100. Results are means ± SE. *P < 0.05 compared with nondiabetic. #P < 0.05, ##P < 0.01 compared with vehicle-treated Ins2Akita mice. †P < 0.05 compared with Ins2Akita mice treated with captopril early. A representative Western blot is shown.
We also measured eNOS phosphorylation using Western blot analysis since eNOS plays a critical role in DN (9, 10, 19, 29, 32, 38, 47). Phosphorylation of eNOS at serine 1177 upregulates eNOS activity (8). Our data demonstrated that eNOS phosphorylation was significantly reduced in vehicle-treated Ins2Akita mice compared with nondiabetic mice (Fig. 3B). Early or late treatment of BEC significantly restored eNOS phosphorylation at 18 wk of age compared with vehicle-treated mice. In contrast, neither early nor late treatment with captopril affected eNOS phosphorylation, which was comparable in levels with vehicle-treated Ins2Akita mice.
Arginase inhibition restores renal medullary blood flow in established DN.
We next measured renal medullary blood flow to evaluate the downstream effect of endothelial dysfunction in DN. As shown in Fig. 4, renal medullary blood flow was significantly reduced in vehicle-treated Ins2Akita mice compared with nondiabetic mice. As expected, early and late treatment with BEC significantly restored renal medullary blood flow at 18 wk of age compared with vehicle-treated mice. In contrast, neither early nor late treatment with captopril affected renal medullary blood flow, which was comparable with vehicle-treated Ins2Akita mice.
Fig. 4.

Late treatment with arginase inhibitor restores renal medullary blood flow in Ins2Akita mice at 18 wk of age. Renal medullary blood flow was measured in Ins2Akita mice at 18 wk of age, and expressed relative to the mean value for nondiabetic mice, which was arbitrarily set at 100. Results are means ± SE. *P < 0.05, **P < 0.01 compared with nondiabetic. #P < 0.05, ##P < 0.01 compared with vehicle-treated Ins2Akita mice. †P < 0.01 compared with Ins2Akita mice treated with captopril early. ‡P < 0.01 compared with Ins2Akita mice treated with captopril late.
Arginase inhibition reduces urinary TBARS in established DN.
Levels of urinary TBARS, which is an indicator of oxidative stress, were significantly elevated in vehicle-treated Ins2Akita mice compared with nondiabetic mice (Fig. 5). Early or late treatment with BEC significantly reduced urinary TBARS levels at 18 wk of age compared with vehicle-treated mice. Similarly, early or late treatment with captopril significantly reduced urinary TBARS levels at 18 wk of age compared with vehicle-treated mice.
Fig. 5.

Late treatment with arginase inhibitor reduces urinary thiobarbituric acid-reactive substances (TBARS) in Ins2Akita mice at 18 wk of age. Urine was collected for measurement of TBARS levels. Results are means ± SE. *P < 0.01, **P < 0.001 compared with nondiabetic. #P < 0.05 compared with vehicle-treated Ins2Akita mice.
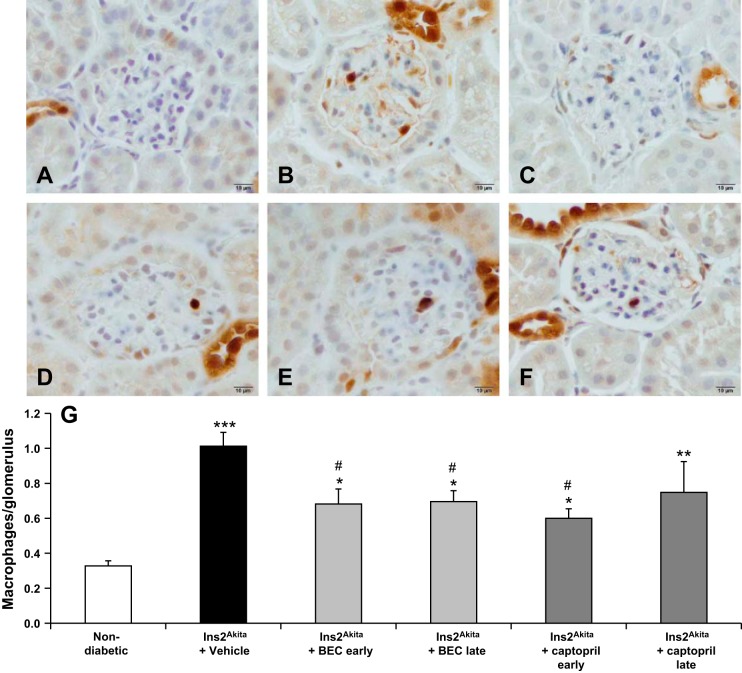
Arginase inhibition decreases glomerular macrophage infiltration in established DN.
Our previous data demonstrated that early BEC treatment resulted in significantly reduced glomerular macrophage infiltration in diabetic mice (28, 42). In this study (Fig. 6), we showed that vehicle-treated Ins2Akita mice had significantly increased glomerular macrophage infiltration compared with nondiabetic mice (P < 0.0001). Early or late treatment with BEC significantly decreased glomerular macrophage infiltration at 18 wk of age (P < 0.05) compared with vehicle-treated mice. In contrast, only early but not late treatment with captopril significantly decreased glomerular macrophage infiltration (P < 0.05) to a similar extent as early treatment with BEC. Late treatment with captopril showed a tendency to reduce glomerular macrophage infiltration but the infiltration was not significantly different from vehicle-treated Ins2Akita mice.
Fig. 6.
Late treatment with arginase inhibitor reduces macrophage infiltration in Ins2Akita mice at 18 wk of age. Mac-2-positive macrophages in glomeruli were identified by immunohistochemical staining at 18 wk of age in nondiabetic (A), vehicle-treated Ins2Akita (B), early BEC-treated (C), late BEC-treated (D), early captopril-treated (E), or late captopril-treated (F) Ins2Akita mice. G: summary data for macrophages/glomerulus. Results are means ± SE. *P < 0.05, **P < 0.001, ***P < 0.0001 compared with nondiabetic. #P < 0.05 compared with vehicle-treated Ins2Akita mice.
Arginase inhibition reduces histological changes in established DN.
PAS staining of kidney sections showed increased glomerular cellularity and mesangial expansion in vehicle-treated Ins2Akita mice compared with nondiabetic mice (P < 0.0001; Fig. 7). Early or late treatment with BEC significantly decreased glomerular cellularity and mesangial expansion at 18 wk of age (P < 0.001 and P < 0.05, respectively) compared with vehicle-treated mice. In contrast, early but not late treatment with captopril significantly decreased glomerular cellularity and mesangial expansion (P < 0.001) to a similar extent as early treatment with BEC.
Fig. 7.
Late treatment with arginase inhibitor reduces renal histological changes in Ins2Akita mice at 18 wk of age. Sections were stained with periodic acid Schiff (PAS) and all glomeruli were graded individually at ×400 magnification after 18 wk of age in nondiabetic (A), vehicle-treated Ins2Akita (B), early BEC-treated (C), late BEC-treated (D), early captopril-treated (E), or late captopril-treated (F) Ins2Akita mice. Images were taken with ×100 (oil) objective with a total magnification of ×1,000. Images are representative of 7–13 mice in each group. G: summary data for glomerular PAS score. Results are means ± SE. *P < 0.01, **P < 0.0001 compared with nondiabetic. #P < 0.05, ##P < 0.001 compared with vehicle-treated Ins2Akita mice.
Arginase inhibition restores nephrin protein expression in established DN.
Our previous publications showed that DN is associated with reduced nephrin expression (41, 43). In the present study, we confirmed this result and showed that vehicle-treated Ins2Akita mice had significantly reduced nephrin expression compared with nondiabetic mice (Fig. 8). Early or late treatment with BEC significantly restored nephrin expression at 18 wk of age compared with vehicle-treated mice. In contrast, neither early nor late treatment with captopril affected the reduction in nephrin expression.
Fig. 8.
Late treatment with arginase inhibitor restores renal nephrin protein expression in Ins2Akita mice at 18 wk of age. Kidney nephrin expression was evaluated using Western blot at 18 wk of age. Quantification was performed by densitometry followed by normalization to GAPDH. The mean nephrin/GAPDH value for nondiabetic mice was arbitrarily set to 100. A representative Western blot is shown. Results are means ± SE. *P < 0.05 compared with nondiabetic. #P < 0.05 compared with vehicle-treated Ins2Akita mice. ‡P < 0.05 compared with Ins2Akita mice treated with captopril late.
DISCUSSION
We demonstrated previously that pharmacological blockade or genetic deficiency of arginase-2 confers kidney protection in diabetic mouse models (28) via an eNOS-dependent mechanism (42). Our previous work focused on early changes of nephropathy. However, the role of arginase inhibition in the progression of established DN remained to be firmly established. Understanding the role of arginases in the progression of DN will have clinical relevance. The current study shows that arginase inhibition not only prevents the development but also the progression of DN in the Ins2Akita mice. First, we show that early or late treatment with BEC reduces albuminuria, histopathological changes, and kidney macrophage infiltration in DN. Second, early or late treatment with BEC restores NO production/action as indicated by increased kidney nitrite + nitrate, eNOS phosphorylation, renal blood flow, and reduced urinary TBARS in DN. Third, arginase inhibition results in restoration of renal nephrin expression in DN. Taken together, our data indicate that arginase inhibition could be a potentially new therapeutic tool for treating diabetic patients.
Reduced NO bioavailability and increased oxidative stress are hallmark characteristics of insulin resistance, diabetes mellitus, hypertension, and DN (9, 10, 19, 29, 32, 38, 47). Specifically, endothelial dysfunction plays a critical role in DN (30); thus, elucidating the basis for vascular dysfunction in DN is critical (3). NO is produced from l-arginine, which is a common substrate of NOS enzymes and arginases. Reduced eNOS expression (38) or genetic depletion of eNOS (47) exacerbates DN (38, 47), indicating the critical role of eNOS in DN. Interestingly, arginase inhibition prevents DN via an eNOS-dependent mechanism (42); thus, restoring NO could be an important therapeutic tool in the prevention and treatment of DN.
Although a significant role for eNOS in DN is well-established, we appreciate that neuronal (n)NOS or inducible (i)NOS may also play a role in DN. In fact, expression of nNOS, which is expressed especially in the collecting duct and macula densa (17, 24), was reduced in several models of progressive chronic kidney disease, including DN (5, 20, 40). On the other hand, some investigators have reported increased nNOS expression in DN (12, 31). Although the current study did not evaluate the impact of diabetes or arginase inhibition on renal nNOS or iNOS expression, we showed previously that diabetes resulted in increased renal expression of iNOS but not of nNOS (43). It is important to note that whether the expression of NOS isoforms changes in a consistent pattern in DN remains a matter of controversy, with various studies demonstrating an increase, no change, or a decrease in their expression in diabetic rats (for a review, see Ref. 21). The variable results likely reflect differences in animal species or strains, methods of inducing diabetes, the duration of diabetes (early or late), and the methods used to evaluate NOS expression.
In addition to NOS restoration, inhibition of arginase could reduce arginase-dependent production of some downstream metabolite(s), such as polyamines (26). Increased plasma levels of polyamines have been shown in chronic kidney disease such as DN (18). In our study, arginase inhibition resulted in less mesangial expansion and glomerular hypercellularity in diabetes, indicating a possible contribution of arginases to the initiation and/or progression of diabetic renal fibrosis. Additionally, inhibition of arginase reduced kidney macrophage infiltration and restored nephrin expression. Macrophages play a pivotal role in inducing renal injuries through potent cytokines such as monocyte chemoattractant protein-1 (15, 33, 37), TNF-α (34), and IL-1 (35), while nephrin expression is a very important protein in the pathophysiology of proteinuria (45).
The present study addressed a clinically relevant question: does arginase inhibition prevent late stage of established DN? The current study not only confirms our previous results that early treatment with arginase inhibition prevents the development of DN (28) but, importantly, extends these observations by demonstrating that arginase inhibition is also effective in treating the progression of established DN.
A second issue addressed in the current study was the efficacy of arginase inhibition compared with treatment with captopril (an ACEi), which is the gold standard for treating diabetic patients. Our data show that early treatment with captopril was almost as effective as early treatment with arginase inhibition in reducing albuminuria, kidney macrophage infiltration, and histopathological changes along with reduced urinary oxidative stress. However, the effect of early treatment with captopril in reducing albuminuria was immediate (within 6 wk of treatment and was maintained thereafter) while the effect of early treatment with arginase inhibition in reducing albuminuria was delayed. Early treatment with captopril, however, lacked any effect on nephrin expression or kidney nitrate + nitrite levels, eNOS phosphorylation, or renal blood flow. Thus, the effect of early treatment with captopril to ameliorate DN is eNOS-independent. This conclusion is supported by reports that captopril remarkably reduced albuminuria in low-dose streptozotocin in eNOS-deficient mice (44) and remarkably reduced glomerular and tubulointerstitial injury in db/db eNOS-deficient mice (46). Interestingly, late treatment with captopril lacked any effect to ameliorate diabetic renal injury. Additional studies to investigate the efficacy of combined BEC and captopril treatments will be needed in the future.
Data from urinary TBARS were not surprising. Early or late treatment with captopril reduced the elevated urinary TBARS associated with DN despite the lack of their effect on NO. A previous report concluded that sulfhydryl group containing ACEi such as captopril may exhibit antioxidant properties (4). The fact that late treatment of captopril failed to protect the kidney in DN despite its effect to reduce urinary TBARS indicates that oxidative stress is a possible consequence rather than a cause of diabetic injury. Captopril was also reported to have anti-inflammatory activity (25). Indeed, early treatment with captopril reduced glomerular macrophage infiltration to a similar extent as early treatment with BEC.
In the current study, we administered the arginase inhibitor BEC systemically via an osmotic mini-pump. It is appropriate to consider whether systemic administration of arginase inhibitors may adversely affect the hepatic urea cycle. Currently available arginase inhibitors are competitive and are not isozyme-specific. As the liver contains a very high amount of arginase 1, enormous amounts of an inhibitor would be required to inhibit liver arginase sufficiently for any inhibition of the urea cycle to become apparent. More importantly, however, the reactions of the urea cycle are not diffusion-controlled but are tightly coupled, such that arginine generated within the urea cycle and used by arginase does not exchange with the free arginine pool within the cell (11). Consequently, a competitive arginase inhibitor will not exchange with arginine generated within the urea cycle and thus should have little or no effect on the urea cycle. It should be noted that competitive arginase inhibitors have been used in many animal studies, and hyperammonemia or other adverse effects have not been reported in any of them. Even mild hyperammonemia would become apparent via altered behavior, but no such effects have been noted.
In conclusion, our study demonstrates that treatment with an arginase inhibitor not only prevents the development but also the progression of established DN. Our data therefore are clinically relevant since identification of targets that retard the progression of established DN is lacking. Results of this study may ultimately lead to novel therapeutic interventions using arginase inhibition in the treatment of diabetic kidney disease.
GRANTS
This study was supported by National Institutes of Health Grants DK094930 and DK094930S1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.Y., T.G., and T.K.C. performed experiments; H.Y., T.G., T.K.C., and A.S.A. analyzed data; H.Y. and A.S.A. interpreted results of experiments; H.Y., T.K.C., and A.S.A. prepared figures; H.Y. and A.S.A. drafted manuscript; H.Y., T.G., T.K.C., S.M.M., and A.S.A. approved final version of manuscript; T.K.C., S.M.M., and A.S.A. edited and revised manuscript; S.M.M. and A.S.A. conception and design of research.
REFERENCES
- 1.Abdel-Rahman EM, Saadulla L, Reeves WB, Awad AS. Therapeutic modalities in diabetic nephropathy: standard and emerging approaches. J Gen Intern Med 27: 458–468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awad AS, Kinsey GR, Khutsishvili K, Gao T, Bolton WK, Okusa MD. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am J Physiol Renal Physiol 301: F1358–F1366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balakumar P, Chakkarwar VA, Krishan P, Singh M. Vascular endothelial dysfunction: a tug of war in diabetic nephropathy? Biomed Pharmacother 63: 171–179, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bartosz M, Kedziora J, Bartosz G. Antioxidant and prooxidant properties of captopril and enalapril. Free Radic Biol Med 23: 729–735, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 294: F1–F9, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Breyer MD, Bottinger E, Brosius FC 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Brosius FC 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T. Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H, Harris RC. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets 14: 22–33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng H, Wang H, Fan X, Paueksakon P, Harris RC. Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int 82: 1176–1183, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung CW, Cohen NS, Raijman L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J Biol Chem 264: 4038–4044, 1989. [PubMed] [Google Scholar]
- 12.Choi KC, Kim NH, An MR, Kang DG, Kim SW, Lee J. Alterations of intrarenal renin-angiotensin and nitric oxide systems in streptozotocin-induced diabetic rats. Kidney Int Suppl 60: S23–S27, 1997. [PubMed] [Google Scholar]
- 13.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Ding S, Guo H, Kats A, Lamb K, Li S, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis 61: A7, e1–476, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 93: 137–188, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Giunti S, Barutta F, Perin PC, Gruden G. Targeting the MCP-1/CCR2 system in diabetic kidney disease. Curr Vasc Pharmacol 8: 849–860, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Gotoh T, Sonoki T, Nagasaki A, Terada K, Takiguchi M, Mori M. Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett 395: 119–122, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62: 91–98, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi K, Ueda S, Yoshida K, Kashiwagi K. Polyamines in renal failure. Amino Acids 31: 477–483, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T. Deficiency of endothelial nitric oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol 170: 1473–1484, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keynan S, Hirshberg B, Levin-Iaina N, Wexler ID, Dahan R, Reinhartz E, Ovadia H, Wollman Y, Chernihovskey T, Iaina A, Raz I. Renal nitric oxide production during the early phase of experimental diabetes mellitus. Kidney Int 58: 740–747, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol 284: F1121–F1137, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic Biol Med 31: 331–335, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Lovell HG. Angiotensin converting enzyme inhibitors in normotensive diabetic patients with microalbuminuria. Cochrane Database Syst Rev 1: CD002183, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD Jr, Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol 298: F1465–F1471, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin MF, Surrall KE, McKenna F, Dixon JS, Bird HA, Wright V. Captopril: a new treatment for rheumatoid arthritis? Lancet 1: 1325–1328, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr 137: 1602S–1609S, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Morris SM Jr, Bhamidipati D, Kepka-Lenhart D. Human type II arginase: sequence analysis and tissue-specific expression. Gene 193: 157–161, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Morris SM Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes 60: 3015–3022, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol 18: 539–550, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, Li Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol 7: 36–44, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin SJ, Lai FJ, Wen JD, Hsiao PJ, Hsieh MC, Tzeng TF, Chen HC, Guh JY, Tsai JH. Neuronal and endothelial nitric oxide synthase expression in outer medulla of streptozotocin-induced diabetic rat kidney. Diabetologia 43: 649–659, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Harris RC. Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. J Diabetes Res 2014: 590541, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang WW, Qi M, Warren JS. Monocyte chemoattractant protein 1 mediates glomerular macrophage infiltration in anti-GBM Ab GN. Kidney Int 50: 665–671, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Tipping PG, Leong TW, Holdsworth SR. Tumor necrosis factor production by glomerular macrophages in anti-glomerular basement membrane glomerulonephritis in rabbits. Lab Invest 65: 272–279, 1991. [PubMed] [Google Scholar]
- 35.Tipping PG, Lowe MG, Holdsworth SR. Glomerular interleukin 1 production is dependent on macrophage infiltration in anti-GBM glomerulonephritis. Kidney Int 39: 103–110, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD. Cloning and characterization of the human type II arginase gene. Genomics 38: 118–123, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda SI, Takasawa K, Yoshimura M, Kida H, Kobayashi KI, Mukaida N, Naito T, Matsushima K, Yokoyama H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58: 1492–1499, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Wang CH, Li F, Hiller S, Kim HS, Maeda N, Smithies O, Takahashi N. A modest decrease in endothelial NOS in mice comparable to that associated with human NOS3 variants exacerbates diabetic nephropathy. Proc Natl Acad Sci USA 108: 2070–2075, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yagihashi N, Nishida N, Seo HG, Taniguchi N, Yagihashi S. Expression of nitric oxide synthase in macula densa in streptozotocin diabetic rats. Diabetologia 39: 793–799, 1996. [DOI] [PubMed] [Google Scholar]
- 41.You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. Am J Physiol Renal Physiol 305: F1719–F1727, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You H, Gao T, Cooper TK, Morris SM Jr, Awad AS. Arginase inhibition mediates renal tissue protection in diabetic nephropathy by a nitric oxide synthase 3-dependent mechanism. Kidney Int 84: 1189–1197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You H, Gao T, Cooper TK, Morris SM Jr, Awad AS. Diabetic nephropathy is resistant to oral l-arginine or l-citrulline supplementation. Am J Physiol Renal Physiol 307: F1292–F1301, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuen DA, Stead BE, Zhang Y, White KE, Kabir MG, Thai K, Advani SL, Connelly KA, Takano T, Zhu L, Cox AJ, Kelly DJ, Gibson IW, Takahashi T, Harris RC, Advani A. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol 23: 1810–1823, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012: 314251, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang MZ, Wang S, Yang S, Yang H, Fan X, Takahashi T, Harris RC. Role of blood pressure and the renin-angiotensin system in development of diabetic nephropathy (DN) in eNOS−/− db/db mice. Am J Physiol Renal Physiol 302: F433–F438, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]