Abstract
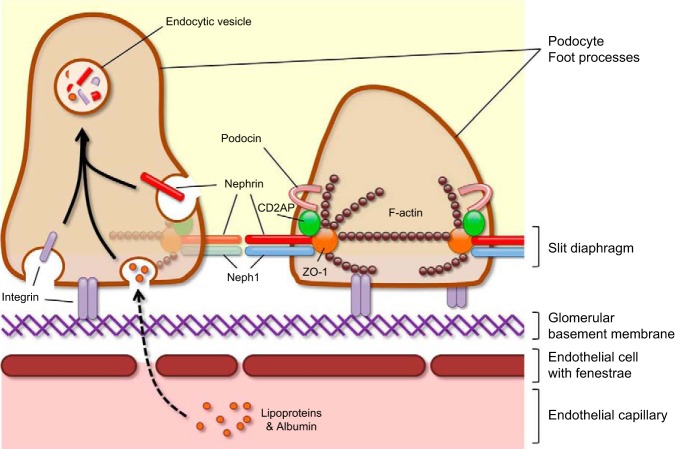
Severe defects in the glomerular filtration barrier result in nephrotic syndrome, which is characterized by massive proteinuria. The podocyte, a specialized epithelial cell with interdigitating foot processes separated by a slit diaphragm, plays a vital role in regulating the passage of proteins from the capillary lumen to Bowman's space. Recent findings suggest a critical role for endocytosis in podocyte biology as highlighted by genetic mouse models of disease and human genetic mutations that result in the loss of the integrity of the glomerular filtration barrier. In vitro podocyte studies have also unraveled a plethora of constituents that are differentially internalized to maintain homeostasis. These observations provide a framework and impetus for understanding the precise regulation of podocyte endocytic machinery in both health and disease.
Keywords: glomerular disease, podocyte, endocytosis, phosphoinositides
the kidney is composed of many different segments that work in unison to filter plasma, generating urine essentially devoid of large-molecular weight proteins and regulating electrolyte balance through tubular secretion and reabsorption. This filtration process begins at the glomerulus, which is composed of podocytes, the glomerular basement membrane, and fenestrated endothelial cells, which collectively compose the plasma-nephron interface. Amino acids, glucose, and electrolytes, which filter through the glomerulus, can be reabsorbed in the proximal tubule by sodium dependent cotransporters. Proximal tubular endocytic machinery located at the brush-border membrane and mediated by receptors gp330/megalin and cubilin can also retrieve albumin and low-molecular weight proteins (10, 18, 19, 111, 116). However, breaches to any part of the filtration barrier can result in nephrotic syndrome as massive proteinuria overwhelms the capacity of the proximal tubule to reabsorb filtered protein. Loss of either megalin or cubilin in a mouse disease model results in increased excretion of albumin and low-molecular weight proteins, suggesting that an intact proximal tubular endocytic process is required to maintain homeostasis (2, 58). These two receptors are also believed to be important in human diseases, as patients with megalin (LRP2; Donnai-Barrow and facio-oculo-acoustico-renal syndromes) and cubilin (CUBN) gene mutations have increased low-molecular weight proteinuria (26, 49, 76).
The hypothesis that podocytes internalize and remove proteins was proposed over 50 years ago. Multiple electron microscopy studies have shown vesicles in podocytes which become more pronounced under pathological conditions (29). This fundamental process appears to be conserved in podocytes. However, unlike the proximal tubules, the physiological relevance of the endocytic uptake mechanisms in podocytes has until recently remained elusive. In this review, we will focus on the current understandings of endocytosis in podocyte biology and explain their significance.
Endocytosis
Endocytosis serves as a portal for internalizing and retrieving plasma membrane components and transmembrane receptors by forming vesicles (93, 98). Endocytosis can be divided into clathrin-dependent and -independent pathways. Interestingly, proteins can often exploit either pathway to allow for internalization. Clathrin-mediated endocytosis is characterized by a membrane invagination, which is lined with a triskelion lattice coat. Clathrin-mediated endocytosis occurs in phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2]-enriched regions of the plasma membrane, and these regions recruit clathrin adaptor proteins such as adaptor protein 2 (AP2). The pit further develops through the recruitment of Bin–Amphiphysin–Rvs (BAR)-containing proteins such as endophilin (34, 61). BAR domain-containing proteins dimerize, allowing for their positively charged amino acids to interact with the negatively charged lipid membrane. These dimers impose their innate crescent-shaped curvature on the membrane, thus functioning as membrane-curving or -sensing proteins (33, 34, 64, 70, 79).
Endocytic Machinery in Podocytes
Endophilin also contains a SH3 domain that binds dynamin, which subsequently oligmerizes to constrict the neck, resulting in fission through GTP hydrolysis (60). Following dynamin-induced fission, clathrin uncoating and shedding is initiated by inositol polyphosphate-5-phosphatases such as synaptojanin 1 (22) and then finalized by the arrival of auxillin/GAK and hsc70 (39, 65). Internalization signals appear to exist for proteins that undergo clathrin-mediated endocytosis. Although there appear to be various endocytic signals, two well-described processes are proteins that contain either a dileucine- or tyrosine-based linear motif that are recognized by AP2, thus coupling the cargo to clathrin (110).
Distinct clathrin-independent endocytosis also plays a critical role in cell trafficking, which can be divided by ultrastructural size by electron microscopy. Large-sized (micrometer) pathways include macropinocytosis and phagocytosis. For example, macropinocytosis is a method of internalization where substances in the extracellular fluid are engulfed and then compartmentalized in vesicles. Small-scale processes (<200 nm) include caveolae, Arf6, flotillin-1, and clathrin-independent carrier (CLIC)/GPI-AP-enriched early endosomal compartment (GEEC)-mediated pathways. Moreover, it has been shown that proteins such as dynamin and endophilin, which are critical for clathrin-mediated endocytosis, also are utilized in clathrin-independent pathways (11, 86). One of the most well-studied clathrin-independent pathway is the caveolar system. Caveolae are cholesterol-abundant invaginations that participate in the uptake of sphingolipids or proteins found in lipid rafts. The major constituent of caveolae is caveolin 1 (Cav1), which oligomerizes to form caveolae (71) before dynamin-dependent fission, which completes its internalization (62). In the GEEC pathway, GPI-anchored protein is internalized in tubular elements. This pathway does not rely on dynamin activity and appears to be responsible for fluid phase uptake (90). Another dynamin-independent pathway is flotillin-1, an integral membrane protein that couples with lipid rafts to select lipid cargo (37). Last, Arf6 is localized at the interface of the plasma membrane and endosomal structure. The cargo proteins of the Arf6-dependent pathway, such as the IL-2 receptor α-subunit, E-cadherin, and β1-integrins, have been identified (14, 77, 85).
Phosphoinositides in Podocytes
Membrane and protein trafficking are both highly coordinated biological processes required for cellular homeostasis. Utilization of endocytic pathways results in the internalization of molecules from the plasma membrane that can be recycled back to the cell surface or degraded in lysosomal compartments. Inositol phospholipids, concentrated on the cytosolic leaflet of membranes, play a central role in orchestrating the fidelity of these systems (24). Reversible phosphorylation of the inositol ring at positions 3, 4, and 5 generates seven distinct phosphoinositide (PI) species, while a host of intracellular kinases and phosphatases govern the discrete localization of these phospholipids in the cell. PIs mediate the recruitment of proteins to membranes using their phosphorylated headgroups while also serving as precursors to secondary messengers. The precision of PI metabolism is a critical feature of vectoral membrane and protein trafficking. For instance, clathrin-mediated endocytosis relies heavily on PI metabolism as PI(4,5)P2 serves to recruit endocytic clathrin adaptor molecules in the early phase of coated-pit formation. Several human diseases are caused by mutations in genes intimately linked to PI metabolism and clathrin-mediated endocytosis (63). Recently, the interrogation of PI metabolism in podocytes revealed that genetic ablation of Synj1, a member of the inositol 5-phosphatase family which hydrolyzes the PI(4,5)P2 on clathrin-coated vesicles (22, 67), results in severe proteinuria, foot process effacement, and a concomitant increase in PI(4,5)P2 levels within the kidney (102). The loss of endophilin, a protein which recruits synaptojanin 1, displays a similar phenotype (102). Despite the presence of several other PI(4,5)P2 phosphatases in the mammalian genome, it is unclear why defects in Synj1 have such a profound effect on the glomerular filtration barrier. For example, mutations in the inositol 5-phosphatase OCRL cause Lowe syndrome, an X-linked disorder characterized by mental retardation, congenital cataracts, and proximal tubulopathy (5, 101), while mutations of inositol 5-phosphatase INPP5E results in Joubert syndrome, where patients develop cystic kidneys (9). However, both diseases fail to affect the glomerulus. Further evidence suggests the importance of PI metabolism in podocyte function. Loss of podocyte class III phosphatidylinositol (PI) 3-kinase [mammalian homolog of yeast vacuolar protein sorting defective 34 (mVps34)], which phosphorylates phosphatidylinositol to generate PI(3)P, results in severe glomerulosclerosis (16). Loss of mVps34 also demonstrates trafficking defects for intracellular vesicles. In particular, a block between early endosomal Rab5 and late endosomal Rab7 compartments is observed after podocyte mVps34 deletion (7). Furthermore, genetic ablation of class II PI 3-kinase C2 α (Pi3kc2a), which contains a clathrin binding domain and generates PI(3)P as well as PI(3,4)P2, results in severe proteinuria and kidney failure (41). These findings suggest that regulation of phosphoinositides is pivotal in maintaining a healthy glomerular filtration barrier.
Relationship of Podocyte Clathrin-Mediated Endocytosis With the Actin Cytoskeleton
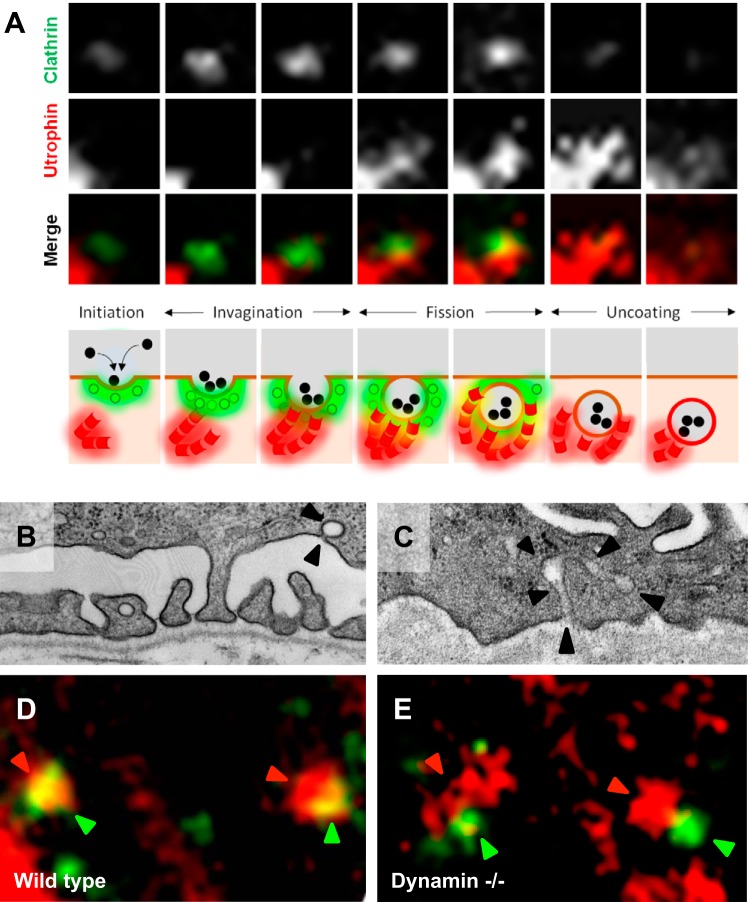
Human genetics research informs our understanding of the actin cytoskeleton's fundamental role in podocyte biology. Mutations causal for focal segmental glomerulosclerosis have been identified in actin-interacting proteins such as α-actinin-4 (ACTN4), CD2AP, INF2, and MYO1E (13, 50, 52, 68, 95). Recent evidence suggests that endocytic protein networks sit at the interface of various actin-regulatory proteins (105). For example, dynamin, a GTPase that mediates the fission reaction during clathrin-mediated endocytosis, has been shown to interact with actin-nucleating protein Arp2/3 (57) and cortactin (66, 69). In vitro, it has been demonstrated that actin nucleation is either stimulated or inhibited depending on the concentration of dynamin (96). Dynamin has also been shown to interact directly with F-actin and has been implicated in the organization of the podocyte actin cytoskeleton (40). It has been postulated that actin may play a critical role in inducing local tension in the membrane, which is necessary for fission to occur. In vivo, mice injected with either a dominant negative dynamin 1 K44A plasmid or dynamin undergoing proteolytic cleavage by cathepsins demonstrate proteinuria (99). Furthermore, podocyte-specific Dnm1 and Dnm2 conditional knockout (KO; Pod-Dnm-DKO) mice exhibit severe proteinuria and foot process effacement (102). Live cell imaging of wild-type podocytes display actin transiently visiting the neck of clathrin-coated pits before the fission reaction (Fig. 1, A and D). The wild-type podocytes demonstrate an Ω-shaped clathrin-coated pit (Fig. 1B). However, podocytes lacking dynamin manifest with extreme accumulation of Arp2/3 and F-actin at clathrin-coated pits (Fig. 1E) (102), resulting in a tubulated morphology similar to that observed in Dnm DKO fibroblasts (31) (Fig. 1C). These observations suggest that dynamin in wild-type podocytes serves as a brake, which prevents continued actin nucleation at the clathrin-coated pit. PI(4,5)P2 has been shown to facilitate actin nucleation. 5′-Phosphatases, such as synaptojanin 1, can reduce actin assembly due to hydrolysis of PI(4,5)P2 bound to actin-regulatory proteins (94). Examination of Synj1 KO podocytes revealed increased ectopic Arp2/3 accumulation, suggesting aberrant actin nucleation. It would be of great interest to determine whether Synj1 KO podocytes have actin comets within the cytoplasm due to inability of clathrin uncoating, similar to what has been observed in fibroblasts isolated from Lowe syndrome patients that lack functional OCRL (65).
Fig. 1.
Link between actin and endocytosis in podocytes. A: spinning disc confocal images of selected frames 4 s apart from time series of a clathrin-coated pit (GFP-CLC) with merged images depicting late actin (mCherry-Utrophin) arrival before membrane fission and the disappearance of the clathrin-coated pit. Cartoon shows the temporal and spatial relationship of actin (red) and the clathrin-coated pit (green; data from experiments conducted in Ref. 102). B: electron microscope image of wild-type podocyte reveals an Ω-shaped clathrin-coated pit (arrowheads). C: electron microscope image of Pod-Dnm-DKO podocyte (data from experiments conducted in Ref. 102) reveals a tubulated clathrin-coated pit (arrowheads). D: wild-type podocytes demonstrate actin (red) and clathrin (green) merging (arrowheads). E: actin (red arrowheads) accumulates near sites of clathrin (green arrowheads) in Pod-Dnm-DKO podocytes.
Proteins previously proven central to the integrity of foot processes, such as Myo1e, CD2AP, and Nck, are also dynamin and synaptojanin 1 interactors (12, 30, 56). Myo1e, an actin-based motor protein, participates in endocytosis through recruitment to the late stages of clathrin-coated pits along with dynamin. Myo1e appears to move in a vectoral manner, driving the clathrin-coated pit inward (17). Loss of Myo1e in cells also reduces clathrin-mediated transferrin endocytosis, reflecting its integral role in the endocytic machinery. Myo1e also colocalizes with F-actin at endocytic pits, suggesting its importance in coalescing actin assembly at sites of endocytosis (102, 105). CD2AP is a close homolog of CIN85, a class of proteins thought to have overlapping cellular functions but partially different tissue distribution. This explains the occurrence of nephrotic syndrome in CD2AP mutant mice and humans, but not in CIN85 mutant mice (100). Some functions of CD2AP/CIN85 are mediated, at least in part, by its direct and indirect interactions with dynamin, synaptojanin 1, and endophilin (12, 80, 103). CD2AP has been shown to visit clathrin-coated pits and late endosomes (114) while also colocalizing with cortactin (117). Nck adaptor protein interacts with the proline rich domain of dynamin and synaptojanin 1 through its SH3 domain (114, 115). Nck also binds to actin-polymerizing protein N-WASP to induce actin tails (43). Given that the loss of key clathrin-mediated regulatory proteins or their interactors results in aberrant actin dynamics, a coordinated role between regulation of actin and endocytosis likely exists in podocytes. Furthermore, targeting the endocytic-actin interface with small molecule Bis-T 23 to promotes dynamin oligmerization and actin stabilization may have potential therapeutic implications (97).
Endocytic Process in Slit Diaphragm Regulation
The slit diaphragm is a modified tight junction that links adjacent podocyte foot processes and serves as a terminal barrier for the retention of circulating macromolecules as blood is filtered in the glomerulus (38, 74). These structures have been identified through the use of electron microscopy, and their biological importance was established through the discovery of the NPHS1 gene. The NPHS1 gene encodes the protein nephrin, a transmembrane protein that belongs to the immunoglobulin superfamily of cell adhesion molecules. Using positional cloning, it was discovered that mutations in NPHS1 result in congenital nephrotic syndrome of the Finnish type, wherein newborns present with massive proteinuria (51, 89). This finding spurred the investigation of slit diaphragm biology. Recent immunogold-tracing electron microscopy and time-lapse fluorescent microscopy experiments suggest that endocytic mechanisms regulate nephrin (Fig. 2, A–D). Following tyrosine phosphorylation by Src family kinases Fyn and Yes, and the binding of Nck protein and the p85 subunit of phosphoinositide 3-OH kinase, nephrin can serve as a signaling platform (42, 47, 73). Nephrin phosphorylation at tyrosine residue 1193 augments podocin interaction, while dephosphorylation at this site induces β-arrestin interaction followed by nephrin internalization (83). Another regulatory mechanism for nephrin endocytosis involves PKC-α (84). Increased PKC-α activity has been demonstrated in mouse models of diabetic nephropathy, where phosphorylation of threonine residues 1120 and 1125 augments β-arrestin interaction and internalization (109). Either treatment with a PKC-α inhibitor or genetic ablation of Prkca results in nephrin retention at the membrane (84). Moreover, induction of the planar cell polarity pathway (PCP) also induces nephrin endocytosis in a β-arrestin-dependent manner (6). Podocyte-specific deletion of Vangl2, a PCP protein, results in reduction of nephrin endocytosis and abnormal glomerulogenesis. These mutant mice also have increased susceptibility to anti-glomerular basement membrane-induced injury (88). Nephrin turnover also involves CIN85/RunkL-mediated ubiquitination in podocytes lacking CD2AP, and it has been postulated that CD2AP can downregulate CIN85 expression due to SUMOylation at lysine 598 (108). Currently, it is unclear whether nephrin uptake occurs via clathrin-mediated or non-clathrin-mediated pathways. One body of evidence suggests that both are involved, as phosphorylated nephrin appears to undergo raft-mediated endocytosis while the dephosphorylated nephrin results in clathrin-mediated endocytosis (82). However, nephrin internalization involving the dynamin-dependent Notch pathway appears to require clathrin, as cholesterol depletion does not induce nephrin internalization (112). Although nephrin retention at the surface appears critical, evidence suggests that reduction of nephrin internalization is also detrimental to the podocyte (102), as slit diaphragm proteins likely require recycling or turnover to contend with the daily rigors of plasma filtration.
Fig. 2.
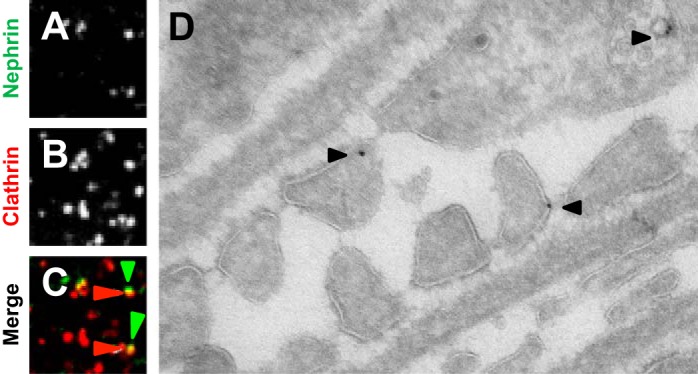
Nephrin visits clathrin-coated pit and endosome. A–C: confocal microscopy images of podocytes with red fluorescence protein (RFP)-clathrin (red arrowheads) and Alexa 488-CD8 nephrin (green arrowheads) merging. D: immunogold labeling of nephrin demonstrates localization at the slit diaphragm and endosome (data from experiments conducted in Ref. 102).
Another slit diaphragm component that appears to be regulated by endocytic proteins is zonula occludens (ZO)-1. The critical importance of ZO-1 in podocyte biology was recently discovered, as mice with Tjp-1 ablated specifically in podocytes developed severe proteinuria and foot process effacement (44). Furthermore, it has been reported that ZO-1 is mislocalized from tight junctions following puromycin-induced podocyte injury (87). Actin-based myosin motor Myo1e not only visits clathrin-coated pits but also interacts with ZO-1 (8). Further validating this point, non-muscle myosin 1c also interacts with PI(4,5)P2 and with slit diaphragm protein Neph1 and nephrin, presumably to maintain their proper localization at the membrane (4). Loss of myo1c in zebrafish results in an abnormal glomerular filtration barrier (3). Thus it is likely that the uptake of endocytic proteins plays a fundamental role in quality control of the slit diaphragm. It is unclear where slit diaphragm proteins are destined following endocytosis, as the cargo can be transferred from early to late endosomes followed by lysosomal degradation, the trans-Golgi network, and to recycling endosomes. This complex process is orchestrated and marked by numerous Rab-GTPases. For example, Rab1 and Rab2 are localized at endoplasmic reticulum (ER) exit sites and the pre-Golgi intermediate compartment, affecting ER-Golgi trafficking. In addition, Rab6, Rab33, and Rab40 are localized in the Golgi to mediate intra-Golgi trafficking, while Rab22 functions to traffic cargo between the trans-Golgi network and early endosomes. Conversely, Rab5 is localized at early endosomes, phagosomes, caveosomes, and the plasma membrane mediating early endosome fusion of clathrin-coated vesicles, allowing for Rab11-dependent recycling and/or Rab7-dependent late endosomal trafficking (104). We have recently found that nephrin visits Rab family proteins Rab11 (recycling endosomes) and Rab7 (late endosomes), indicating that pools of nephrin are either recycled or degraded (Inoue K and Ishibe S, unpublished observations).
Lipoproteins and Albumin Endocytosis
Plasma membrane and soluble receptors bind to signaling molecules outside the cell and subsequently activate signal transduction pathways. When a ligand binds to a receptor, this can often induce endocytosis of the receptor-ligand complex. Recent evidence suggests that sFLT-1, a soluble VEGF receptor-1 localized at lipid rafts, is internalized and then found in compact punctae that colocalize with endosomal proteins, flotillin, and Rab5 (46). This process appears to be dynamin dependent, as pharmacological inhibition with dynasore abrogates this process. It was discovered that sFLT-1 associates with sphingolipids at the lipid raft domains, inducing endocytosis. Podocyte-specific deletion of sFLT-1 results in severe proteinuria and foot process effacement, suggesting sFLT-1′s role in the maintenance of the glomerular filtration barrier. The recent discovery of clathrin-dependent internalization of apolipoprotein L1 also underscores the importance of this fundamental process (59). Patients of African ancestry who have mutations in the gene APOL1 have higher risks of various forms of glomerular diseases such as focal segmental glomerulosclerosis and human immunodeficiency virus-associated nephropathy (32, 35, 36). However, it is unclear whether the uptake of apolipoprotein L1 is differentially regulated to induce podocyte injury in affected patients who carry the APOL1 risk alleles.
It is proposed that podocytes are able to internalize and eliminate proteins filtered throughout the glomerular filtration barrier. Immunogold ultrastructural studies have shown that albumin is taken up by rat podocytes following puromycin aminonucleoside administration (53). In addition, albumin endocytosis through caveolae has been observed in cultured podocytes, which are degraded by lysosomes (15, 25). Furthermore, new evidence also suggests that free fatty acid bound to albumin undergoes endocytosis in podocytes (78) and directly elicits injury (1, 75). During nephrotic syndrome in vivo, the fatty acid-bound albumin may augment macropinocytosis due to the free fatty acid, G protein-coupled receptor's Gβ/Gγ subunit (20), which may lead to podocyte dysfunction directly or indirectly through activation of angiopoietin-like 4, a protein secreted by podocytes that induces proteinuria (21).
Integrins and Transmembrane Receptor Endocytosis
Integrins are transmembrane receptors that organize interaction between the cell matrix and extracellular matrix (ECM). There are 24 distinct integrins, each consisting of 1 of 18 α-subunits and 1 of 8 β-subunits (72). The principal integrin that mediates a podocyte's adhesion to the glomerular basement membrane is the laminin binding integrin αβ (55, 92). Podocyte-specific deletion of Itgb1 or Itga3 in mice induces severe proteinuria and foot process effacement (81, 91). Furthermore, deletion of integrin interactors' tetraspanin CD151 (Cd151), talin1 (Tln1), integrin-linked kinase (Ilk), and α3β1 ligand β-laminin (Lamb2) results in severe proteinuria (23, 27, 45, 48, 91, 107). Current evidence suggests that clathrin-mediated endocytosis of activated β1-integrins and dynamin involvement with focal adhesion kinase may facilitate focal adhesion disassembly (28, 106). This process may induce cell movement, akin to foot processes undergoing effacement. Activation of the urokinase plasminogen activator receptor (uPAR) has been linked to the pathogenesis of focal segmental glomerulosclerosis (113). A recent study found that podocyte injury occurs through plasminogen activator inhibitor type 1 (PAI-1) mediated endocytosis of both β1-integrin and the uPAR (54). These findings indicate the potential importance of understanding the connection between integrin proteins, focal adhesion turnover, and endocytosis.
Concluding Remarks
The endocytic process in podocytes plays a fundamental role in the development and maintenance of the glomerular filtration barrier. The loss of key molecular machinery results in defective actin regulation and faulty slit diaphragm maintenance, while increases in the uptake of lipoproteins or integrins may have deleterious effects on podocyte health (Fig. 3). These current findings provide important insight into podocyte biology and motivate further investigation to garner evidence to understand the complexities displayed by endocytic regulation of diverse cellular processes.
Fig. 3.
Endocytosis in the podocyte model proposing the roles of the podocyte endocytic machinery regulating intricate cellular processes to maintain the glomerular filtration barrier.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK083294 and DK093629.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.I. and S.I. prepared figures; K.I. and S.I. drafted manuscript; K.I. and S.I. edited and revised manuscript; K.I. and S.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Arnaud Marlier for assistance with graphic art.
REFERENCES
- 1.Agrawal S, Guess AJ, Chanley MA, Smoyer WE. Albumin-induced podocyte injury and protection are associated with regulation of COX-2. Kidney Int 86: 1150–1160, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arif E, Kumari B, Wagner MC, Zhou W, Holzman LB, Nihalani D. Myo1c is an unconventional myosin required for zebrafish glomerular development. Kidney Int 84: 1154–1165, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arif E, Wagner MC, Johnstone DB, Wong HN, George B, Pruthi PA, Lazzara MJ, Nihalani D. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol Cell Biol 31: 2134–2150, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358: 239–242, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Babayeva S, Rocque B, Aoudjit L, Zilber Y, Li J, Baldwin C, Kawachi H, Takano T, Torban E. Planar cell polarity pathway regulates nephrin endocytosis in developing podocytes. J Biol Chem 288: 24035–24048, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechtel W, Helmstadter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, Schell C, Kretz O, Liu S, Geist F, Kerjaschki D, Walz G, Huber TB. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol 24: 727–743, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi J, Chase SE, Pellenz CD, Kurihara H, Fanning AS, Krendel M. Myosin 1e is a component of the glomerular slit diaphragm complex that regulates actin reorganization during cell-cell contact formation in podocytes. Am J Physiol Renal Physiol 305: F532–F544, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41: 1032–1036, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow TE, Moestrup SK, Christensen EI. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 105: 1353–1361, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucrot E, Ferreira AP, Almeida-Souza L, Debard S, Vallis Y, Howard G, Bertot L, Sauvonnet N, McMahon HT. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 517: 460–465, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Brett TJ, Traub LM, Fremont DH. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure 10: 797–809, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol 154: 1007–1017, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson JM, Okamura K, Wakashin H, McFann K, Dobrinskikh E, Kopp JB, Blaine J. Podocytes degrade endocytosed albumin primarily in lysosomes. PLoS One 9: e99771, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Chen MX, Fogo AB, Harris RC, Chen JK. mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol 24: 196–207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng J, Grassart A, Drubin DG. Myosin 1E coordinates actin assembly and cargo trafficking during clathrin-mediated endocytosis. Mol Biol Cell 23: 2891–2904, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 280: F562–F573, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Christensen EI, Nielsen S, Moestrup SK, Borre C, Maunsbach AB, de Heer E, Ronco P, Hammond TG, Verroust P. Segmental distribution of the endocytosis receptor gp330 in renal proximal tubules. Eur J Cell Biol 66: 349–364, 1995. [PubMed] [Google Scholar]
- 20.Chung JJ, Huber TB, Godel M, Jarad G, Hartleben B, Kwoh C, Keil A, Karpitskiy A, Hu J, Huh CJ, Cella M, Gross RW, Miner JH, Shaw AS. Albumin-associated free fatty acids induce macropinocytosis in podocytes. J Clin Invest 125: 2307–2316, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clement LC, Avila-Casado C, Mace C, Soria E, Bakker WW, Kersten S, Chugh SS. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17: 117–122, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99: 179–188, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17: 2164–2175, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Dobrinskikh E, Okamura K, Kopp JB, Doctor RB, Blaine J. Human podocytes perform polarized, caveolae-dependent albumin endocytosis. Am J Physiol Renal Physiol 306: F941–F951, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnai D, Barrow M. Diaphragmatic hernia, exomphalos, absent corpus callosum, hypertelorism, myopia, and sensorineural deafness: a newly recognized autosomal recessive disorder? Am J Med Genet 47: 679–682, 1993. [DOI] [PubMed] [Google Scholar]
- 27.El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol 17: 1334–1344, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 7: 581–590, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Farquhar MG, Vernier RL, Good RA. An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. J Exp Med 106: 649–660, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fawcett JP, Georgiou J, Ruston J, Bladt F, Sherman A, Warner N, Saab BJ, Scott R, Roder JC, Pawson T. Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc Natl Acad Sci USA 104: 20973–20978, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, O'Toole E, De Camilli P. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17: 811–822, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 21: 1422–1426, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell 137: 191–196, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J 25: 2898–2910, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, Needham AW, Lazarus R, Pollak MR. A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 78: 698–704, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol 8: 46–54, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm–from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Greener T, Zhao X, Nojima H, Eisenberg E, Greene LE. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J Biol Chem 275: 1365–1370, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Gu C, Yaddanapudi S, Weins A, Osborn T, Reiser J, Pollak M, Hartwig J, Sever S. Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J 29: 3593–3606, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris DP, Vogel P, Wims M, Moberg K, Humphries J, Jhaver KG, DaCosta CM, Shadoan MK, Xu N, Hansen GM, Balakrishnan S, Domin J, Powell DR, Oravecz T. Requirement for class II phosphoinositide 3-kinase C2alpha in maintenance of glomerular structure and function. Mol Cell Biol 31: 63–80, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstadt H, Shaw AS, Walz G, Benzing T. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humphries AC, Donnelly SK, Way M. Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote N-WASP-dependent actin polymerisation. J Cell Sci 127: 673–685, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Itoh M, Nakadate K, Horibata Y, Matsusaka T, Xu J, Hunziker W, Sugimoto H. The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PLoS One 9: e106621, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin J, Sison K, Li C, Tian R, Wnuk M, Sung HK, Jeansson M, Zhang C, Tucholska M, Jones N, Kerjaschki D, Shibuya M, Fantus IG, Nagy A, Gerber HP, Ferrara N, Pawson T, Quaggin SE. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151: 384–399, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH Jr, Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 584–593, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, Chassaing N, Lacombe D, Devriendt K, Teebi A, Loscertales M, Robson C, Liu T, MacLaughlin DT, Noonan KM, Russell MK, Walsh CA, Donahoe PK, Pober BR. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet 39: 957–959, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Kinugasa S, Tojo A, Sakai T, Tsumura H, Takahashi M, Hirata Y, Fujita T. Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase. Kidney Int 80: 1328–1338, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi N, Ueno T, Ohashi K, Yamashita H, Takahashi Y, Sakamoto K, Manabe S, Hara S, Takashima Y, Dan T, Pastan I, Miyata T, Kurihara H, Matsusaka T, Reiser J, Nagata M. Podocyte injury-driven intracapillary PAI-1 accelerates podocyte loss via uPAR-mediated β1-integrin endocytosis. Am J Physiol Renal Physiol 308: F614–F626, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Krendel M, Osterweil EK, Mooseker MS. Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett 581: 644–650, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell 14: 1085–1096, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361–1370, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, Saleem MA, Satchell SC, Banas B, Mathieson PW, Kretzler M, Hemal AK, Rudel LL, Petrovic S, Weckerle A, Pollak MR, Ross MD, Parks JS, Freedman BI. Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 26: 339–348, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410: 231–235, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N. Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J 25: 2889–2897, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayor S, Parton RG, Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol 6: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 24: 8–16, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMahon HT, Boucrot E. Membrane curvature at a glance. J Cell Sci 128: 1065–1070, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533, 2011. [DOI] [PubMed] [Google Scholar]
- 66.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol 151: 187–198, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature 379: 353–357, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R, Krendel M, Bettoni S, Morigi M, Delledonne M, Pecoraro C, Abbate I, Capobianchi MR, Hildebrandt F, Otto E, Schaefer F, Macciardi F, Ozaltin F, Emre S, Ibsirlioglu T, Benigni A, Remuzzi G, Noris M. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 365: 295–306, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121: 593–606, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Mim C, Unger VM. Membrane curvature and its generation by BAR proteins. Trends Biochem Sci 37: 526–533, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell 6: 911–927, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science 324: 895–899, 2009. [DOI] [PubMed] [Google Scholar]
- 73.New LA, Keyvani Chahi A, Jones N. Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J Biol Chem 288: 1500–1510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.New LA, Martin CE, Jones N. Advances in slit diaphragm signaling. Curr Opin Nephrol Hypertens 23: 420–430, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Okamura K, Dummer P, Kopp J, Qiu L, Levi M, Faubel S, Blaine J. Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS One 8: e54817, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ovunc B, Otto EA, Vega-Warner V, Saisawat P, Ashraf S, Ramaswami G, Fathy HM, Schoeb D, Chernin G, Lyons RH, Yilmaz E, Hildebrandt F. Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol 22: 1815–1820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and Chinese hamster ovary cells: the initial fate of unbound E-cadherin. J Biol Chem 278: 21050–21057, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Pawluczyk IZ, Pervez A, Ghaderi Najafabadi M, Saleem MA, Topham PS. The effect of albumin on podocytes: the role of the fatty acid moiety and the potential role of CD36 scavenger receptor. Exp Cell Res 326: 251–258, 2014. [DOI] [PubMed] [Google Scholar]
- 79.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 416: 187–190, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L. Beta-arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci USA 103: 14110–14115, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quack I, Woznowski M, Potthoff SA, Palmer R, Konigshausen E, Sivritas S, Schiffer M, Stegbauer J, Vonend O, Rump LC, Sellin L. PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J Biol Chem 286: 12959–12970, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol 139: 49–61, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Renard HF, Simunovic M, Lemiere J, Boucrot E, Garcia-Castillo MD, Arumugam S, Chambon V, Lamaze C, Wunder C, Kenworthy AK, Schmidt AA, McMahon HT, Sykes C, Bassereau P, Johannes L. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 517: 493–496, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rico M, Mukherjee A, Konieczkowski M, Bruggeman LA, Miller RT, Khan S, Schelling JR, Sedor JR. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol Renal Physiol 289: F431–F441, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Rocque BL, Babayeva S, Li J, Leung V, Nezvitsky L, Cybulsky AV, Gros P, Torban E. Deficiency of the planar cell polarity protein vangl2 in podocytes affects glomerular morphogenesis and increases susceptibility to injury. J Am Soc Nephrol 26: 576–586, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 96: 7962–7967, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2: 411–423, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175: 33–39, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sachs N, Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat Rev Nephrol 9: 200–210, 2013. [DOI] [PubMed] [Google Scholar]
- 93.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol 4: a005645, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sakisaka T, Itoh T, Miura K, Takenawa T. Phosphatidylinositol 4,5-bisphosphate phosphatase regulates the rearrangement of actin filaments. Mol Cell Biol 17: 3841–3849, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanna-Cherchi S, Burgess KE, Nees SN, Caridi G, Weng PL, Dagnino M, Bodria M, Carrea A, Allegretta MA, Kim HR, Perry BJ, Gigante M, Clark LN, Kisselev S, Cusi D, Gesualdo L, Allegri L, Scolari F, D'Agati V, Shapiro LS, Pecoraro C, Palomero T, Ghiggeri GM, Gharavi AG. Exome sequencing identified MYO1E and NEIL1 as candidate genes for human autosomal recessive steroid-resistant nephrotic syndrome. Kidney Int 80: 389–396, 2011. [DOI] [PubMed] [Google Scholar]
- 96.Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA. Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol 12: 1852–1857, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schiffer M, Teng B, Gu C, Shchedrina VA, Kasaikina M, Pham VA, Hanke N, Rong S, Gueler F, Schroder P, Tossidou I, Park JK, Staggs L, Haller H, Erschow S, Hilfiker-Kleiner D, Wei C, Chen C, Tardi N, Hakroush S, Selig MK, Vasilyev A, Merscher S, Reiser J, Sever S. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat Med 21: 601–609, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmid SL, Sorkin A, Zerial M. Endocytosis: past, present, future. Cold Spring Harb Perspect Biol 6: a022509, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sever S, Altintas MM, Nankoe SR, Moller CC, Ko D, Wei C, Henderson J, del Re EC, Hsing L, Erickson A, Cohen CD, Kretzler M, Kerjaschki D, Rudensky A, Nikolic B, Reiser J. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest 117: 2095–2104, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimokawa N, Haglund K, Holter SM, Grabbe C, Kirkin V, Koibuchi N, Schultz C, Rozman J, Hoeller D, Qiu CH, Londono MB, Ikezawa J, Jedlicka P, Stein B, Schwarzacher SW, Wolfer DP, Ehrhardt N, Heuchel R, Nezis I, Brech A, Schmidt MH, Fuchs H, Gailus-Durner V, Klingenspor M, Bogler O, Wurst W, Deller T, de Angelis MH, Dikic I. CIN85 regulates dopamine receptor endocytosis and governs behaviour in mice. EMBO J 29: 2421–2432, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silver DN, Lewis RA, Nussbaum RL. Mapping the Lowe oculocerebrorenal syndrome to Xq24-q26 by use of restriction fragment length polymorphisms. J Clin Invest 79: 282–285, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, Volpicelli-Daley L, Tian X, Wu Y, Ma H, Son SH, Zheng R, Moeckel G, Cremona O, Holzman LB, De Camilli P, Ishibe S. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122: 4401–4411, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416: 183–187, 2002. [DOI] [PubMed] [Google Scholar]
- 104.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009. [DOI] [PubMed] [Google Scholar]
- 105.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol 9: e1000604, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teckchandani A, Toida N, Goodchild J, Henderson C, Watts J, Wollscheid B, Cooper JA. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol 186: 99–111, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, Inoue K, Balkin DM, Hassan H, Son SH, Lee Y, Moeckel G, Calderwood DA, Holzman LB, Critchley DR, Zent R, Reiser J, Ishibe S. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124: 1098–1113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, Schiffer M. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem 285: 25285–25295, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tossidou I, Teng B, Menne J, Shushakova N, Park JK, Becker JU, Modde F, Leitges M, Haller H, Schiffer M. Podocytic PKC-alpha is regulated in murine and human diabetes and mediates nephrin endocytosis. PLoS One 5: e10185, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol 163: 203–208, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verroust PJ, Kozyraki R. The roles of cubilin and megalin, two multiligand receptors, in proximal tubule function: possible implication in the progression of renal disease. Curr Opin Nephrol Hypertens 10: 33–38, 2001. [DOI] [PubMed] [Google Scholar]
- 112.Waters AM, Wu MY, Huang YW, Liu GY, Holmyard D, Onay T, Jones N, Egan SE, Robinson LA, Piscione TD. Notch promotes dynamin-dependent endocytosis of nephrin. J Am Soc Nephrol 23: 27–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wei CL, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang QY, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Welsch T, Endlich N, Gokce G, Doroshenko E, Simpson JC, Kriz W, Shaw AS, Endlich K. Association of CD2AP with dynamic actin on vesicles in podocytes. Am J Physiol Renal Physiol 289: F1134–F1143, 2005. [DOI] [PubMed] [Google Scholar]
- 115.Wunderlich L, Farago A, Buday L. Characterization of interactions of Nck with Sos and dynamin. Cell Signal 11: 25–29, 1999. [DOI] [PubMed] [Google Scholar]
- 116.Zhai XY, Nielsen R, Birn H, Drumm K, Mildenberger S, Freudinger R, Moestrup SK, Verroust PJ, Christensen EI, Gekle M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int 58: 1523–1533, 2000. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J, Bruck S, Cemerski S, Zhang L, Butler B, Dani A, Cooper JA, Shaw AS. CD2AP links cortactin and capping protein at the cell periphery to facilitate formation of lamellipodia. Mol Cell Biol 33: 38–47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]