Abstract
Major plasma membrane components of the tumor cell, ion channels, and integrins play crucial roles in metastasis. Glioma cells express an amiloride-sensitive nonselective cation channel composed of acid-sensing ion channel (ASIC)-1 and epithelial Na+ channel (ENaC) α- and γ-subunits. Inhibition of this channel is associated with reduced cell migration and proliferation. Using the ASIC-1 subunit as a reporter for the channel complex, we found a physical and functional interaction between this channel and integrin-β1. Short hairpin RNA knockdown of integrin-β1 attenuated the amiloride-sensitive current, which was due to loss of surface expression of ASIC-1. In contrast, upregulation of membrane expression of integrin-β1 increased the surface expression of ASIC-1. The link between the amiloride-sensitive channel and integrin-β1 was mediated by α-actinin. Downregulation of α-actinin-1 or -4 attenuated the amiloride-sensitive current. Mutation of the putative binding site for α-actinin on the COOH terminus of ASIC-1 reduced the membrane localization of ASIC-1 and also resulted in attenuation of the amiloride-sensitive current. Our data suggest a novel interaction between the amiloride-sensitive glioma cation channel and integrin-β1, mediated by α-actinin. This interaction may form a mechanism by which channel activity can regulate glioma cell proliferation and migration.
Keywords: Deg/ENaC, cytoskeleton, amiloride, ASIC-1
glioblastoma multiforme is the most common and most aggressive form of diffusely infiltrating adult brain tumors. A major characteristic of gliomas, which have high rates of proliferation and migration, is their ability to invade normal brain tissue, along with blood vessels and white matter tracts. Multiple previous studies have focused on the signaling biology of gliomas, but the role of ion channels and their interaction with other membrane receptors, cytoskeletal proteins, and signaling pathways in the regulation of glioma cell migration and proliferation is not well understood. We previously showed that World Health Organization (WHO) grade III and IV glioma cells express a nonselective amiloride-sensitive cation current that is absent in grade I and II gliomas and normal astrocytes (7, 8). This cation current is due to expression of a heteromeric channel complex formed by members of the degenerin (Deg)/epithelial Na+ channel (ENaC) superfamily, i.e., acid-sensing ion channel (ASIC) type 1, α-ENaC, and γ-ENaC. Knockout of any one of these subunits was associated with loss of the amiloride-sensitive current (26). The signaling mechanism linking glioma cation channel activity to these cellular downstream effects is not known; however, it has been shown that glioma cell migration and proliferation can be regulated by integrin-mediated activation of signaling pathways (39).
Integrins are heterodimeric transmembrane adhesion receptors composed of α- and β-subunits. They not only function as ligands for extracellular matrix (ECM) proteins, but they also regulate cell behavior by modulating signaling pathways involved in cytoskeletal organization (24). Glioma cells express multiple integrin subunits that have functional importance in glioma invasion. Fibronectin (integrin-α5β1), vitronectin (integrin-αVβ3), and laminin (integrin-α6β1) receptors are critical mediators of glioma invasion, with integrin-β1 playing an important role because of its ability to bind multiple substrates (30, 39). There is an increasing body of evidence demonstrating frequent physical and functional communication between cell adhesion receptors and ion channels and, in particular, between channels and integrin-β1 (3, 13, 14, 29). The integrin-ion channel interaction may also involve other membrane and cytosolic signaling proteins to activate downstream regulatory pathways. In arteriolar smooth muscle, integrin-β1 and a Ca2+ (CaL) channel form a macromolecular signaling complex that also includes c-Src and protein kinase A and modulates CaL channel function (13). It has also been reported that a direct correlation between α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor surface expression and adhesion to the ECM is mostly mediated by the higher surface level of glycosylated integrin-β1 (36). These observations suggest that integrins interact with ion channels to impact cellular physiology by regulating channel localization or by modulating integrin and integrin-associated protein signaling. Interestingly, a complex containing ENaC, voltage-gated Ca2+ channels, and the Na+-K+-ATPase has been shown to colocalize with integrin-β1 in limb-bud chondrocytes (43). However, functional interactions between ENaCs or ASICs and integrins have not been reported.
In the present study we tested the hypothesis that ASIC-1, as a representative marker for the glioma-specific cross-clade channel complex, interacts with integrin-β1 and that this interaction is required to maintain the amiloride-sensitive current. We report that integrin-β1 coimmunoprecipitates with ASIC-1 and that expression of integrin-β1 is required for surface expression of ASIC-1 and the glioma-specific cation current at the plasma membrane. We also found that a fibronectin-induced increase in surface ASIC-1 expression is dependent on expression of integrin-β1 and that the interaction between these membrane proteins is mediated through α-actinin.
EXPERIMENTAL PROCEDURES
Cell culture.
Experiments were performed on the glioma cell lines D54MG (derived from a WHO grade IV tumor; a kind gift of Dr. D. Bigner, Duke University, Durham, NC) and U87MG, U251, and U373MG (American Type Culture Collection).1 The cells were cultured and maintained in Dulbecco's modified Eagle's medium/Ham's F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Rockford, IL) in the absence of antibiotics. Cells were split onto flame-sterilized coverslips in 35-mm dishes 24 h before electrophysiological recording. In the case of transfection, cells were split 24 h previously and used after 48–72 h of transfection.
Generation of stable cell lines.
Stable cell lines were generated by transfection with 2 μg of eGFP-ASIC-1 or truncated eGFP-ASIC-1 cDNA (termed A1DN) as previously described (26). After transfection, cells were cultured for 72 h, transferred to a T75 flask, and selected with G418 (500 μg/ml; Mediatech, Manassas, VA). After initial antibiotic selection, GFP-positive cells were sterile-sorted by FACS. Stable transfectants were maintained in Dulbecco's modified Eagle's medium/Ham's F-12 medium with 10% fetal bovine serum.
Stable downregulation by RNAi.
HuSH 29mer short hairpin (shRNA) kits providing vector control, scrambled negative control, and four target variants (OriGene, Rockville, MD) were used to downregulate integrin-β1 (TG320392: locus ID 3688; GAAGGAATGCCTACTTCTGCACGATGTGA), α-actinin-1 (TF314970: locus ID 87; CTCATCTTCGACAACAAGCACACCAACTA), and α-actinin-4 (TG314967: locus ID 81; GAGCACCTGATGGAGGACTACGAGAAGCT). The cells were transfected using Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol and selected with puromycin (1–2 μg/ml; Sigma), and positive clones were screened by Western blot analysis.
Western blotting and coimmunoprecipitation.
Cells were washed three times with cold PBS and lysed in buffer [150 mM NaCl, 5 mM EDTA, 50 mM Tris (pH 7.4), 1% Triton X-100, and Complete protease inhibitor cocktail (Roche, Indianapolis, IN)] for 30 min at 4°C. In the case of the experiments examining ERK phosphorylation, cells were synchronized for 48 h as previously described (41). Lysis buffer used to prepare cells for ERK phosphorylation experiments also included HALT phosphatase inhibitor (Thermo Fisher Scientific). Cell lysates were passed 10 times through a 22-gauge needle and centrifuged (13,200 rpm, 30 min, 4°C). Ten to 25 μg of protein lysates were used per lane for SDS-PAGE and immunoblotting. Lysates were heated at 95°C for 6–7 min in 1× Laemmli sample buffer (Bio-Rad) and subjected to SDS-PAGE over 8% or 12% separating gels, and proteins were transferred to Immobilon-P membranes (Millipore, Hayward, CA). After transfer, membranes were blocked for 1 h with 5% nonfat dry milk or BSA (for phospho-specific antibody) in Tris-buffered saline [100 mM Tris (pH 7.5) and 150 mM NaCl] with 0.1% Tween 20 (TBS-T; Bio-Rad, Hercules, CA) for 1 h at room temperature and probed with primary antibodies in 5% milk or BSA in TBS-T overnight at 4°C. Blots were washed with TBS-T (3 times, 5 min) and probed with secondary antibodies conjugated to horseradish peroxidase (Thermo Fisher Scientific) in 5% milk in TBS-T. The blots were developed in Luminata Crescendo horseradish peroxidase substrate and exposed to X-ray film. The X-ray films were scanned using a Syngene G-Box, and images were analyzed for densitometry by GeneTools software (Syngene). In coimmunoprecipitation experiments, 2 μg of antibody were added to 500 μg of cell lysates, which were incubated overnight at 4°C and then with 70 μl of protein A beads (for rabbit IgG) or protein G beads (for mouse IgG) for 4 h. The beads were pelleted and washed three to four times with lysis buffer at 4°C. The immune complexes were eluted after boiling in 1× Laemmli sample buffer for 7–8 min and loaded onto SDS gels (8%) for immunoblotting.
Cell surface biotinylation.
All steps were performed at 4°C or on ice. The cells were washed three to four times with ice-cold PBS and then incubated for 30 min in PBS containing 1 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Thermo Fisher Scientific). The biotinylation reaction was quenched by incubation for 15 min with 1× PBS containing 100 mM glycine. The cells were then washed three times with cold PBS, and cell lysates were prepared as described above. Total cell lysates (250 μg of protein) were immunoprecipitated with streptavidin beads (Thermo Fisher Scientific) by incubation overnight at 4°C. The beads were pelleted and washed three times with cell lysis buffer. Biotinylated proteins were eluted from the beads by boiling with 1× Laemmli sample buffer for 7–8 min and loaded onto SDS gels (8%) for immunoblotting.
Antibodies and drugs.
The following antibodies were used: mouse anti-GFP (AM1009a, Abgent, San Diego, CA; 1:5,000 dilution), rabbit anti-ASIC-1 (sc28756, Santa-Cruz Biotechnology, Santa Cruz, CA; 1:500 dilution), mouse anti-integrin-β1 (MAB2000, Millipore, Billerica, MA; 1:10,000 dilution), mouse anti-α-actinin-1 (sc135819, Santa Cruz Biotechnology; 1:1,000 dilution), goat anti-α-actinin-4 (sc49333, Santa Cruz Biotechnology; 1:1,000 dilution), mouse pan-anti-actinin (sc17829, Santa Cruz Biotechnology; 1:1,000), mouse anti-actin (MAB1501, Millipore; 1:200,000 dilution), and mouse anti-α1-Na+-K+-ATPase (ab67671, Abcam, Cambridge, MA; 1:3,000 dilution). Amiloride and benzamil hydrochloride was obtained from Sigma-Aldrich.
Site-directed mutagenesis.
To replace three different amino acids in the α-actinin-binding site (484LSLDDVK490) on the COOH terminus of ASIC-1, site-directed mutagenesis was performed using the QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies), sense and antisense primers [5′-ggcgtggccctcagcgcggacgccgtcgcaagacacaacccgtgcgagagcc-3′ (forward) and 5′-ggctctcgcacgggttgtgtcttgcgacggcgtccgcgctgagggccacgcc-3′ (reverse)], and pEGFP-N1-hASIC1 as template DNA. The primers contained the necessary base replacement to mutate the desired leucines (L), aspartate (D), and lysines (K) to alanines (A). All generated mutations were confirmed by DNA sequencing (Heflin Genetics Center, University of Alabama at Birmingham).
Electrophysiology.
Whole cell current recordings were obtained using the amphotericin B perforated-patch technique as previously described (1). Cells were mounted in a flow-through chamber on the stage of an inverted microscope (model DMIRB, Leica Microsystems, Heidelberg, Germany). Bath solution exchange was achieved using a pinch-valve control system converging on an 8-1 manifold. Tips of borosilicate recording pipettes (5–7 mΩ) were backfilled with pipette solution [150 mM KCl and 10 mM HEPES, pH 7.2 (Tris·HCl)] and then with the same solution containing ∼0.2 mg/ml amphotericin B (Sigma-Aldrich). Currents were obtained using a patch-clamp amplifier (Axopatch 200B, Axon Instruments/Molecular Devices, Sunnyvale, CA), with voltage commands and data acquisition controlled by Clampex software (pClamp 10, Axon Instruments), and digitized (Digidata 1440A interface, Axon Instruments) at a sampling frequency of 2 kHz. Current-voltage relationships were obtained using a pulse protocol in which cells were stepped from a −40-mV holding potential between −100 and +80 mV in 20-mV increments for 250 ms. Mean currents were obtained from the average of three sweeps during the 200- to 250-ms period of each sweep using Clampfit 10 software (Axon Instruments). Whole cell conductance was obtained during the linear portion of the current-voltage relationship between −100 and 0 mV. Bath solution contained (in mM) 140 NaCl, 4.0 KCl, 1.8 CaCl2, 1.0 MgCl2, 10 glucose, and 10 HEPES, pH 7.4 (NaOH/HCl). Appropriate vehicle controls were performed.
Data analysis and statistics.
Statistical analysis was done with Microsoft Excel. All experiments were performed at least three times. Values are means ± SD, except for patch-clamp data, which are means ± SE. Data were analyzed using Student's t-tests (unpaired or paired) or one-way ANOVA with Dunnett's post hoc test.
RESULTS
Integrin-β1 and ASIC-1 coimmunoprecipitate in glioma cells.
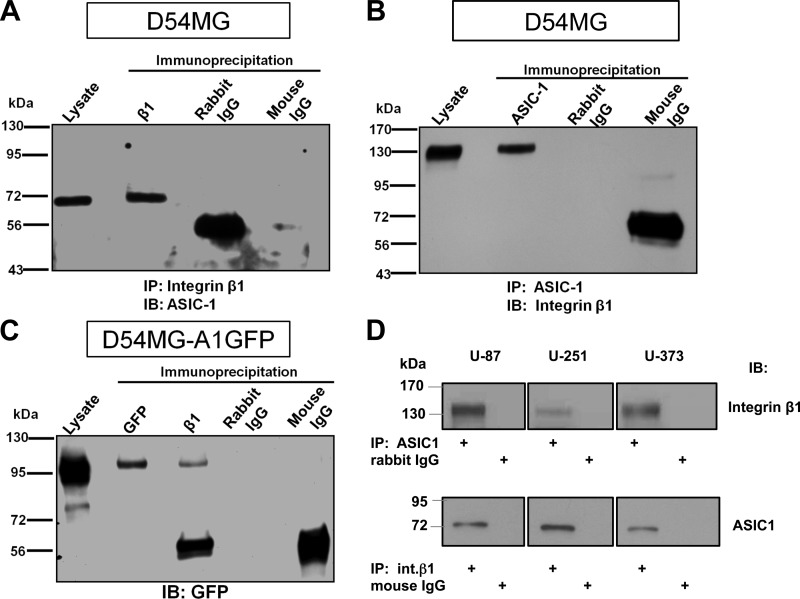
Consistently overexpressed in glioma cells, integrins are crucial regulators of glioma cell migration and downstream signaling molecules via activation of the Ras/mitogen-activated protein kinase (MAPK) pathway (18, 20). Integrins interact with several cation channels, with the earliest examples from studies on human ether-à-go-go (hERG) channels in leukemic and neuronal cells, where cell differentiation, migration, and neurite extension were studied (2, 4). Importantly, this regulation can be a two-way street, in that integrins can activate the channel, while the channel can, in turn, modulate signaling pathways regulated by the integrin (37). Thus we hypothesized that glioma cells might exhibit a similar interaction between integrin-β1 and the glioma ASIC/ENaC. To examine this potential interaction, cell lysates from D54MG, U87, U251, and U373 glioma cells were subjected to coimmunoprecipitation using anti-integrin-β1 or anti-ASIC-1 antibodies. Immunoprecipitation of integrin-β1 revealed a band consistent with the expected size of full-length ASIC-1 at ∼70 kDa (Fig. 1, A and D). Similarly, immunoprecipitation of ASIC-1 pulled down a band migrating at ∼130 kDa, consistent with the expected size of integrin-β1 (Fig. 1, B and D). We also generated a stable D54MG cell line overexpressing ASIC-1 epitope-tagged with GFP at the COOH terminus to facilitate immunoprecipitation and immunoblotting in some experiments. Immunoprecipitation of integrin-β1 from these cells identified a band migrating at ∼100 kDa, consistent with the predicted molecular weight of ASIC-1-GFP (Fig. 1C).
Fig. 1.
Physical association of acid-sensing ion channel (ASIC)-1 with integrin-β1 in glioma cells. A: cell lysates were immunoprecipitated (IP) with mouse anti-integrin-β1 (β1) antibody and immunoblotted (IB) with rabbit anti-ASIC-1. Immunoprecipitation of integrin-β1 pulled down ASIC-1 at ∼70 kDa. Lysate refers to total lysate (25 μg of protein) prepared from cells. B: immunoprecipitation of ASIC-1 using rabbit anti-ASIC-1 from D54MG cell lysates pulled down integrin-β1 detected by mouse anti-integrin-β1 at ∼130 kDa. C: in D54MG cells stably overexpressing GFP-tagged ASIC-1 (D54MG_A1GFP), immunoprecipitation of integrin-β1 pulled down GFP-ASIC-1 consistent with the expected size of GFP-tagged ASIC-1 at ∼100 kDa. Nonimmune rabbit IgG and mouse IgG did not immunoprecipitate any specific bands (n ≥ 3). D: ASIC-1 and integrin-β1 also coprecipitated from 3 additional glioma cell lines (U87, U251, and U373).
Stable knockdown of integrin-β1 attenuates the amiloride-sensitive ion conductance.
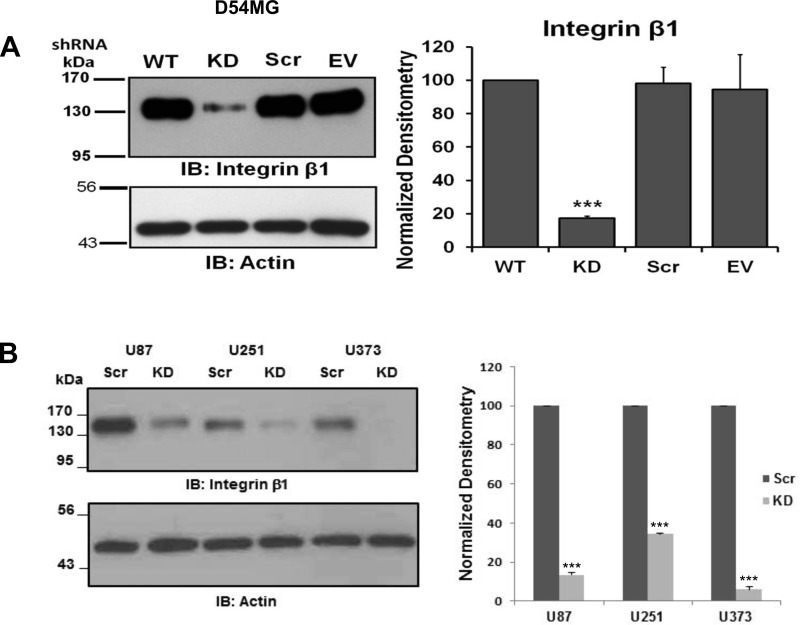
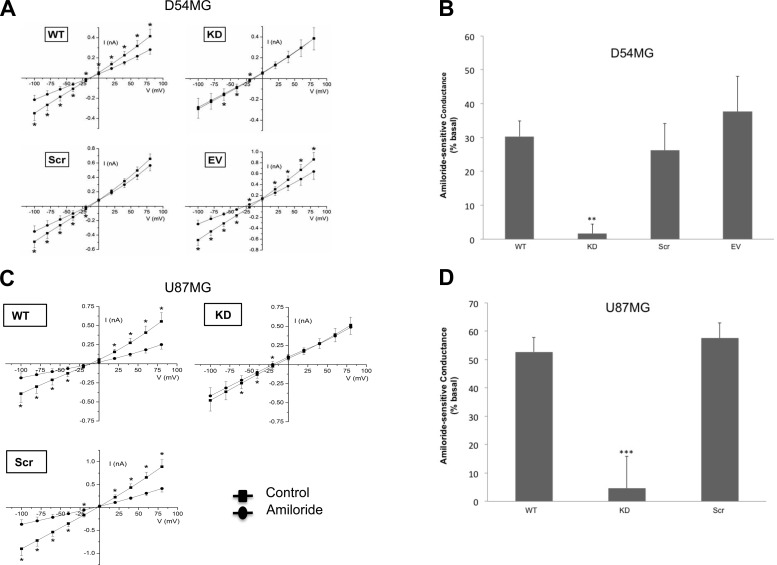
We next performed whole cell patch-clamp experiments to determine if a functional relationship existed between the channel and integrin-β1. To minimize variability in transfections due to the low transfection efficiency of glioma cells, we generated stable cell line variants transfected with a shRNA directed against integrin-β1 [knockdown (KD)], a scrambled (Scr) shRNA, or (in D54MG cells) an empty vector. The specific shRNA reduced integrin-β1 expression by 65–95% in the different cell lines (Fig. 2). The functional consequence of knockdown of integrin-β1 was determined by recording amiloride-sensitive currents. In nontransfected wild-type D54MG cells, 30.33 ± 4.6% (n = 14) of the basal conductance was amiloride-sensitive (Fig. 3B). In contrast, in D54MG cells in which integrin-β1 was depleted, only 1.63 ± 2.9% (n = 9) of the basal conductance was amiloride-sensitive (Fig. 3B). Stable D54MG cell lines transfected with the scrambled shRNA construct or the empty vector exhibited wild-type currents (Fig. 3A). Similarly, in wild-type U87 cells, 52.66 ± 5.1% (n = 6) of the basal conductance was amiloride-sensitive, whereas when integrin-β1 was knocked down, only 4.54 ± 11.4% (n = 4) of the basal conductance was amiloride-sensitive (Fig. 3D). Thus, reduced expression of integrin-β1 correlated with attenuation of basal amiloride-sensitive currents.
Fig. 2.
Knockdown of integrin-β1 in glioma cells. A: lysates from untransfected wild-type (WT) D54MG cells and D54MG cells stably transfected with shRNA for integrin-β1 [knockdown (KD)], scrambled shRNA (Scr), or empty vector (EV). Expression of integrin-β1 was significantly decreased in KD cells compared with WT cells (n ≥ 4). B: expression of integrin-β1 in 3 additional glioma cell lines (U87, U251, and U373) was also significantly reduced by shRNA. ***P < 0.001 by ANOVA and Dunnett's post hoc test (A) and Student's t-test (B).
Fig. 3.
Knockdown of integrin-β1 inhibits amiloride-sensitive current in glioma cells. A: current-voltage relationships show significant attenuation of amiloride-sensitive whole cell current after knockdown of integrin-β1 (n ≥ 6). B: average amiloride-sensitive conductance in D54MG cells. C: current-voltage relationships recorded from U87MG cells transfected with shRNA targeting integrin-β1. D: average amiloride-sensitive conductance in U87MG cells. **P < 0.01; ***P < 0.001 by ANOVA and Dunnett's post hoc test.
Integrin-β1 is required for surface expression of ASIC-1.
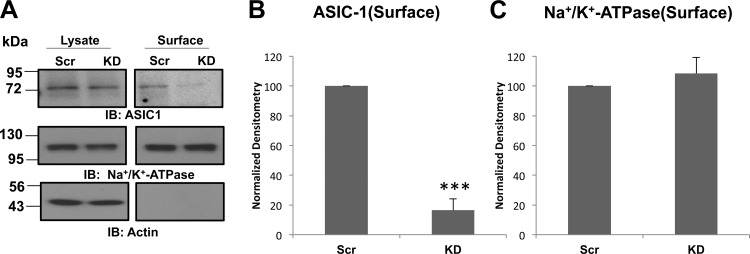
After identifying a functional dependence of the amiloride-sensitive conductance on the expression of integrin-β1, we determined if ASIC-1 required integrin-β1 for proper membrane localization. We biotinylated D54MG cells in which integrin-β1 had been stably knocked down or D54MG cells that were stably transfected with the scrambled shRNA construct. As shown in Fig. 4, membrane localization of ASIC-1 was significantly reduced (by 75 ± 16%, n ≥ 4) in integrin-β1-depleted glioma cells, supporting the concept that integrin-β1 facilitates membrane expression of the cation channel. To control for nonspecific effects of stable knockdown of integrin-β1 on the surface expression of other membrane proteins, we reprobed the blot with an antibody directed against the Na+-K+-ATPase α1-subunit. However, there was no difference in surface expression of the Na+ pump between cells in which integrin-β1 had been knocked down and cells expressing the scrambled construct. β-Actin served as a negative marker for biotinylation of surface proteins, as well as a loading control for whole cell lysates. In contrast, knockdown of ASIC-1 had no significant effect on the surface expression of integrin-β1 (data not shown). These results suggest that integrin-β1 has an important role in maintaining the surface expression of ASIC-1 and that loss of channel surface expression likely accounts for the reduction of amiloride-sensitive current in the integrin-β1 knockout cells.
Fig. 4.
Surface expression of ASIC-1 requires integrin-β1. A: after surface biotinylation, lysates (10 μg) and the surface fraction from D54MG cells in which integrin-β1 was knocked down (KD) and control (Scr) cells (transfected with ASIC-1-GFP) were analyzed. Membrane expression of ASIC-1 was significantly inhibited in KD cells compared with Scr cells, but there was no significant change in total expression of ASIC-1 (n ≥ 4). B and C: normalized densitometry of surface ASIC-1 and Na+-K+-ATPase expression in KD and Scr D54MG cells. Blots were stripped and reimmunoblotted for Na+-K+-ATPase and β-actin as a loading control for surface proteins. ***P < 0.001.
Fibronectin-mediated cell adhesion increased membrane localization of ASIC-1.
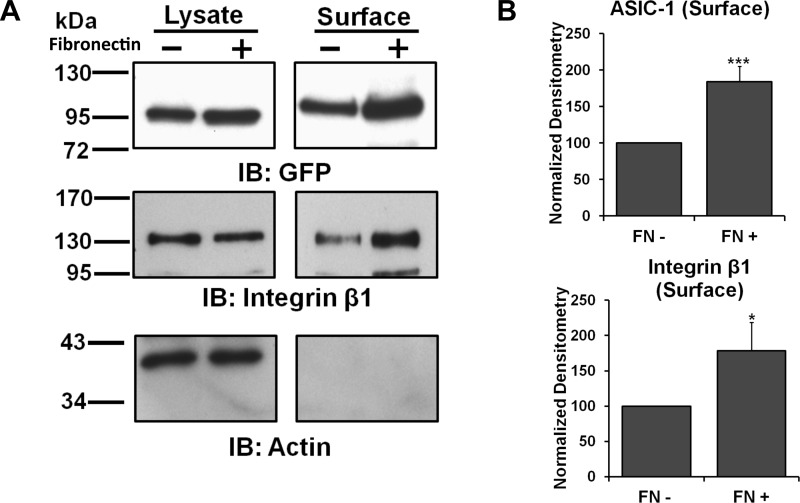
The involvement of integrin-β1 in the surface stability of ASIC-1 provoked us to determine if the composition of the ECM would affect the membrane expression of ASIC-1. D54MG cells, in this case stably transfected with ASIC-1-GFP, were split into six-well plates with no additional matrix or coated with fibronectin (100 μg/ml). After 24 h of incubation, the cells were biotinylated and immunoblotted for GFP and integrin-β1. Membrane localization of ASIC-1 was significantly increased (by 72 ± 1%, n ≥ 3), as was membrane expression of integrin-β1, in the presence of fibronectin (Fig. 5). To confirm that the effect of fibronectin on the membrane localization of ASIC-1 was specific, we repeated this experiment using plates coated with poly-l-lysine (100 μg/ml). Under these conditions, membrane localization of ASIC-1 and integrin-β1 was not altered (n ≥ 3; data not shown).
Fig. 5.
Cell adhesion through fibronectin increased membrane expression of ASIC-1. A: total and surface proteins were immunoblotted for GFP and integrin-β1 in D54MG cells. Membrane localization of ASIC-1 was significantly increased and total and membrane expression of integrin-β1 was higher following 24 h of incubation on fibronectin (FN)-coated plates (n ≥ 3). B: normalized densitometry of membrane ASIC-1 and integrin-β1 in cells incubated on fibronectin-coated dishes (FN+) or on uncoated plates (FN−) for 24 h. β-Actin was used as a loading control. *P < 0.05; ***P < 0.001.
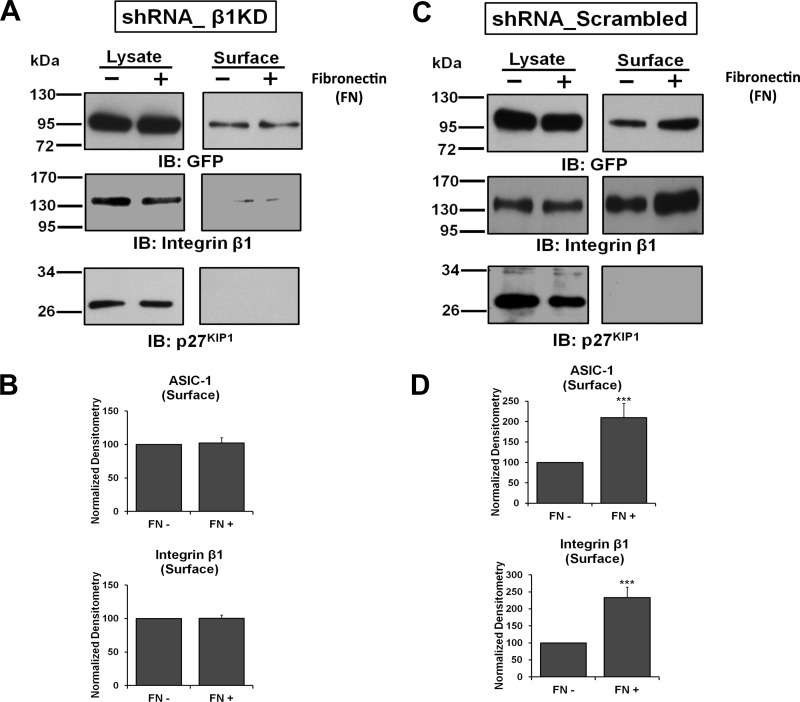
To determine if the effect of fibronectin on membrane localization of ASIC-1 was mediated through integrin-β1 or was a direct effect of fibronectin on the channel subunit, we evaluated the surface expression of ASIC-1 in the integrin-β1 knockdown cells. Both stable D54MG cell lines (β1-KD and β1-Scr) were transiently transfected with ASIC-1-GFP and, after 72 h, seeded onto six-well plates and biotinylated as described above. As shown in Fig. 6, A and B, no significant change in membrane expression of ASIC-1 was observed under these conditions. As expected, the surface expression of integrin-β1 in this cell line was also diminished, and no appreciable change was observed in the presence of fibronectin. In contrast, in D54MG cells stably expressing the scrambled integrin-β1 shRNA, we found a significant increase (by 109 ± 35%, n ≥ 3) in the membrane localization of ASIC-1 as well as integrin-β1 (Fig. 6, C and D). These results suggest that the increased surface localization of ASIC-1 in response to fibronectin is dependent on integrin-β1.
Fig. 6.
Upregulation of ASIC-1 membrane expression by cell adhesion to fibronectin requires integrin-β1. A and C: stable D54MG cell lines β1-KD and β1-Scr were transfected with ASIC-1-GFP. After 72 h, cells were incubated on fibronectin-coated dishes or on uncoated plates for 24 h; then total, as well as biotinylated, surface proteins were immunoblotted for GFP and integrin-β1. No increase in plasma membrane expression of ASIC-1 was observed in β1-KD cells with fibronectin-mediated cell adhesion (n ≥ 3). In contrast, a significant increase (n ≥ 3) in membrane localization of ASIC-1, as well as integrin-β1, after fibronectin-mediated integrin activation was observed in β1-Scr cells (C). p27KIP1 was used as a loading control and to demonstrate lack of biotinylation of intracellular proteins. B and D: normalized densitometry of membrane ASIC-1, integrin-β1, and Na+-K+-ATPase in D54MG β1-KD and β1-Scr cells transfected with ASIC-1-GFP and incubated on fibronectin-coated dishes or on uncoated plates for 24 h. ***P < 0.001.
α-Actinin-1 and -4 are required for physical and functional interaction between ASIC-1 and integrin-β1.
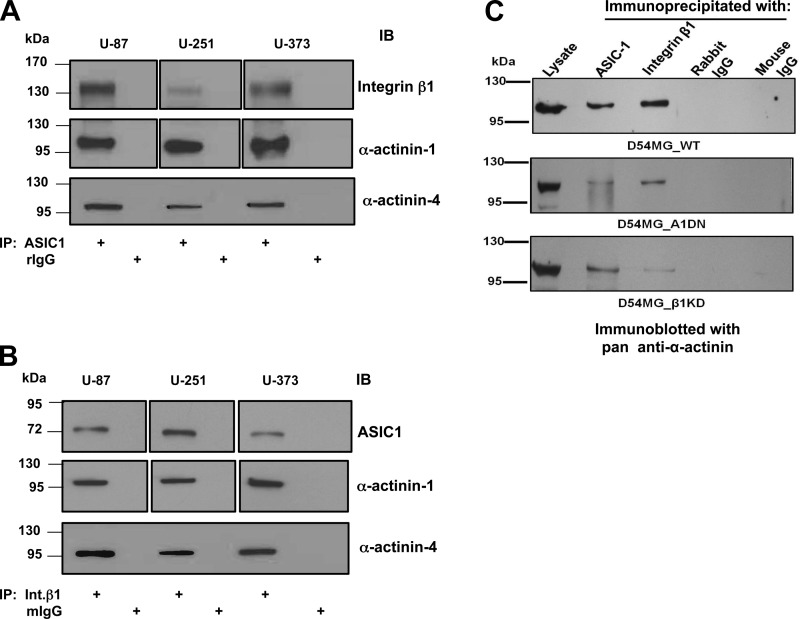
After identifying the association of ASIC-1 with integrin-β1, we sought to determine the nature (direct vs. indirect) of this interaction. α-Actinin, an actin-binding cytoskeletal protein found in the macromolecular complex of proteins associated with integrin-mediated focal adhesions, has been shown to interact with integrin-β1 through its spectrin repeat domains (27, 35). The nonmuscle α-actinin-1 and -4 have been reported to modulate whole cell current properties of homomeric ASIC-1 in heterologously expressing cells and hippocampal neurons (42). In the present study, using coimmunoprecipitation, we found an association of ASIC-1, integrin-β1, and α-actinins in glioma cells. As shown in Fig. 7, A and B, ASIC-1 and integrin-β1 coimmunoprecipitated with α-actinin-1 and -4. Depletion of ASIC-1 or integrin-β1 reduced the interaction of each of these proteins with α-actinins, while leaving the interaction between α-actinin (as determined using a pan-actinin antibody) and the nondepleted protein relatively unaffected (Fig. 7C). These results suggest that integrin-β1 and ASIC-1 independently interact with α-actinin in glioma cells.
Fig. 7.
Immunoprecipitation of ASIC-1 and integrin-β1 requires α-actinin. A and B: lysates from U87, U251, and U373 cells were immunoprecipitated with antibodies against ASIC-1 and blotted for integrin-β1 and actinin-1 and -4 or precipitated with antibodies against integrin-β1 (Intβ1) and blotted with antibodies against ASIC-1 and actinin-1 and -4. C: D54MG cells [wild-type (D54MG_WT)], stable D54MG cells with truncated ASIC-1 (D54MG_A1DN), and stable D54MG cells with shRNA for integrin-β1 (D54MG_β1KD) were immunoprecipitated with rabbit anti-ASIC-1 or mouse anti-integrin-β1 antibody and blotted with a rabbit pan-anti-α-actinin. Immunoprecipitation of ASIC-1 and integrin-β1 pulled down α-actinin at ∼110 kDa in D54MG cells. Coimmunoprecipitation of ASIC-1 and α-actinin was decreased in D54MG_A1DN cells, and coimmunoprecipitation of α-actinin with integrin-β1 was reduced in D54MG_β1KD cells but coimmunoprecipitation between ASIC-1 and α-actinin was not affected. Lysate refers to total lysate (25 μg of protein) prepared from these cells. Nonimmune rabbit IgG (rIgG) and mouse IgG (mIgG) immunoprecipitation showed specificity of rabbit ASIC-1, mouse integrin-β1, and rabbit α-actinin antibody (n ≥ 3).
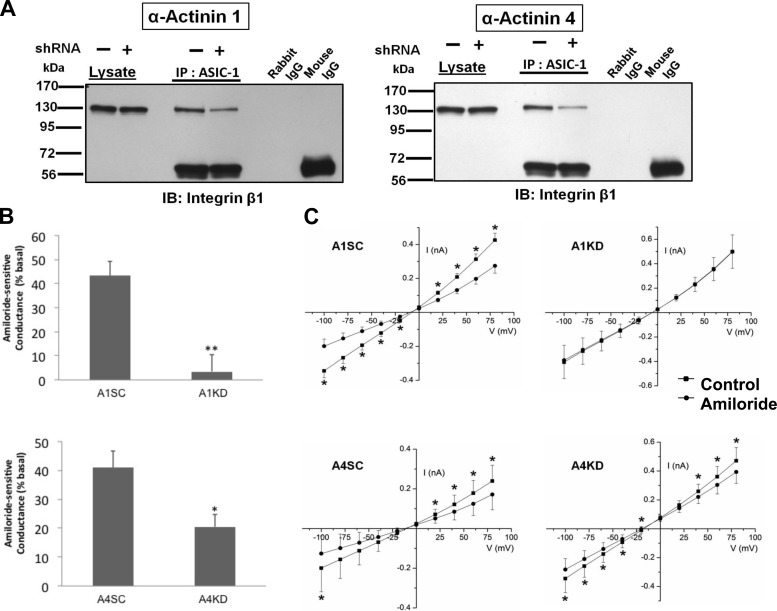
Using shRNAs, we next generated stable D54MG cell lines with diminished α-actinin-1 or -4 expression. To confirm the knockdown, the lysates from these stable cell lines were immunoblotted with antibodies directed against α-actinin-1 or -4 (data not shown). We transfected these stable cell lines with ASIC-1-GFP as described above and immunoprecipitated ASIC-1 using an anti-GFP antibody and immunoblotted for integrin-β1. In each case, knockdown of α-actinin-1 or -4 diminished the coimmunoprecipitation of integrin-β1 (Fig. 8A). These data are consistent with involvement of α-actinin-1 and -4 in linking ASIC-1 and integrin-β1, likely via the cytoskeleton. We also found a significant attenuation of amiloride-sensitive whole cell conductance when we knocked down α-actinin-1 (to 3.47 ± 7.1%, n = 5) or α-actinin-4 (to 20.44 ± 4.4%, n = 6) of the amiloride-sensitive current recorded in the corresponding scrambled shRNA control cells (Fig. 8B). The current-voltage relationships for each experimental condition are shown in Fig. 8C.
Fig. 8.
Knockdown of α-actinin-1 and -4 reduces amiloride-sensitive current in D54MG cells. A: knockdown of α-actinin-1 and -4 reduces coimmunoprecipitation of ASIC-1 and integrin-β1. B: average amiloride-sensitive conductance of D54MG cells stably transfected with scrambled or knockdown shRNA for α-actinin-1 (A1SC and A1KD, n ≥ 5) and scrambled or knockdown shRNA for α-actinin-4 (A4SC and A4KD, n ≥ 4) at −80 mV. C: current-voltage relationships show significant attenuation of amiloride-sensitive whole cell conductance after knockdown of α-actinin-1 and -4. Knockdown of individual α-actinin-1 or -4 diminished coimmunoprecipitation of ASIC-1 and integrin-β1 compared with individual shRNA with the scrambled sequence (n ≥ 3). *P < 0.05; **P < 0.01 by Student's t-test.
α-Actinin-1 and -4 are required for surface expression of ASIC-1.
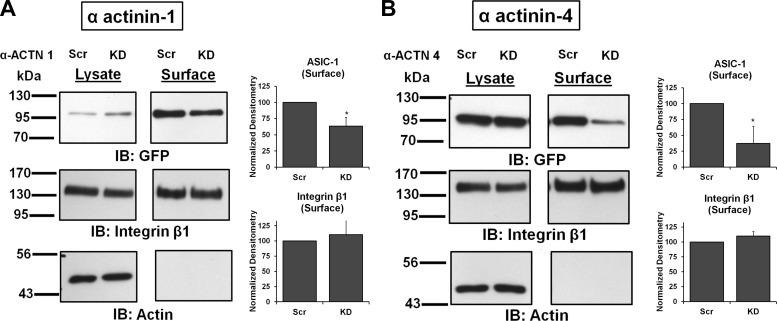
Next we examined if the reduction of whole cell current following knockdown of the α-actinins was due to a change in the surface expression of ASIC-1-GFP. Using cell surface biotinylation, we found that membrane expression of ASIC-1 was significantly reduced in α-actinin-1 (by 37 ± 14%, n ≥ 4; Fig. 9A) and α-actinin-4 (to 43 ± 27%, n ≥ 4; Fig. 9B) knockdown cells compared with the scrambled shRNA control cells. In this experiment, the inhibition of surface expression of ASIC-1 was integrin-β1-independent, as there was no appreciable difference in surface integrin-β1 expression in these stable cell lines.
Fig. 9.
Surface expression of ASIC-1 also requires α-actinin-1 and -4. A: stable D54MG cell lines α-ACTN 1-KD and α-ACTN 1-Scr were transfected with ASIC-1-GFP; after 72 h of surface biotinylation, lysates (10 μg) and surface fraction from stable D54MG cells transfected with KD and Scr shRNA for α-actinin-1 were analyzed. Membrane expression of ASIC-1 was significantly inhibited in KD cells compared with Scr cells, but there was no significant change in total expression of ASIC-1 (n ≥ 4). Blots were stripped and immunoblotted for integrin-β1 and β-actin. B: membrane expression of ASIC-1 was significantly inhibited in KD cells compared with Scr cells when α-actinin-4 was stably knocked down (n ≥ 4). Normalized densitometry shows membrane ASIC-1 and integrin-β1 in KD and Scr cells. *P < 0.05.
An α-actinin-binding site in the COOH terminus of ASIC-1 is essential for surface expression of ASIC-1 and its association with integrin-β1.
A putative binding site for α-actinin on the COOH terminus of ASIC-1 has been identified (42). To determine whether this COOH-terminal binding motif was required for the interaction between α-actinin-1 and -4 and ASIC-1 in glioma cells, we established stable cell lines expressing ASIC-1-GFP containing a mutation in the binding motif (from 484LSLDDVK490 to 484LSADAVA490) as previously described (42). The effect of mutation of the α-actinin-binding motif was first examined by determining the surface expression of ASIC-1 and integrin-β1. As shown in Fig. 10, we found that the expression of ASIC-1 at the surface of D54MG cells was significantly decreased (by 93 ± 10%, n ≥ 4), while the membrane integrin-β1 level was unchanged, in cells transfected with ASIC-1-GFP containing the COOH-terminal mutation. Similar results were obtained in U373 cells (Fig. 10, C and D). This lack of surface expression was not due to transcriptional or translational failure, as protein at the correct size for ASIC-1-GFP was easily detectable in total lysates using anti-GFP antibodies.
Fig. 10.
Putative binding site for α-actinin on the COOH terminus of ASIC-1 is required for surface expression. A and B: surface expression of ASIC-1 was significantly inhibited in D54MG cells expressing the mutant construct compared with wild-type D54MG cells, while membrane integrin-β1 level was unchanged (n ≥ 4). Blots were stripped and reblotted for integrin-β1 and β-actin. C and D: similar results in U373 cells, where mutation of the COOH terminus of ASIC-1 significantly reduced surface expression of ASIC-1. ***P < 0.001.
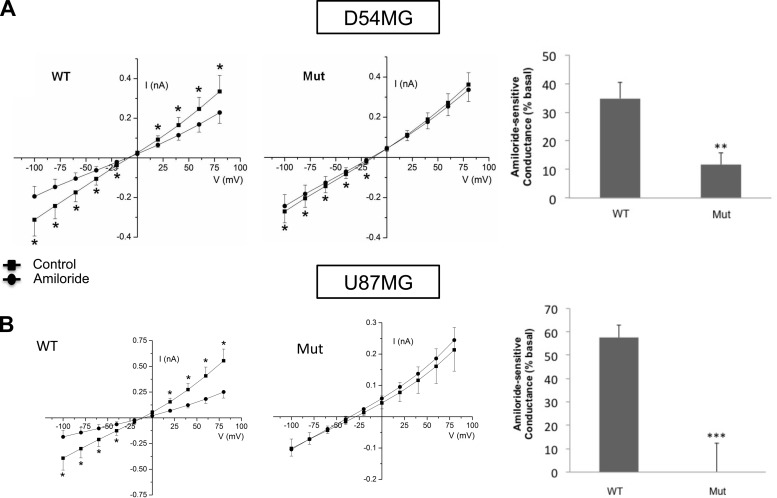
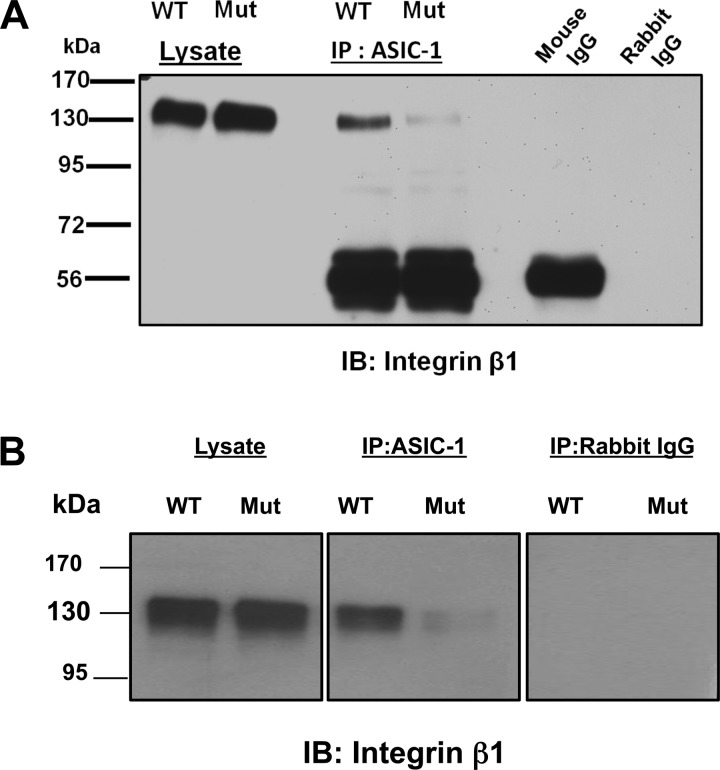
Consistent with these observations, we found a significant attenuation of amiloride-sensitive whole cell conductance in ASIC-1 mutant cells compared with wild-type ASIC-1-overexpressing D54MG [34.82 ± 5.74%, n = 7 (control); 11.6 ± 4.27%, n = 9, (mutant)] and U87 [57.54 ± 5.29%, n = 7 (control); −0.61 ± 12.37%, n = 6, (mutant)] cells (Fig. 11). As the mutation at the COOH terminus affected the membrane expression of ASIC-1, we sought to determine if the migration properties of these cells would be affected. Using a Transwell migration assay, we also observed a significant decrease (by 43 ± 13%, n = 4) in migration in D54MG cells expressing the mutant ASIC-1 construct, consistent with our earlier observations that a functional amiloride-sensitive channel is required for glioma cell migration (26, 41, 47) (data not shown). As knockdown of α-actinins in glioma cells prevented the functional association of ASIC-1 with integrin-β1, we next wanted to determine if this binding motif was required for the coimmunoprecipitation between ASIC-1 and integrin-β1. As shown in Fig. 12, mutation of the three amino acids on the binding motif on the COOH terminus of ASIC-1 essentially eliminated the coimmunoprecipitation of ASIC-1 and integrin-β1.
Fig. 11.
Deletion of the α-actinin-binding site in ASIC-1 attenuates amiloride-sensitive currents. A and B: current-voltage relationship shows significant attenuation of amiloride-sensitive whole cell conductance in D54MG and U87MG cells expressing mutation of the α-actinin-binding site compared with ASIC-1-overexpressing (WT) cells (n ≥ 7 in each case). **P < 0.01; ***P < 0.001 by Student's t-test.
Fig. 12.
Deletion of the α-actinin-binding site in ASIC-1 prevents coimmunprecipitation of ASIC-1 and integrin-β1. Immunoprecipitation of lysates from D54MG (A) and U373 (B) cells with antibodies directed against ASIC-1 and immunoblotting for integrin-β1 showed significant inhibition of association of ASIC-1 with integrin-β1 in the mutant cells (n ≥ 4).
Inhibition of actinin binding decreases phosphorylation of ERK1/2.
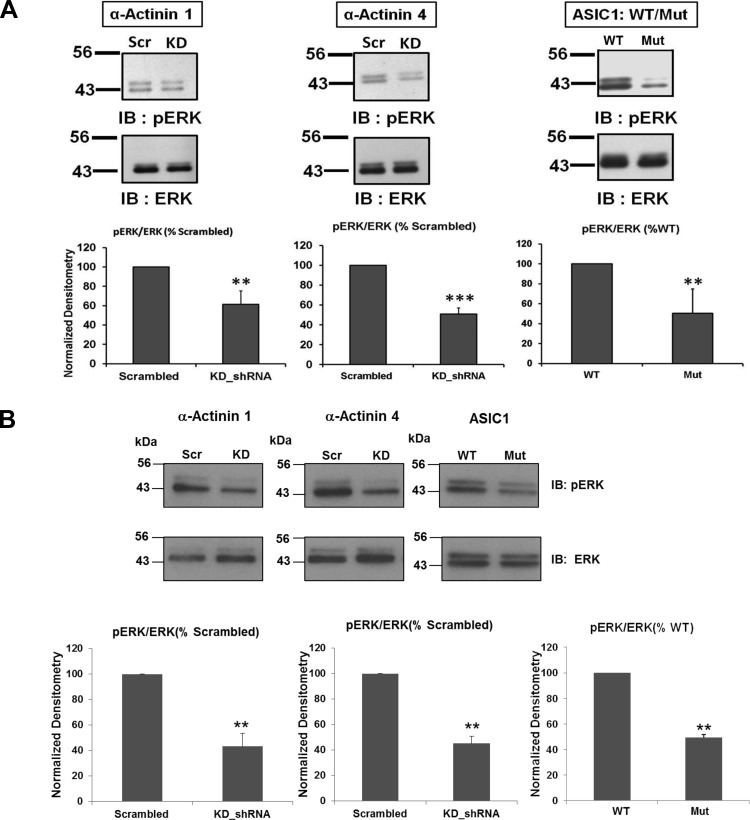
As we previously found that knockdown of ASIC-1 decreased phosphorylation of ERK1/2 (41), we wanted to explore whether knockdown of α-actinin or mutation of the α-actinin-binding site on ASIC-1 also reduced phosphorylation of ERK1/2. Phosphorylation of ERK1/2 was significantly downregulated by knockdown of actinin-1 (by 39 ± 14%, n ≥ 3) and actinin-4 (49 ± 6%, n ≥ 3; Fig. 13). Similarly, mutation of the α-actinin-binding site in ASIC-1 also reduced ERK1/2 phosphorylation by 50 ± 24% (n ≥ 6; Fig. 13).
Fig. 13.
Inhibition of ERK1/2 phosphorylation. Immunoblot analysis of lysates from D54MG (A) and U373 (B) cells stably transfected with shRNA for scrambled or KD shRNA for α-actinin-1 or -4 or transfected with the ASIC-1 COOH-terminal mutant. Blots were probed for phosphorylated ERK1/2 and then stripped and reprobed for total ERK1/2 (n ≥ 3). Phosphorylation of ERK was significantly inhibited in each case compared with cells expressing WT ASIC-1 (n ≥ 6). **P < 0.01; ***P < 0.001.
DISCUSSION
The cross-clade ASIC-1/α/γ-ENaC/Deg channel complex that we have described is associated with migration and proliferation in gliomas (7, 26, 47). These studies are consistent with those in other cell systems, such as vascular smooth muscle cells (22, 23), trophoblasts (19), and corneal epithelium (15), that have shown a role for ENaC and ASIC proteins in cell migration. We previously showed that inhibition of the amiloride-sensitive current slowed progression of glioma cells through the cell cycle, most likely as a result of inhibition of ERK1/2 phosphorylation and subsequent accumulation of the cyclin-dependent kinase inhibitor proteins p21Cip1 and p27Kip1 (41). We therefore sought to determine how changes in ion channel activity at the membrane could be transduced to the MAPK pathway. One candidate for the link between the channel and downstream signaling events was integrin-β1. Integrins regulate numerous cellular activities, including cell proliferation, migration, and invasion. Activation of integrins by binding to components of the ECM results in the formation of large multiprotein signaling complexes at the plasma membrane, cross-linked to the cytoskeleton (24). At the same time, downstream signaling pathways are activated as a consequence of recruitment of intermediates to the complex (46, 49).
Our present results support a novel interaction between integrin-β1 and the ASIC-1 component of the glioma cation channel. The loss of both current and surface expression of the ASIC-1 subunit following knockdown of integrin-β1 expression suggests that this integrin is required to maintain expression of the channel at the plasma membrane. The fibronectin-dependent increase in surface expression of ASIC-1 only in cells expressing integrin-β1 is also consistent with the concept that activation of the integrin is required. However, because none of the glioma cation channel components contain an RGD motif [unlike, for example, the G protein-coupled inwardly rectifying K+ channels (33)], the interaction between the glioma channel and the integrin was most likely indirect.
The formation and retention of ion channel-containing complexes within the plasma membrane domain are governed by interactions with other membrane proteins, including the actin cytoskeleton. Evidence supporting an interaction between cytoskeletal proteins and ENaC has come from a number of studies showing acute activation of Na+ channels by depolymerization of actin filaments with cytochalasins or by addition of monomeric or filamentous actin to excised membrane patches (10, 11). Studies in planar lipid bilayers, heterologously expressing Madin-Darby canine kidney cells, and Xenopus oocyte expression systems have shown that cytoskeletal proteins can modify ENaC gating and behavior (5, 6, 31). Short F-actin filaments have also been shown to bind directly and specifically to the COOH terminus of α-ENaC (31). In addition to actin, several other cytoskeletal-associated proteins, including filamin (48), cortactin (25), and intermediate filaments, have been proposed to interact with ENaC (12). Similar effects of actin on ASIC-1 have not been reported. However, Schnizler et al. (42) showed that α-actinin could modify the behavior of acid-sensitive ASIC-1 channels in heterologously expressing cells and cortical neurons. Coexpression of ASIC-1 with α-actinin-4, but not α-actinin-1, reduced the current density and increased the pH sensitivity without affecting the surface expression of the channel. In hippocampal neurons, simultaneous knockdown of α-actinin-1, -2, and -4 increased current density and reduced pH sensitivity of ASIC-1 (42).
In the present study, stable knockdown of α-actinin-1 and -4 or mutation of the putative actinin-binding site of ASIC-1 attenuated the whole cell conductance by reducing the membrane localization of ASIC-1. The more dramatic results in the glioma cells than the human embryonic kidney (HEK) cells and neurons used by Schnizler et al. (42) may reflect differences between the cross-clade heteromeric channel complex formed by ASIC-1/α/γ-ENaC and the homomeric ASIC-1 channel expressed in the HEK cells. Both muscle (subunits 2 and 3) and neuronal (subunits 1 and 4) forms of α-actinin share a common structure with functionally distinct domains (44). The neuronal α-actinins have been implicated in anchoring ion channels to the cytoskeleton. For example, cell surface expression of the metabotropic glutamate receptor type 5b is dependent on its binding with α-actinin-1 via its COOH-terminal domain, whereas mutation of the actin-binding site of α-actinin-1 inhibited the membrane expression of this receptor, suggesting a requirement for the actin cytoskeleton for proper membrane localization (9). Integrin-β1 has also been shown to interact with α-actinin, and interaction between these two molecules is critical in the development of focal adhesions required for cell migration (17, 27, 40).
After determining the association of ASIC-1 and integrin-β1 with α-actinin (Fig. 6A), we found that stable knockdown of α-actinin-1 or -4 attenuated the glioma-specific current (Fig. 7B). Interestingly, current was more effectively reduced by knockdown of actinin-1 than by knockdown of actinin-4, whereas knockdown of actinin-4 seemed to have a greater effect on the interaction between ASIC-1 and the integrin. These data suggest that actinin-1 may directly and positively affect the glioma channel, in addition to any role in cross-linking the channel to the cytoskeleton, and that actinin-4 may be required for the correct distribution of integrin-β1. Loss of actinin-4 may also be compensated by actinin-1, and/or loss of actinin-1 may cause internalization of the channel. These data are also consistent with a previous report that actinin-4 was more closely associated with the cytoskeleton in glioma cells than was actinin-1 (38). The role of α-actinin in membrane expression of ASIC-1 was further supported by the reduction of surface ASIC-1 expression after the mutation of a putative α-actinin-binding site located at the COOH terminus of ASIC-1 (Fig. 9).
The data demonstrating that phosphorylation of ERK1/2 was reduced in cells that lacked surface expression of ASIC-1 (Fig. 12) also clearly support our previous findings that the glioma cation conductance can influence phosphorylation of this kinase. Cell adhesion to ECM proteins by binding of integrins at the cell surface is known to activate the MAPK signaling pathway and involves several signaling molecules forming a macromolecular complex with other cytoskeletal proteins, including the α-actinins (18). α-Actinin can also stably interact with ERK via its calponin homology domain to retain ERK2 in the cytoplasm (16, 28). Actinin may therefore provide a link between ASIC-1 and ERK1/2. ERK1/2 phosphorylation is a key signaling process controlling migration and proliferation of tumor cells, including gliomas (21, 32, 34), and this kinase is also known to regulate several ion channels, including ENaC (45, 50). Alternately, loss of channel surface localization could also modify integrin phosphorylation, thereby reducing cell migration.
In summary, our data suggest that the association of ion channel subunit, integrin, and cytoskeletal protein is required to maintain the amiloride-sensitive glioma cell cation current. This study supports the association of ASIC-1 with integrin-β1 in glioma cells and shows that the association of ASIC-1 with integrin-β1 is dependent on the presence of α-actinin. More than 50 proteins are thought to interact with integrins at the cell surface to form multiprotein signaling complexes that regulate cell migration, adhesion, and proliferation. Similarly, ENaC has been shown to participate in membrane signaling complexes. It is therefore plausible that the glioma cation channel may also be part of such a complex. The most parsimonious interpretation of our data is that the cation channel couples to the cytoskeleton via binding of α-actinin to the COOH terminus of ASIC-1; α-actinin serves a similar role in linking integrin-β1 to the cytoskeleton, thus enabling the coimmunoprecipitation of these components. The loss of ASIC-1 surface expression under conditions of integrin-β1 or α-actinin knockdown or mutation of the ASIC-1 actinin-binding site may reflect a failure of ASIC-1 trafficking to the membrane or increased degradation as a misfolded protein. Additional studies are required to distinguish between these different possibilities.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-37206.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.R., C.M.M., and C.M.F. developed the concept and designed the research; A.K.R., Z.L., and C.M.M. performed the experiments; A.K.R., Z.L., C.M.M., and C.M.F. analyzed the data; A.K.R., Z.L., C.M.M., and C.M.F. interpreted the results of the experiments; A.K.R., Z.L., C.M.M., and C.M.F. prepared the figures; A.K.R., Z.L., C.M.M., and C.M.F. drafted the manuscript; A.K.R., C.M.M., and C.M.F. edited and revised the manuscript; A.K.R., Z.L., C.M.M., and C.M.F. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Melissa McCarthy for excellent cell culture assistance and Drs. Edlira Clark, Bakhrom Berdiev, Niren Kapoor, Sandeep Chand Chaudhary, Bhaskar Roy, and Yawar Qadri for helpful discussions.
Present address of A. K. Rooj: Dept. of Neurosurgery, Brigham and Women's Hospital, Harvard Medical School, Boston MA 02115.
Footnotes
On the basis of an American Type Culture Collection statement, there is a high likelihood that U251 and U373MG are identical cell lines. Thus, U251 cells have been used in only a limited number of experiments. For further information see http://www.atcc.org/Products/Cells_and_Microorganisms/Cell_Lines/Misidentified_Cell_Lines.as.
REFERENCES
- 1.Akaike N, Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol 44: 433–473, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Arcangeli A, Becchetti A, Mannini A, Mugnai G, De Filippi P, Tarone G, Del Bene MR, Barletta E, Wanke E, Olivotto M. Integrin-mediated neurite outgrowth in neuroblastoma cells depends on the activation of potassium channels. J Cell Biol 122: 1131–1143, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artym VV, Petty HR. Molecular proximity of Kv1.3 voltage-gated potassium channels and β1-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J Gen Physiol 120: 29–37, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becchetti A, Arcangeli A, Del Bene MR, Olivotto M, Wanke E. Response to fibronectin-integrin interaction in leukaemia cells: delayed enhancing of a K+ current. Proc R Soc Lond B Biol Sci 248: 235–240, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Berdiev BK, Latorre R, Benos DJ, Ismailov II. Actin modifies Ca2+ block of epithelial Na+ channels in planar lipid bilayers. Biophys J 80: 2176–2186, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berdiev BK, Prat AG, Cantiello HF, Ausiello DA, Fuller CM, Jovov B, Benos DJ, Ismailov II. Regulation of epithelial sodium channels by short actin filaments. J Biol Chem 271: 17704–17710, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem 278: 15023–15034, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bubien JK, Keeton DA, Fuller CM, Gillespie GY, Reddy AT, Mapstone TB, Benos DJ. Malignant human gliomas express an amiloride-sensitive Na+ conductance. Am J Physiol Cell Physiol 276: C1405–C1410, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Cabello N, Remelli R, Canela L, Soriguera A, Mallol J, Canela EI, Robbins MJ, Lluis C, Franco R, McIlhinney RA, Ciruela F. Actin-binding protein α-actinin-1 interacts with the metabotropic glutamate receptor type 5b and modulates the cell surface expression and function of the receptor. J Biol Chem 282: 12143–12153, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Cantiello HF. Role of the actin cytoskeleton on epithelial Na+ channel regulation. Kidney Int 48: 970–984, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Cantiello HF, Stow JL, Prat AG, Ausiello DA. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol Cell Physiol 261: C882–C888, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Cerecedo D, Martinez-Vieyra I, Alonso-Rangel L, Benitez-Cardoza C, Ortega A. Epithelial sodium channel modulates platelet collagen activation. Eur J Cell Biol 93: 127–136, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Chao JT, Gui P, Zamponi GW, Davis GE, Davis MJ. Spatial association of the Cav1.2 calcium channel with α5β1-integrin. Am J Physiol Cell Physiol 300: C477–C489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherubini A, Hofmann G, Pillozzi S, Guasti L, Crociani O, Cilia E, Di Stefano P, Degani S, Balzi M, Olivotto M, Wanke E, Becchetti A, Defilippi P, Wymore R, Arcangeli A. Human ether-à-go-go-related gene 1 channels are physically linked to β1-integrins and modulate adhesion-dependent signaling. Mol Biol Cell 16: 2972–2983, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chifflet S, Hernandez JA, Grasso S. A possible role for membrane depolarization in epithelial wound healing. Am J Physiol Cell Physiol 288: C1420–C1430, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Christerson LB, Vanderbilt CA, Cobb MH. MEKK1 interacts with α-actinin and localizes to stress fibers and focal adhesions. Cell Motil Cytoskeleton 43: 186–198, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Craig DH, Zhang J, Basson MD. Cytoskeletal signaling by way of α-actinin-1 mediates ERK1/2 activation by repetitive deformation in human Caco2 intestinal epithelial cells. Am J Surg 194: 618–622, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Abaco GM, Kaye AH. Integrins: molecular determinants of glioma invasion. J Clin Neurosci 14: 1041–1048, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Del Monaco SM, Marino GI, Assef YA, Damiano AE, Kotsias BA. Cell migration in BeWo cells and the role of epithelial sodium channels. J Membr Biol 232: 1–13, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10: 9–22, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glassmann A, Reichmann K, Scheffler B, Glas M, Veit N, Probstmeier R. Pharmacological targeting of the constitutively activated MEK/MAPK-dependent signaling pathway in glioma cells inhibits cell proliferation and migration. Int J Oncol 45: 2587–2595, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Grifoni SC, Gannon KP, Stec DE, Drummond HA. ENaC proteins contribute to VSMC migration. Am J Physiol Heart Circ Physiol 291: H3076–H3086, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res 75: 202–210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer 2: 91–100, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Ilatovskaya DV, Pavlov TS, Levchenko V, Negulyaev YA, Staruschenko A. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J 25: 2688–2699, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem 284: 24526–24541, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly DF, Taylor KA. Identification of the β1-integrin binding site on α-actinin by cryoelectron microscopy. J Struct Biol 149: 290–302, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Leinweber BD, Leavis PC, Grabarek Z, Wang CL, Morgan KG. Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. Biochem J 344: 117–123, 1999. [PMC free article] [PubMed] [Google Scholar]
- 29.Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, Attali B, Lider O. Extracellular K+ and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and β1-integrins. J Exp Med 191: 1167–1176, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196: 395–406, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzochi C, Bubien JK, Smith PR, Benos DJ. The carboxyl terminus of the α-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J Biol Chem 281: 6528–6538, 2006. [DOI] [PubMed] [Google Scholar]
- 32.McLendon RE, Turner K, Perkinson K, Rich J. Second messenger systems in human gliomas. Arch Pathol Lab Med 131: 1585–1590, 2007. [DOI] [PubMed] [Google Scholar]
- 33.McPhee JC, Dang YL, Davidson N, Lester HA. Evidence for a functional interaction between integrins and G protein-activated inward rectifier K+ channels. J Biol Chem 273: 34696–34702, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Nakabayashi H, Shimizu K. HA1077, a Rho kinase inhibitor, suppresses glioma-induced angiogenesis by targeting the Rho-ROCK and the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signal pathways. Cancer Sci 102: 393–399, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Otey CA, Pavalko FM, Burridge K. An interaction between α-actinin and the β1-integrin subunit in vitro. J Cell Biol 111: 721–729, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piao Y, Lu L, de Groot J. AMPA receptors promote perivascular glioma invasion via β1-integrin-dependent adhesion to the extracellular matrix. Neuro Oncol 11: 260–273, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillozzi S, Arcangeli A. Physical and functional interaction between integrins and hERG1 channels in cancer cells. Adv Exp Med Biol 674: 55–67, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Quick Q, Skalli O. α-Actinin 1 and α-actinin 4: contrasting roles in the survival, motility, and RhoA signaling of astrocytoma cells. Exp Cell Res 316: 1137–1147, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Riemenschneider MJ, Mueller W, Betensky RA, Mohapatra G, Louis DN. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am J Pathol 167: 1379–1387, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. Proc Natl Acad Sci USA 110: E1361–E1370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooj AK, McNicholas CM, Bartoszewski R, Bebok Z, Benos DJ, Fuller CM. Glioma-specific cation conductance regulates migration and cell cycle progression. J Biol Chem 287: 4053–4065, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnizler MK, Schnizler K, Zha XM, Hall DD, Wemmie JA, Hell JW, Welsh MJ. The cytoskeletal protein α-actinin regulates acid-sensing ion channel 1a through a C-terminal interaction. J Biol Chem 284: 2697–2705, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shakibaei M, Mobasheri A. β1-Integrins co-localize with Na,K-ATPase, epithelial sodium channels (ENaC) and voltage activated calcium channels (VACC) in mechanoreceptor complexes of mouse limb-bud chondrocytes. Histol Histopathol 18: 343–351, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sjoblom B, Salmazo A, Djinovic-Carugo K. α-Actinin structure and regulation. Cell Mol Life Sci 65: 2688–2701, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soundararajan R, Melters D, Shih IC, Wang J, Pearce D. Epithelial sodium channel regulated by differential composition of a signaling complex. Proc Natl Acad Sci USA 106: 7804–7809, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration—the actin connection. J Cell Sci 122: 199–206, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vila-Carriles WH, Kovacs GG, Jovov B, Zhou ZH, Pahwa AK, Colby G, Esimai O, Gillespie GY, Mapstone TB, Markert JM, Fuller CM, Bubien JK, Benos DJ. Surface expression of ASIC2 inhibits the amiloride-sensitive current and migration of glioma cells. J Biol Chem 281: 19220–19232, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Dai XQ, Li Q, Tuli J, Liang G, Li SS, Chen XZ. Filamin interacts with epithelial sodium channel and inhibits its channel function. J Biol Chem 288: 264–273, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wehrle-Haller B. Structure and function of focal adhesions. Curr Opin Cell Biol 24: 116–124, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's β- and γ-subunit. J Biol Chem 281: 9859–9868, 2006. [DOI] [PubMed] [Google Scholar]