Abstract
Cystic fibrosis (CF) has a profound impact on airway physiology. Accumulating evidence suggests that intercellular junctions are impaired in CF. We examined changes to CF transmembrane conductance regulator (CFTR) function, tight junctions, and gap junctions in NuLi-1 (CFTRwt/wt) and CuFi-5 (CFTRΔF508/ΔF508) cells. Cells were studied at air-liquid interface (ALI) and compared with primary human bronchial epithelial cells. On the basis of fluorescent lectin binding, the phenotype of the NuLi-1 and CuFi-5 cells at week 8 resembled that of serous, glycoprotein-rich airway cells. After week 7, CuFi-5 cells possessed 130% of the epithelial Na+ channel activity and 17% of the CFTR activity of NuLi-1 cells. In both cell types, expression levels of CFTR were comparable to those in primary airway epithelia. Transepithelial resistance of NuLi-1 and CuFi-5 cells stabilized during maturation in ALI culture, with significantly lower transepithelial resistance for CuFi-5 than NuLi-1 cells. We also found that F508del CFTR negatively affects gap junction function in the airway. NuLi-1 and CuFi-5 cells express the connexins Cx43 and Cx26. While both connexins were properly trafficked by NuLi-1 cells, Cx43 was mistrafficked by CuFi-5 cells. Cx43 trafficking was rescued in CuFi-5 cells treated with 4-phenylbutyric acid (4-PBA), as assessed by intracellular dye transfer. 4-PBA-treated CuFi-5 cells also exhibited an increase in forskolin-induced CFTR-mediated currents. The Cx43 trafficking defect was confirmed using IB3-1 cells and found to be corrected by 4-PBA treatment. These data support the use of NuLi-1 and CuFi-5 cells to examine the effects of F508del CFTR expression on tight junction and gap junction function in the context of serous human airway cells.
Keywords: normal lung, cystic fibrosis, cell model, differentiation, air-liquid culture, gap junction, connexin
cystic fibrosis (CF) is one of the most common genetic disorders affecting primarily Caucasians. CF causes defects in secretory epithelia throughout the body but has particular impact on lung function (54). Over 70% of patients with CF have at least one chromosomal copy of F508del CFTR [loss of the amino acid residue F508 in the CF transmembrane conductance regulator (CFTR) protein], making it an attractive disease target that has significant impact among 30,000 American patients and 70,000 patients world-wide (52).
The airway consists of a complex mosaic of cell types, including ciliated and nonciliated columnar, goblet, club, basal, and neuroendocrine epithelial cells. Cells are arranged in a pseudostratified epithelium that lines the airway, and cell type composition differs with distance from the trachea, advancing through the bronchi and bronchioles (47, 67). These cells function as a biological physical barrier that protects the lower airway from pathogens (12, 62). Key components of this barrier are the tight junctions, which are intercellular contacts that form the physical basis for regulating paracellular permeability of water, ions, and metabolites between cells. Tight junctions thereby play a critical role in the separation of solutes and ions between the apical and basolateral faces of the epithelial cell layer (43).
In vitro airway model systems have been used to study several aspects of the molecular regulation of tight junctions (61). Cells in vitro have been used to show that airway epithelial cells expressing wild-type (WT) CFTR exhibit higher transepithelial resistance (TER) than CFTR-null cells or cells expressing F508del CFTR, supporting a role for CFTR and tight junctions regulating ion transport (7, 34, 44, 66). However, conflicting data in the literature suggesting that TER is more sensitive to the selected cell type than to expression of normal or abnormal CFTR (40) underscore the point that cell model selection is critical for interpretable experiments.
Another class of intercellular contacts, called gap junctions, are composed of channel-forming proteins known as connexins. Gap junctions mediate cellular communication by forming intercellular channels that provide a means for direct diffusion of ions and metabolites between juxtaposed cells in a tissue. In the airway, gap junctions contribute to Ca2+ wave propagation (3), ciliary beat frequency (35), coordinated secretion of airway surface liquid (46) and mucous proteins (4), and innate immune functions of the epithelial layer (32, 38, 67). CFTR has been described to interact with connexins to regulate protein trafficking and ion regulation (6). However, the means by which gap junctions are impaired in the CF airway are not well understood. Critical to understanding the effects of disease-related gap junction biology is the importance of finding cell models where disease physiology is retained and comparable to that observed in primary cells. In particular, cell models should express physiological levels of CFTR, as well as native connexins, which reflect the appropriate cell phenotype.
As mentioned above, each distinct area of the airway contains numerous cell types. As a result, tissue samples from different airway segments produce cell cultures with distinct characteristics (47). Among the various cell line models used for CF research, only a few of the nonciliated upper airway epithelial cell lines originate from the human airway. There is an inherent advantage to using near-physiological, noncancerous airway epithelial cells to elucidate the physiological roles of all cell types in the healthy human airway. However, the majority of airway research has been done using model cells isolated from cancerous tissue, models from viral-transduced overexpression, or models focused on ciliated cells that may obscure normal cell functions of nonciliated cells in the airway.
Procurement of high-quality, bio-similar primary cell samples is frequently challenging and requires knowledge of how to characterize, harvest, isolate, expand, and use them for experimentation. By contrast, cell lines of cancerous origin are easier to use in experiments; however, they often obscure normal, noncancerous disease physiology because of chromosomal instability and/or interference with normal signal transduction. This can be demonstrated with studies observing metabolism related to normal, noncancerous, and cancerous cell models (49). Cell lines such as the human bronchiolar epithelial (hBE) cell variants CFBE41o- and 16HBE14o- can vary significantly in target gene and protein expression, which confounds interpretation of results obtained using these cells compared with in vitro primary human airway cells (16, 17, 40). Therefore, well-controlled and bio-similar noncancerous cell models are ideal for research related to noncancerous diseases and to normal cell function and physiology.
One method of cell line creation that blends the advantages of primary cell phenotype with the utility of a cell line involves the use of telomerase and human papilloma virus-16 E6 and E7 cell cycle effector proteins for cell immortalization (2, 41). Telomerase acts to extend the ends of the chromosomes, preventing cellular crisis, while E6 and E7 degrade p53 and retinoblastoma protein, respectively, allowing cell cycling to continue. This immortalization method may provide a unique opportunity to study cellular processes that are obscured by cancer-related physiological processes resulting from the use of cancer-derived cell line models.
The NuLi and CuFi cell lines are telomerase-immortalized human airway epithelial cells that represent a system with features such as genomic stability and known age, sex, and donor genotype (69). NuLi and CuFi cells are particularly advantageous, because they are fairly easy to propagate in culture and, additionally, have the ability to differentiate when cultured at an air-liquid interface (ALI) on Transwell permeable supports. These cells also appear to exhibit near-physiological levels of expression for CFTR mRNA and protein (69), making them particularly well suited to CF research. Despite what is known about NuLi and CuFi cells, only a handful of studies have utilized these human airway cell models. Moreover, comparison between these studies is difficult because of varying maturation conditions. Several studies have used NuLi and CuFi cells differentiated on Transwell permeable supports at ALI for 4–6 wk (1, 19, 27, 37, 45, 57, 58, 63), although cells at ALI for 2–3 wk have also been used (5, 64, 69), as have cells on collagen-coated tissue culture plastic (8, 13, 25, 39, 57, 58).
Given that maturation time and culture conditions can have a significant effect on cell function, we phenotypically profiled the NuLi-1 (CFTRwt/wt) and CuFi-5 (CFTRΔF508/ΔF508) cell lines at weeks 3, 5, and 7 of ALI culture. We then focused on the utility of these cells for studying tight junctions and gap junctions. The electrophysiological profile and CFTR expression were also assessed at different times following ALI culture. Since primary cells are known to take weeks to months to fully differentiate, we compared the transcriptional and phenotypic changes that occurred with time in ALI culture in NuLi-1 and CuFi-5 cells with changes in primary patient-derived hBE cells from non-CF lungs (NhBE cells) and CF lungs homozygous for CFTRΔF508 (CFhBE cells). We found that NuLi-1 and CuFi-5 cells continue to differentiate in ALI culture and require 6–7 wk to fully stabilize, which has implications for their composition in culture and their proper optimization for use in airway research. We also posit that NuLi-1 and CuFi-5 cell lines are utilizable in gap junction functional studies, as we have demonstrated that expression of F508del CFTR in these cell lines impairs gap junction function.
MATERIALS AND METHODS
Cell lines and culture methods.
Established normal lung University of Iowa 1 (NuLi-1) and CF University of Iowa 5 (CuFi-5) cell lines (CRL-4011 and CRL-4016, respectively, American Type Culture Collection) were grown as described by Zabner et al. (69) with modifications described here. NuLi-1 cells are from a 36-yr-old nondiseased human male donor. CuFi-5 cells are from a 32-yr-old CFTRΔF508 homozygous human male donor. Growth repression was relieved via expression of both human telomerase reverse transcriptase and human papilloma virus-16 E6/E7 genes in both cell lines. Cells were grown on collagen-coated (60 μg/ml, human placental type IV; catalog no. C7521, Sigma-Aldrich) T75 flasks (catalog no. 353136, BD Corning) in bronchial epithelial growth medium (catalog no. CC-3170, Lonza; with all supplied supplements except gentamicin and amphotericin B) in a humidified HEPA-filtered cell culture incubator supplemented with 5% CO2. NuLi-1 and CuFi-5 cells between passages 5 and 17 were used. Proliferating cells (i.e., cells on plastic) were split once per week on Monday and fed bronchial epithelial growth medium on Monday, Wednesday, and Friday. NuLi-1 and CuFi-5 cells in 10 ml of medium were seeded onto plastic T75 flasks at 3.5 × 105 and 3.8 × 105 cells/flask, respectively, to obtain roughly equivalent confluence after 1 wk in culture. After trypsinization, cells were collected and seeded onto semipermeable filters in DMEM/F-12 medium (catalog no. 51445C, Sigma-Aldrich) containing 5% FBS (catalog no. S11550, Atlanta Biologicals). Transwell (catalog nos. 3450 and 3460, Corning) or Snapwell (catalog no. 3801, Corning) semipermeable supports were used to induce differentiation by seeding each collagen-coated support with 1.2 × 105 and 2.4 × 105 cells/cm2 for NuLi-1 and CuFi-5, respectively, to facilitate confluence within 1 wk. Cells were allowed to grow at liquid-liquid interface for 2 days in DMEM/F-12 medium + 5% FBS; then the medium was changed to bilateral DMEM/F-12 medium + 2% Ultroser G (catalog no. 15950-017, Crescent Chemical/Pall-BioSpera) “differentiation medium” until a confluent monolayer was achieved (∼5–7 days total). Then ALI culture was achieved by complete removal of the apical medium and replacement of the basolateral medium with fresh differentiation medium. At all phases of growth, medium was replaced every Monday, Wednesday, and Friday. We recommend use of cells after ≥7 wk of culture at ALI. Primary cells were obtained from Cystic Fibrosis Foundation Therapeutics and cultured in 2% Ultroser G according to their directions without changes.
Quantitative PCR.
mRNA was harvested from duplicate Transwell permeable supports containing each cell line, at the indicated time points, using the RNeasy Plus Mini Kit (catalog no. 74134, Qiagen). The isolated mRNA was then quantified with a spectrophotometer (NanoDrop, Thermo Scientific), and 1 μg of mRNA was used to generate cDNA using the iScript cDNA synthesis kit (catalog no. 170-8890, Bio-Rad) with random hexamer and poly(dT) primers. Quantitative RT-PCR was performed using a customized, validated, and commercially available 96-well plate assay (PrimePCR system, Bio-Rad) that contained lyophilized and validated quantitative PCR primer sets sufficient for 20 μl SYBR Green Taq (catalog no. 170-8882, Bio-Rad) assays with an annealing temperature of 60°C. Generated cDNA was diluted 1:10 with molecular biology-grade water and used for quantitative RT-PCR analysis with 2 μl of diluted cDNA sample per 20 μl of SYBR Green assay using an iQ5 iCycler multicolor detection system (Bio-Rad) with auto cycle threshold (CT) determination. In this analysis, CT of 35 for any amplicon is considered below the reasonable detection limit, reflecting undetectably low expression. Each assay contained internal controls and reference wells for inter- and intra-assay comparisons utilizing the normalized CT number and relative expression analysis. The related equations are as follows: ΔCT = CT(target gene) − CT[reference gene (ACTB)]; ΔΔCT = average ΔCT(NhBE) − individual ΔCT(CFhBE) or ΔCT(NuLi-1) or ΔCT(CuFi-5); and relative expression for each amplicon = 2−ΔCT, while relative fold expression for each amplicon = 2−ΔΔCT. Values are means ± SE of three biological replicates, unless otherwise noted.
Fluorescent lectin cell phenotype analysis.
Cells grown on Transwell permeable supports were placed into a 4°C refrigerator for 15 min, washed five times with ice-cold Ca2+/Mg2+-containing Dulbecco's phosphate-buffered saline (DPBS++), fixed with ice-cold 4% paraformaldehyde for 15 min at 4°C, quenched with 1 M glycine in DPBS++ for 5 min at room temperature, washed three times with DPBS++, and then incubated with different combinations of dye-coupled lectins and 4′,6-diaminido-2-phenylindole (DAPI) for 15 min at room temperature. The lectins and the cells they stain are as follows: jack fruit lectin-FITC (jacalin/Artocarpus integrifolia; catalog no. A3590-12C, US Biological) at 10 μg/ml, which labels secretory goblet cells; MPL lectin-Texas Red (Maclura pomifera; catalog no. 21761041-1, Bioworld) at 10 μg/ml, which labels club cells; peanut lectin-Alexa Fluor 594 (PNA/Arachus hypogaea; catalog no. L-32459, Life Technologies) at 1 μg/ml, which labels ciliated and columnar cells; and BSI-B4 lectin-FITC (Bandeiraea simplicifolia; catalog no. L2895, Sigma) at 10 μg/ml, which labels basal cells. All lectins were diluted in DPBS++ just prior to use, and stained cells were mounted in ProLong Gold Antifade with DAPI (catalog no. P36931, Invitrogen). Images were captured with an inverted fluorescence microscope (Olympus IX70) equipped with a Hamamatsu digital camera.
Antibodies, Western blotting, and immunofluorescence.
Cells grown on permeable supports were scraped into DPBS++ supplemented with Complete Mini tablet protease inhibitor cocktail (catalog no. 11836153001, Roche). Multiple permeable supports were combined for protein processing. Proteins were isolated by whole cell lysis with RIPA buffer (catalog no. 9806, Cell Signaling Technologies) with PMSF, NaF, and Na2VO3 according to the manufacturer's instructions, quantitated by bicinchoninic acid assay (catalog no. 23225, Pierce Thermo Scientific), and then mixed with reducing SDS-containing protein sample buffer. Twenty to 120 μg of protein from each cell line sample were loaded into TGX gels (Bio-Rad), run with Tris-glycine buffer, and transferred with the Trans-Blot turbo transfer system (Bio-Rad) according to the manufacturer's directions. For blot development, gels were transferred to nitrocellulose or low-fluorescence polyvinylidene difluoride, blocked with Odyssey blocking buffer (catalog no. 927-40000 or 927-50000, LI-COR), and used for two-color fluorescence imaging with the Odyssey Classic imager (LI-COR). Blots are presented as single-channel gray-scale images.
Antibodies used for Western blotting included CFTR at 1:1,000 dilution for 1 h at room temperature (mouse; CFF596, lot 596TJ03082013, University of North Carolina), actin at 1:20,000 dilution (mouse; catalog no. A5441, Sigma), Cx26 at 1:500 dilution (catalog no. 71-0500, Life Technologies), Cx43 at 1:5,000 dilution (catalog no. C6219, Sigma), zonula occludens 1 (ZO-1) at 1:500 dilution (catalog no. 33-9100, Life Technologies), claudin (Cldn)-1 at 1:500 dilution (catalog no. 37-4900, Life Technologies), and Cldn-4 at 1:500 dilution (catalog no. 32-9400, Life Technologies). Primary antibodies were diluted in a 1:1 mixture of DPBS++ and Odyssey blocking buffer with 0.2% (vol/vol) Tween 20. Fluorescent secondary antibodies (LI-COR) were as follows: goat anti-mouse 680RD, goat anti-mouse 800CW, goat anti-rabbit 680RD, and goat anti-rabbit 800CW, each diluted at 1:20,000 in a 1:1 mixture of DPBS++ and Odyssey blocking buffer with 0.2% (vol/vol) Tween 20.
Cells grown on permeable supports were fixed and immunolabeled accordingly with the antibodies described above. Cells were fixed in 4% paraformaldehyde in DPBS++ for 15 min at room temperature or 50:50 acetone-methanol for 2 min at room temperature, permeabilized with 0.5% Triton X-100, blocked in 1% bovine serum albumin in DPBS++ with 0.05% Tween 20 (PBT buffer) for 30 min at room temperature, and then incubated with primary antibodies overnight in 0.1% bovine serum albumin in PBT. For labeling of ZO-1 (1:200 dilution; catalog no. 40-2200, Invitrogen), cells were fixed in 100% methanol for 8 min at −20°C and labeled as described above. Fluorescent secondary antibodies conjugated to Alexa Fluor 488 or 594 or Texas Red (Jackson ImmunoResearch) were used at 1:1,000 dilution in PBT for 1 h at room temperature. Wash steps were performed with PBT buffer, and after the final wash, each filter was briefly rinsed in 70% ethanol prior to trimming and mounting in ProLong Gold Antifade reagent with DAPI. Permeable supports were imaged using a Nikon epifluorescence inverted microscope or a Zeiss 4 laser confocal microscope. Resulting images were processed using FUJI (NIH ImageJ).
TER measurements and Ussing chamber analysis.
TER was measured with a chopstick ohmmeter (Precision Instruments) in Ringer solution on the Monday of each week prior to feeding, for consistency. On the day of experimentation, cells were washed with DPBS++, and transepithelial currents were recorded using a voltage-clamp amplifier (model VCC-MC6, Warner Instruments), which is controlled using the Acquire and Analyze software provided by the manufacturer. Currents were recorded in a solution containing (in mM) 140 NaCl, 5 KCl, 0.36 K2HPO4, 0.44 KH2PO4, 1.3 CaCl2, 0.5 MgCl2, 4.2 HEPES, and 10 glucose, with pH adjusted to 7.4 with HCl. Prior to recording, voltage offset and fluid resistance compensations were adjusted according to the manufacturer's instructions. During recording, solutions were bubbled with 95 O2-5% CO2, and the chamber was heated to 37°C using a circulating water bath. The clamping paradigm consisted of ±5-mV voltage steps for 20 ms, cycling every second. In each experiment, epithelial Na+ channel (ENaC) current was blocked by addition of 10 μM amiloride to the apical chamber. Ca2+-activated Cl− channel activity was suppressed by apical application of 10 μM DIDS. CFTR currents were elicited with a basolateral application of 100 μM 3-isobutyl-1-methylxanthine (IBMX) + 10 μM forskolin (FSK) and blocked by apical application of 20 μM glycine hydrazide (GlyH)-101. Any remaining current was inhibited by basolateral application of 100 μM bumetanide. All reagents were obtained from EMD-Millipore. Apical-to-basolateral cation flow is shown as a negative current. Values are means ± SE. Data were analyzed using Prism v5.0 software.
Scrape-loading dye-transfer studies.
NuLi-1 and CuFi-5 cells were basolaterally treated with 0, 3, or 5 mM 4-phenylbutyric acid (4-PBA) for 24 h at 37°C. Cells were then vigorously washed with 300 μl of warmed (37°C) Ringer solution (with glucose) to remove loose cells, and four parallel scalpel scrapes were made. Dye solution (200 μl) consisting of 0.25% (wt/vol) calcein + 0.01% (wt/vol) Texas Red dextran (10 kDa), dissolved in Ringer solution and prewarmed to 37°C, was placed onto the washed apical surface. Transwell filters with apical dye were placed into prewarmed Ringer solution in a 100-mm tissue culture dish and placed into the incubator for labeling. After 15 min of labeling, the cells were removed from the incubator, dye was removed and discarded, and apical surfaces were washed 5–10 times with Ringer solution until liquid was clear (≤2 min). Transwell filters were then immediately imaged with U-MWIBA blue/green (band-pass 460–490 nm/dichroic mirror 505 nm/barrier 515–550 nm) 485-nm and U-MNG green/red (band pass 530–550 nm/dichroic mirror 570/barrier 590 to ≥800 nm) 594-nm filter packs for calcein and Texas Red dextran, respectively, as well as phase contrast. Dye transfer was quantified as average distance of dye transfer from the edge of the scrape to the background levels as assessed by gray-scale line scans, with a minimum intensity cutoff of 80–100 on a scale of 0–255. Statistical significance was assessed by two-tailed Student's t-test.
4-PBA treatment of IB3 cells.
IB3-1 cells were seeded onto glass coverslips for imaging. They were treated with 0 or 5 mM 4-PBA overnight at 37°C in MEM + 10% FBS. Cells were fixed with methanol-acetone for 2 min at room temperature, washed with DPBS++, and labeled as described above or analyzed by microinjection dye transfer.
Dye transfer via single-cell microinjection.
Dye-transfer in IB3-1 cells was studied using a method similar to that described by Koval et al. (30). Cells were cultured on glass coverslips 2 days before microinjection experiments. The coverslips were mounted in a tissue chamber (Medical Systems, Greenvale, NY) on an epifluorescence microscope, covered with culture medium, and maintained at 37°C in a 5% CO2 atmosphere. Cells were microinjected with solutions containing 2 mg/ml calcein in 200 mM KCl (Invitrogen) using 1,100-1,200 psi applied for 0.2 s with an Eppendorf Transjector (model 5246). At 5 min after injection, cells were visualized and photographed. A cell was scored as positive if it had a representative area with an average fluorescence intensity of ≥10% of the microinjected cell fluorescence intensity as determined with Image-Pro. Data are presented as number of dye-labeled cells. Values are means ± SE. Statistical significance was assessed by two-tailed Student's t-test.
RESULTS
Lectin phenotyping reveals that NuLi-1 and CuFi-5 are serous cells.
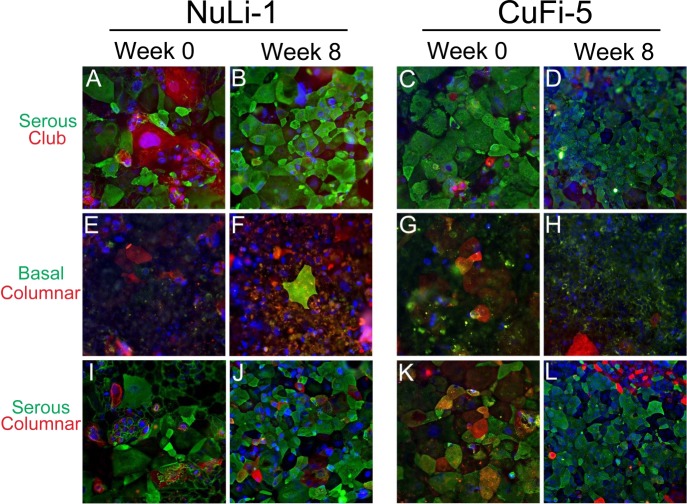
To assess the phenotype of the NuLi-1 and CuFi-5 cell models, carbohydrate-binding lectins were used to characterize cells on the basis of cell type-specific enrichment of surface carbohydrates, as previously described (11, 23, 24, 50, 59, 68). We analyzed NuLi-1 cells at initial ALI (week 0) and at week 8 of ALI culture to study the effects of differentiation time on epithelial cell type population. By lectin analysis, NuLi-1 and CuFi-5 cells display a predominantly pseudostratified serous cell phenotype, discriminated by a multinuclear cellular staining pattern characteristic of pseudostratified epithelia with intense staining by jacalin lectin (Fig. 1). However, not all cells stained at similar intensities, most likely due to variants in cell type and the abundance of glycoproteins on the cell surface. Furthermore, >80% of NuLi-1 and CuFi-5 cells stained positive for jacalin lectin, a goblet cell marker, suggesting that the cells are secretory cells most likely closely related to goblet or mucus-producing cells. Differences in the various phenotypes of lectin intensity with respect to cell size and maturation over the 8-wk ALI culture condition were also observed. At week 0, cells were uniformly large and the cell borders were diffuse. At week 8, cells took on a more regular shape, with defined cell borders and various apical cell sizes compared with the diffuse and larger cell types observed at week 0.
Fig. 1.
NuLi-1 and CuFi-5 cells display a serous cell phenotype at week 8 of air-liquid interface (ALI) culture. NuLi-1 and CuFi-5 cells display a predominantly pseudostratified serous cell phenotype according to lectin analysis, and apical cell surfaces of these cells preferentially bind lectins that associate with serous cell types, such as goblet and club cells. Cells were analyzed at initial shift to ALI (week 0) and at week 8 post-ALI. Cell type-specific sugar-binding lectins were used to phenotype cells. Lectins and the cells they stain were as follows: jack fruit lectin (jacalin/A. integrifolia), serous cells; MPL lectin (M. pomifera), club cells; peanut lectin (PNA, A. hypogaea), columnar cells; BSI-B4 lectin (B. simplicifolia), basal cells. A–D: cells labeled with jacalin (green, serous) and MPL (red, club). E–H: cells stained with BSI-B4 (green, basal) and PNA (red, columnar). I–L: cells stained with jacalin (green, serous) and PNA (red, columnar). Nuclei are stained with DAPI (blue). Not all cells stained at similar intensities, most likely due to specific characteristics of each cell type, such as abundance of sugars on the cell surface. Since at week 8 not all cells had comparable staining intensity, there may be multiple cell types, such as serous pseudostratified and columnar cells, within the cultures. Also note the various phenotypes and sizes of cells grown with submerged culture conditions at week 0 (A/C, E/G, and I/K) compared with week 8 (B/D, F/H, and J/L) post-ALI, where cells appear more disorganized at week 0 and more regular at week 8. Cells at passages 5–17 were used in this analysis. Magnification ×20 for all images.
NuLi-1 and CuFi-5 tight junctions require time to stabilize in ALI culture.
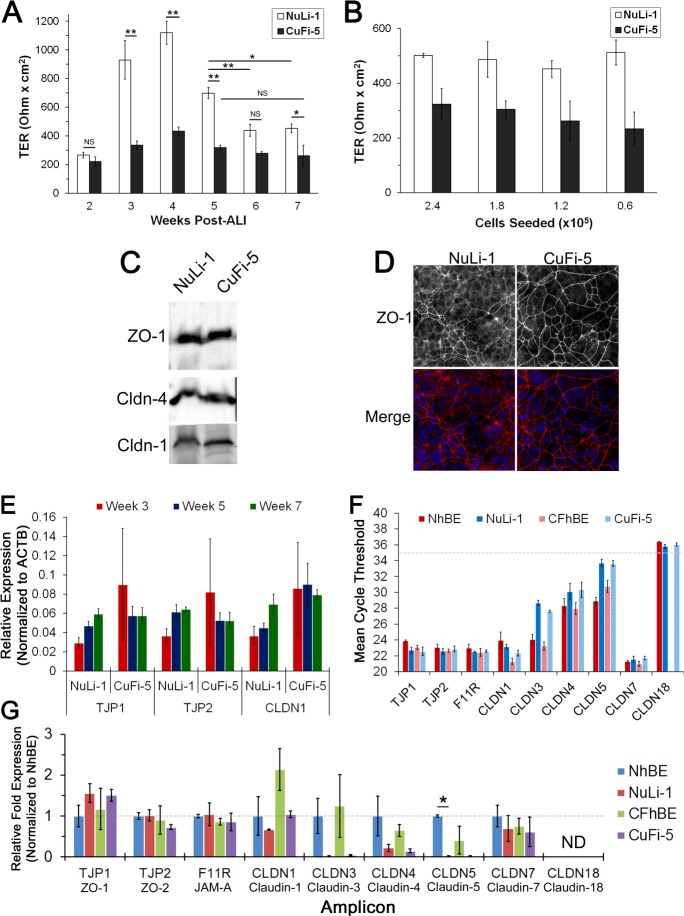
To assess when NuLi-1 and CuFi-5 cell models acquire tight junction barrier stability, we sampled the cell models over 7 wk of culture at ALI. Parameters that define barrier function, such as TER, electrophysiological properties, and tight junction protein gene expression, were measured. Measurements of the NuLi-1 and CuFi-5 cell lines in 2% Ultroser culture conditions revealed that TER changed over time. NuLi-1 cell TER was ∼270 Ω·cm2 at week 2, peaked at ∼1,100 Ω·cm2 at week 4, and stabilized at ∼450 Ω·cm2 at week 6, whereas CuFi-5 cell TER was ∼220 Ω·cm2 at week 2, peaked at ∼430 Ω·cm2 at week 4, and stabilized at ∼250 Ω·cm2 at week 5 (Fig. 2A). Notably, TER was consistently lower at all time points in F508del CFTR-expressing CuFi-5 than NuLi-1 cells. These changes were not dependent on plating densities, suggesting that they represent an inherent difference between the cell lines (Fig. 2B). Stable barrier formation was dependent on maturation time in culture (Fig. 2A) but independent of cell density (Fig. 2B).
Fig. 2.
NuLi-1 and CuFi-5 cell tight junctions change with time in culture. A: transepithelial resistance (TER) was measured at each week of ALI culture to assess overall barrier function of tight junctions. TER did not stabilize until weeks 4 and 6 of ALI culture for CuFi-5 and NuLi-1 cells, respectively. Data are from cells at passage 15 (2.4 × 105 cells/filter initial density). Values are means ± SE (n = 3 filters for each measurement). *P ≤ 0.05, **P ≤ 0.01 (by 1-way ANOVA with Bonferroni's correction); NS, no significant difference. B: TER of week 7 cells plated at various cell densities did not change significantly for NuLi-1 cells but differed slightly for CuFi-5 cells, where higher density correlated with higher TER. Data are from cells at passage 15. Values are means ± SD (n = 3 filters for each density). C: NuLi-1 and CuFi-5 cells express zonula occludens 1 (ZO-1), claudin (Cldn)-1, and Cldn-4 tight junction proteins as determined by immunoblotting. D: week 7 NuLi-1 and CuFi-5 cells were fixed and labeled for ZO-1 and stained with DAPI to demonstrate cell morphology, as well as continuity of the tight junction barrier. There are no noticeable differences in ZO-1 staining between NuLi-1 and CuFi-5 cells; however, the cells displayed a varied pseudostratified appearance as determined by multiple nuclei within a ZO-1-labeled cell border. Magnification ×20 for all images. E: 3 representative amplicons for tight junction protein (TJP) genes were compared with each other relative to calculated expression values for weeks 3, 5, and 7. Transcript abundances for ZO-1 (TJP1), ZO-2 (TJP2), and Cldn-1 (CLDN1) increased over time for NuLi-1, but not CuFi-5, cells. Data from cells at passages 7–10 are presented as relative expression normalized to β-actin (ACTB). Values are means ± SE [n = 2 filters from each of 3 passages (6 filters total) for each time point]. F: to assess abundance of transcripts, amplicon cycle threshold (CT) values were averaged and plotted to gauge overall level of expression, where a low CT value indicates high mRNA expression. CT ≥35 (dashed line) is considered unreliable for detection by PrimePCR assay and, therefore, treated as undetected transcript. No transcript was detectable in 40 cycles for CLDN18 in human bronchial epithelial (hBE) cells from CF lungs homozygous for CFTRΔF508 (CFhBE cells). NhBE cells, hBE cells from non-CF lungs. Data from cells at passages 7–10 are presented as average nonnormalized CT values [n = 2 filters from each of 3 passages (6 filters total) for each time point]. G: expression levels of each gene amplicon at week 7 of ALI culture relative to NhBE. Amplicons for Cldn-18 (CLDN18) were undetected or not reliable and, therefore, not included in this analysis. Data from cells at passages 7–10 are presented as normalized, averaged relative fold expression. Values are means ± SE [n = 2 filters from each of 3 passages (6 filters total) for each time point]. *P < 0.05 (by ANOVA with Bonferroni's correction); ND, not detected.
NuLi-1 and CuFi-5 cells express airway-specific tight junction proteins comparable to primary hBE cells.
Immunoblotting showed that NuLi-1 and CuFi-5 cells at week 7 express the tight junction proteins ZO-1, Cldn-1, and Cldn-4 (Fig. 2C). Immunofluorescence of ZO-1 confirmed the presence of tight junctions in both cell types at week 7 (Fig. 2D). Gene expression analysis of tight junction proteins (TJP) showed increasing gene expression of ZO-1 (TJP1), ZO-2 (TJP2), and Cldn-1 (CLDN1) beginning at week 3 in NuLi-1 cells, whereas CuFi-5 cells exhibited no significant differences in gene expression over all time points (Fig. 2E). Interestingly, at week 7 of ALI culture, the gene expression profiles of NuLi-1 and CuFi-5 cells were remarkably similar to those of ciliated NhBE and CFhBE primary human airway cells (Fig. 2F). However, as indicated by higher CT values, expression levels for Cldn-3, Cldn-4, and Cldn-5 were lower for NuLi-1 and CuFi-5 than NhBE and CFhBE cells. Since the reliable detection limit for the PrimePCR assay system is 35 cycles of amplification (CT = 35), Cldn-18 is considered below detectable levels. We did not expect to find Cldn-18 in these airway cells, since it is found mainly in alveolar epithelia, and not in the airway (28). We used NhBE gene expression as a benchmark and calculated the relative fold expression for each of the tight junction gene amplicons detected (Fig. 2G). For all the claudins, except Cldn-7, mRNA expression was less by NuLi-1 and CuFi-5 cells than by the equivalent primary cell type. Despite low levels of Cldn-1 and Cldn-4 mRNA expression by NuLi-1 and CuFi-5 cells, protein expression for these two claudins was readily detected by immunoblotting (Fig. 2C), and the cells formed high-resistance paracellular barriers (Fig. 2A).
CFTR expression and function of NuLi-1 and CuFi-5 cells develop over time in culture.
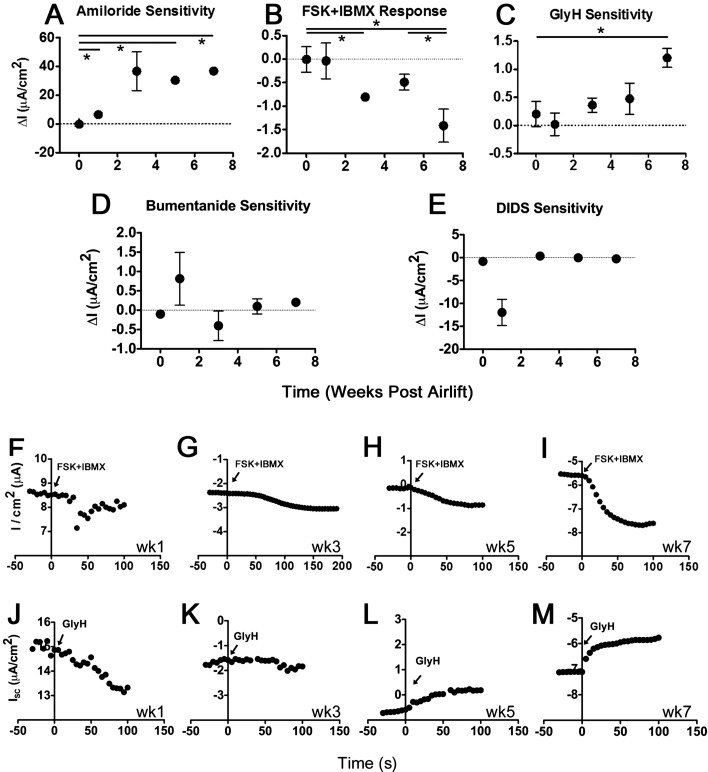
We found that ENaC and CFTR activity differ between the NuLi-1 and CuFi-5 cell lines and primary airway cells (69). These differences in electrophysiological function and CFTR gene and protein levels were measured using Ussing chamber analysis (Fig. 3), quantitative PCR, and immunoblotting (Fig. 4). We observed currents with pharmacological characteristics expected of differentiated airway epithelial-derived cells after week 7 at ALI. To assess the time dependence of current development in NuLi-1 and CuFi-5 cells, we performed Ussing chamber studies on cells from week 0 to week 7 post-airlift. Airlift begins after removal of apical media to create an air-cell interface on top of the cell monolayer. Week 0 represents 1–3 days after airlift. Amiloride-inhibited ENaC currents first appeared in NuLi-1 cells at week 1 post-airlift (Fig. 3A) at a low, but detectable, current of 6.5 ± 1.2 μA/cm2. ENaC currents developed a marked change in amplitude at week 3, averaging 36.7 ± 13.5 μA/cm2, with a maximum observed current of 36.8 ± 3.0 μA/cm2 at week 7. The NuLi-1 cell response to basolaterally applied CFTR agonists forskolin (10 μM) and IBMX (100 μM) (FSK + IBMX) at week 1 was −0.037 ± 0.38 μA/cm2 (Fig. 3B), with appreciable currents appearing at week 3 (−0.81 ± 0.096 μA/cm2) and increasing to −1.42 ± 0.35 μA/cm2 by week 7. In general, GlyH blocked the FSK + IBMX-stimulated current (Fig. 3, C and J–M), suggesting that CFTR functional expression continues to increase through week 7 in NuLi-1 cells. FSK + IBMX-stimulated CFTR currents developed over time in NuLi-1 cells (Fig. 3, B and F–I), with a near doubling in current every 2 wk of maturation. Bumetanide had a minimal effect following addition of amiloride and GlyH, suggesting that ENaC and CFTR are the major contributors to currents in the NuLi-1 and CuFi-5 cells (Figs. 3D and 4B). DIDS (10 μM) had little effect in NuLi-1 or CuFi-5 cells (Figs. 3E and 4B), consistent with minimal basal activity of Ca2+-activated Cl− channels at most time points tested.
Fig. 3.
Ussing chamber analysis indicates that CFTR channel function in Nuli-1 cells continues to increase until week 7 of ALI culture. A–C: representative change in current elicited in recordings from cells treated with 10 μM amiloride to block epithelial Na+ channel (ENaC) current, 10 μM forskolin (FSK) + 100 μM 3-isobutyl-1-methylxanthine (IBMX) to invoke cystic fibrosis transmembrane conductance regulator (CFTR) currents, and 20 μM glycine hydrazide (GlyH) to block CFTR currents in NuLi-1 cell filters at weeks 1, 3, 5, and 7 post-ALI (negative current represents movement of anions in basolateral-to-apical direction or movement of cations in apical-to-basolateral direction). FSK + IBMX-stimulated and GlyH-inhibited CFTR currents developed with time in ALI culture at a rate of ∼0.5 μA per 2 wk to a maximum of 1.5 μA within 7 wk. *P < 0.05 (by 2-tailed t-test). D and E: bumetanide, a Na+-K+-Cl− cotransporter inhibitor, and DIDS, a blocker of anion exchangers and some non-CFTR Cl− channels, applied after GlyH, failed to induce appreciable change in currents at each time point, indicating that the contribution of Ca2+-activated Cl− channels to the overall current was minimal and that FSK + IBMX-stimulated currents were due to CFTR. F–I: representative traces of time-dependent development of FSK + IBMX-stimulated CFTR currents at weeks 1, 3, 5, and 7. Drugs were added at time 0. J–M: representative NuLi-1 cell responses to GlyH at weeks 1, 3, 5, and 7. GlyH responses begin to develop at weeks 5 and 7, correlating with FSK + IBMX responses in NuLi-1 cells. Arrowheads indicate addition of GlyH. Values are means ± SE (n = 3–4 filters for each time point).
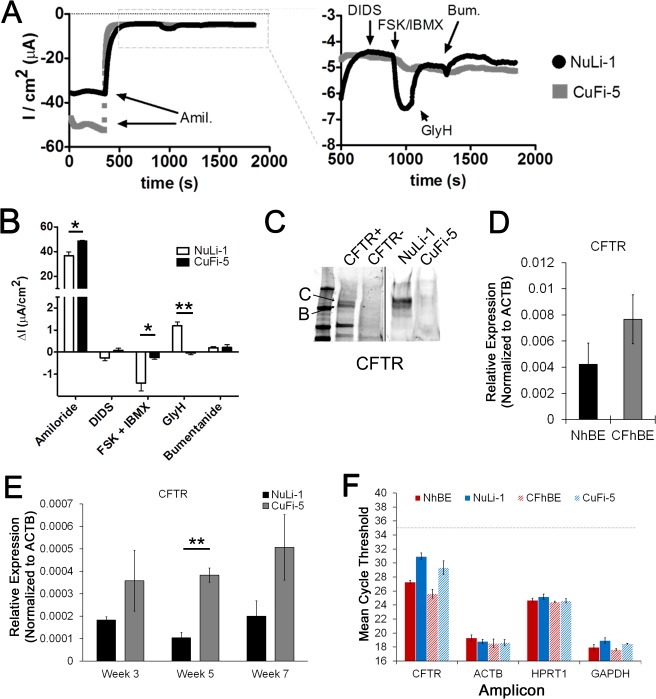
Fig. 4.
Ussing chamber traces of NuLi-1 vs. CuFi-5 cells reflect low CFTR transcript expression at week 7 of ALI culture. A: representative transepithelial recordings of NuLi-1 and CuFi-5 filters at week 7 post-ALI, recorded simultaneously. Left: entire trace. Addition of 10 μM amiloride (Amil) to the apical chamber resulted in a rapid decrease in negative current, consistent with a reduction in ENaC-mediated Na+ current. Right: magnification of the stabilized current after addition of amiloride. Addition of 10 μM DIDS, to block Ca2+-activated Cl− channels, to the apical side did not change current in either filter. CFTR agonists, 10 μM FSK and 100 μM IBMX, added to the basolateral side increased negative current in NuLi-1, but not CuFi-5, cells, consistent with activation of membrane-localized CFTR. FSK/IBMX-stimulated current was inhibited by addition of 20 μM GlyH to the apical chamber. Addition of 100 μM bumetanide (Bum) did not induce further inhibition of the current. B: week 7 results complementing trace in A. CuFi-5 cells were unresponsive to CFTR agonists and antagonists but displayed increased amiloride-sensitive current at baseline, characteristic of primary CF epithelia (15, 60). Values are means ± SE (n = 3–4 filters for each time point). C: representative Western blot of CFTR expression in NuLi-1 and CuFi-5 cells. Exogenous expression of wild-type CFTR in HeLa cells was used for antibody control (CFTR+, contrast enhanced for clarity). CFTR bands B and C are designated as such. D: gene expression analysis of CFTR in primary hBE cells grown on permeable supports. Exression levels of CFTR gene trends higher in CFhBE than NhBE cells. E: gene expression analysis of CFTR in NuLi-1 and CuFi-5 cell model indicates that gene expression stably trends higher over time in CuFi-5 than NuLi-1 cells. **P ≤ 0.01 (by 1-way ANOVA with Bonferroni's correction). F: mean CT values of CFTR and 3 housekeeping genes for reference and comparison. Expression trends and data corroborate electrophysiological properties of the cells (A and B), where Cl− currents are ≥10-fold lower than published expected Cl− currents for primary ciliated airway epithelial cells (15, 60).
As expected, CuFi-5 cells did not develop a FSK + IBMX-responsive current (Fig. 4, A and B). Figure 4A demonstrates the overall current profile where basal currents in CuFi-5 cells consisted of relatively large amiloride-sensitive components of −48.5 ± 1.32 μA/cm2. Basolateral addition of FSK + IBMX elicited a rapidly developing negative current in the NuLi-1 cells, but not in the CuFi-5 cells (−0.243 ± 0.068 μA/cm2), as expected, given the respective CFTR genotypes (Fig. 4, A and B). GlyH had minimal effects in CuFi-5 cells, consistent with the apparent lack of CFTR-mediated current (Fig. 4, A and B). Figure 4B summarizes the data collected in the Ussing chamber experiments. CFTR protein expression in NuLi-1 and CuFi-5 cells paralleled gene expression and Ussing chamber measurements, in that expression levels of CFTR are much lower in CuFi-5 than NuLi-1 cells (Fig. 4C).
The CFTR gene expression trends of the NuLi-1 and CuFi-5 cells (Fig. 4E) at week 7 are similar to those of the NhBE and CFhBE cells (Fig. 4D). Interestingly, the levels of CFTR transcripts were nearly twice as high in the CFhBE than NhBE cells (Fig. 4, D–F). Critically, the expression levels were almost eightfold lower in NuLi-1 and CuFi-5 cells than in primary cells (Fig. 4F). This is an important feature of NuLi-1 and CuFi-5 cells, in that they do not overexpress CFTR, which is a confounding factor characteristic of most other immortalized airway cell lines.
NuLi-1 and CuFi-5 cells express airway-specific gap junction proteins comparable to primary hBE cells.
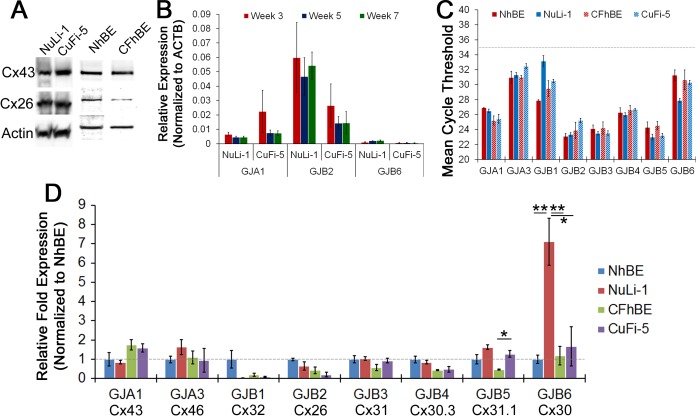
The connexins are the protein constituents of gap junction channels (31). We confirmed the expression of Cx43 (an α-type connexin) and Cx26 (a β-type connexin) by immunoblotting (Fig. 5A) and gene transcription (Fig. 5, B and C). NuLi-1 and CuFi-5 cells express Cx43 mRNA (GJA1) at similar quantities at week 7 (Fig. 5B) but differ greatly in their expression of Cx26 mRNA (GJB2) throughout ALI culture (Fig. 5D). NuLi-1 cells express five times as much Cx26 as CuFi-5 cells at week 7 (Fig. 5B); however, Western blot analysis shows similar amounts of protein in NuLi-1 and CuFi-5 cells (Fig. 5A).
Fig. 5.
NuLi-1 and CuFi-5 cells express airway-specific connexins similar to primary hBE cells. A: Western blots showing expression of Cx26 and Cx43 proteins in NuLi-1, CuFi-5, NhBE, and CFhBE cells. B: relative expression levels of 3 representative connexins (Cx43/GJA3, Cx26/GJB2, and Cx30/GJB6) did not change over time and are expressed at similar levels over time within groups for each connexin; however, GJB2 expression was ∼4-fold greater in NuLi-1 than CuFi-5 cells at all time points. No significant difference was observed by 1-way ANOVA with Bonferroni's correction. C: analysis of mean CT values as an approximation to relative quantitation of each gene amplicon indicates that GJB1 and GJB6 transcript levels differed widely between NhBE and NuLi-1 cells but were not different between CFhBE and CuFi-5 cells. Gene transcripts trend almost identically between the primary and NuLi/CuFi cell models in regard to abundance of connexin transcripts. D: relative fold expression normalized to NhBE transcript levels shows the difference between NhBE and NuLi-1 expression of GJB1 and GJB6, while all others were similar in expression level. Expression of Cx43 (GJA1) was 2-fold higher in CF-affected than normal cells. Data from cells at passages 7–10 are presented as normalized, relative fold expression. Values are means ± SE [n = 2 filters from each of 3 passages (6 filters total) for each time point]. *P ≤ 0.05, **P ≤ 0.01 (by 1-way ANOVA with Bonferroni's correction).
Interestingly, the gene expression of gap junction mRNA in NuLi-1/CuFi-5 cells at maturity is very similar to that in mature primary ciliated NhBE/CFhBE airway cells. Abundance of transcripts for Cx43 (GJA1), Cx46 (GJA3), Cx31 (GJB3), Cx30.3 (GJB4), and Cx31.1 (GJB5) is similar (Fig. 5C) in each paired model. However, Cx32 (GJB1) and Cx30 (GJB6) transcript abundances are markedly different between NhBE and NuLi-1 cells, yet they are similar in CFhBE and CuFi-5 cells (Figs. 5, C and D). GJB1 expression is 16-fold lower in NuLi-1 than NhBE cells, and GJB6 expression is 7.5-fold higher in NuLi-1 than NhBE cells (Fig. 5D). These results demonstrate that airway cells have a complex pattern of connexin expression and point out the possibility that cell-cell communication is likely to be important for function of airway epithelial tissue.
F508del CFTR-expressing cells display a Cx43-specific trafficking anomaly rescued by 4-PBA.
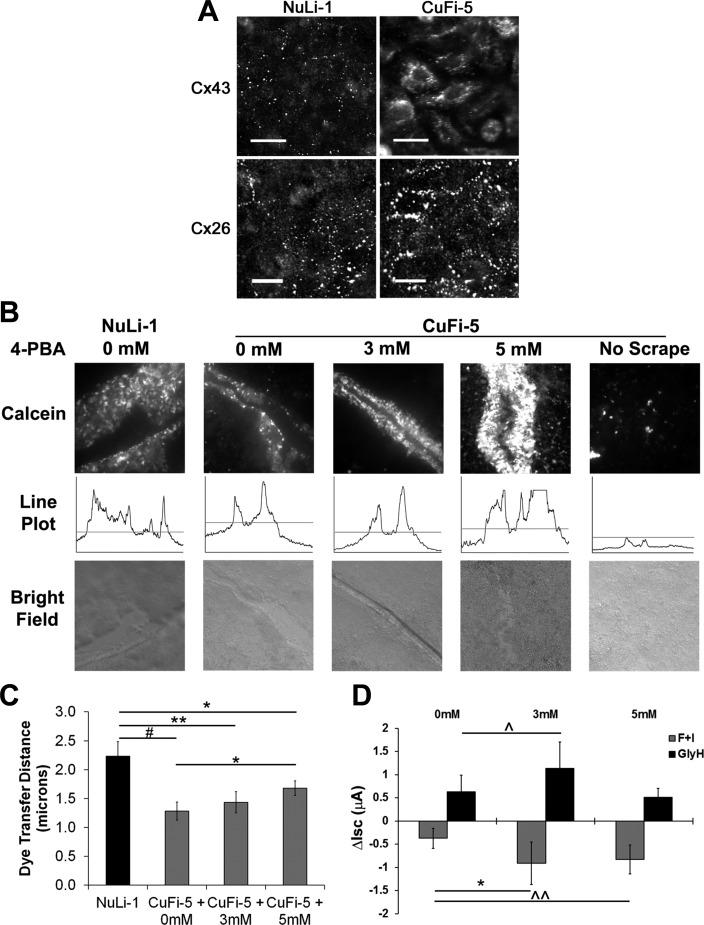
Using immunofluorescence, we confirmed the expression of Cx43 and Cx26 by NuLi-1 and CuFi-5 cells (Figs. 6A). Interestingly, in the CF-affected CuFi-5 cells, but not in the wild-type NuLi-1 cells, we observed trafficking defects of Cx43, but not Cx26 (Fig. 6A). This finding suggests a specific effect on α-type connexins (e.g., Cx43) due to expression of F508del CFTR, but not β-type connexins (e.g., Cx26). Treatment of CuFi-5 cells with 4-PBA, which is known to partially promote folding of mutant CFTR (51, 53, 55) and connexins (9, 26, 65), resulted in a dose-dependent increase in gap junctional communication measured by intercellular transfer of the fluorescent tracer calcein (Fig. 6, B and C). A significant dose-dependent increase in dye transfer was observed between untreated CuFi-5 cells and cells treated with 5 mM 4-PBA, although dye transfer by 4-PBA-treated CuFi-5 cells did not reach the level of dye transfer by NuLi-1 cells (Fig. 6C). Additionally, 4-PBA induced a significant increase in forskolin-responsive, CFTR-mediated Cl− currents (Fig. 6D), confirming that 4-PBA is able to rescue the proper folding of F508del CFTR.
Fig. 6.
F508del CFTR-expressing cells display a Cx43-specific trafficking anomaly rescued by 4-phenylbutyric acid (4-PBA). A: NuLi-1 and CuFi-5 cells were fixed and immunostained for Cx43 and Cx26 proteins. Normal appearance of gap junction plaques is shown by punctate staining along cell borders. Cx43 and Cx26 appeared normal in NuLi-1 cells, but Cx43 showed perinuclear localization in CuFi-5 cells. Cx26 localization was unaffected in CuFi-5 cells and appeared normal. Scale bars = 20 μm. B: to assess gap junction function, calcein (0.6 kDa) and Texas Red-labeled dextran (10 kDa) were scrape-loaded into NuLi-1 and CuFi-5 cells grown on filters at week 8 of ALI. CuFi-5 cells were analyzed by fluorescence microscopy after addition of 0, 3, or 5 mM 4-PBA to the basolateral media chamber for 24 h prior to scrape-loading for 15 min at 37°C. In CuFi-5 cells treated with 5 mM 4-PBA, dye transfer was increased, presumably through rescued Cx43 channels. Line plots are representative of the distance data used to calculate values shown in C. C: dye transfer by scrape-loading was quantified using the line scan measure tool in NIH ImageJ to measure distance the dye traveled from the scrape edge on either side of the scrape. Although complete rescue was not achieved, a dose response was observed in cells treated with 5 mM 4-PBA for 24 h, indicating a significant rescue effect on Cx43 gap junction function. #P = 0.001, NuLi-1 vs. CuFi-5 at 0 mM (n = 24, n = 36); **P = 0.012, NuLi-1 vs. CuFi-5 at 3 mM (n = 24, n = 27); *P = 0.032, NuLi-1 vs. CuFi-5 at 5 mM (n = 24, n = 42); *P = 0.050, CuFi-5 at 0 mM vs. CuFi-5 at 5 mM (by 2-tailed Student's t-test). D: effect of 4-PBA rescue on F508del CFTR Cl− currents. Ussing chamber experiments were conducted in 8-wk-old CuFi-5 cell filters basolaterally treated with 0, 3, or 5 mM 4-PBA for 24 h before analysis for forskolin and IBMX (F + I)-induced currents. Changes in short-circuit currents (ΔIsc) for induction and inhibition experiments indicate that 4-PBA had a positive effect on CFTR-mediated Cl− currents (n = 4 filters each). *P = 0.022, ^P = 0.064, ^^P = 0.055 (by 2-tailed Student's t-test).
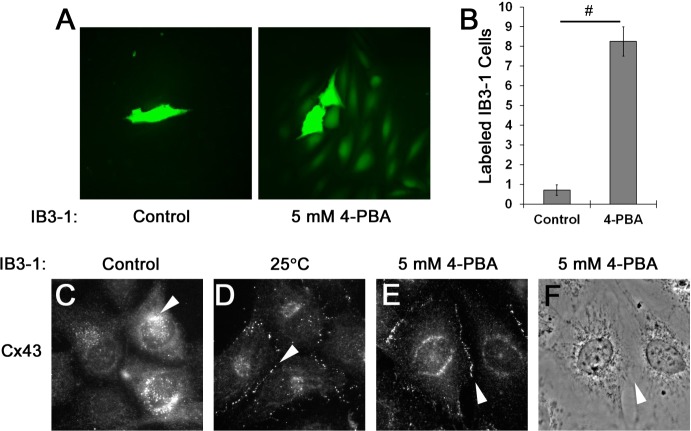
To confirm the observation that Cx43 mistrafficking in CuFi-5 cells was due to F508del CFTR expression, we examined Cx43 expression, localization, and gap junction-mediated dye transfer in IB3-1 cells, a different airway cell line that expresses one copy of F508del CFTR and one copy of a truncated CFTR, W1282X (Fig. 7). We measured intercellular communication between gap junctions by dye transfer using microinjected calcein as a fluorescent small-molecule tracer (Fig. 7A). IB3-1 cells treated overnight with 5 mM 4-PBA showed an eightfold increase in dye transfer compared with untreated control cells, suggesting a rescue of function that correlated with increased Cx43 trafficking to the plasma membrane (Fig. 7B). Consistent with our observation in CuFi-5 cells, Cx43 was retained in the perinuclear region of the cell under control conditions at baseline (Fig. 7C, arrowhead). Consistent with correction of CFTR trafficking by temperature shift, correction of Cx43 trafficking was observed upon a shift in the IB3 cells from 37°C to 25°C (Fig. 7D, arrowhead) compared with the untreated control (Fig. 7C) and was comparable to treatment with 5 mM 4-PBA overnight at 37°C (Fig. 7E, arrowhead). The processes that simultaneously control CFTR and connexin trafficking are unknown; however, these results suggest that both proteins use a shared quality-control pathway that is modulated by 4-PBA (10, 51, 56).
Fig. 7.
Cx43 gap junction function is rescued by 4-PBA treatment of heterozygous F508del/W1282X CFTR-expressing IB3-1 cells. A: rescue of gap junction function by 4-PBA in the IB3-1 cell line. B: calcein was microinjected into single cells, and the number of cells to which the dye was propagated through functional gap junctions was counted. Dye transfer labeling of IB3-1 cells increased 8-fold upon treatment with 4-PBA, indicating rescue of Cx43 gap junction function by 4-PBA within these cells. Values are means ± SE (n = 4 and 7 separate injections for control and 4-PBA treatment, respectively), presented as number of dye-containing cells following microinjection. #P ≤ 0.001 (by 2-tailed t-test). C–F: immunofluorescence images indicating membrane expression of Cx43 after treatment with 5 mM 4-PBA or temperature shift to 25°C. Arrowheads point to immunostained Cx43, located at the perinuclear region (C) or the point at which 2 adjacent cell membranes meet (D–F). Temperature shift to 25°C culture conditions is known to at least partially rescue CFTR trafficking (14).
DISCUSSION
To date, studies of NuLi and CuFi cell behavior have used varying culture conditions on different substrates. The data presented here suggest that NuLi-1 and CuFi-5 cells continue to differentiate over time and that time post-airlift is a critical parameter that needs to be considered when these cell models are utilized for airway research. A number of desired characteristics that define the NuLi-1 and CuFi-5 cell lines (matching epithelial type, age, sex, genotype, immortalization method, ability to culture, and availability) made this cell model attractive for study of the contributions of nonciliated airway cells to CF and airway diseases. We analyzed the genotypic and phenotypic changes that occur in NuLi-1 (CFTRwt/wt) and CuFi-5 (CFTRΔF508/ΔF508) cells during ALI culture. These telomerase-immortalized cell models are advantageous for several reasons, including the ability to propagate on plastic, differentiation into pseudostratified monolayers on Transwell permeable supports, and physiological levels of expression for CFTR message, protein, and channel function. Importantly, these cell models depict a high degree of similarity with, but do not completely recapitulate, primary ciliated hBE cells.
Instead, NuLi-1 and CuFi-5 cells represent pseudostratified epithelia enriched for serous columnar cells (Fig. 1). This finding has considerable utility, since recent research has shown that CF disease impairs diverse airway cells, such as goblet cells, wherein mutant CFTR channels impair detachment of mucus (20). NuLi-1 and CuFi-5 cells also allow for studies in a system that does not rely on overexpression of CFTR protein or CFTR-expressing cancer cells. The effects of low homozygous F508del CFTR expression, rather than overexpression to nonphysiological levels, can now be examined for the impacts of F508del CFTR on overall cell physiology, including physiological processes such as protein secretion, endoplasmic reticulum (ER) stress, innate immunity, and inflammatory response, and on pharmacological correction of defective CFTR protein in cell types other than ciliated cells.
Tight junction and gap junction gene signatures for NuLi-1 and CuFi-5 cells stabilized at weeks 5 and 7; the signatures at week 7 were most similar to ciliated primary airway hBE cells (Figs. 2 and 5). This correlated with development of stable TER values and increasing CFTR-specific Cl− currents, which were detectable in NuLi-1, but not CuFi-5, cells, increasing each week beginning at weeks 1, 3, and 5, while exhibiting the largest response at week 7 post-airlift (Fig. 3). Importantly, the response to the CFTR channel blocker GlyH increased with time in ALI culture (Fig. 3). The maximum current elicited by a combination of CFTR agonists, FSK + IBMX, was 1.42 ± 0.35 μA/cm2 at week 7 post-ALI, while GlyH caused a decrease of 1.20 ± 0.17 μA/cm2 in Cl− current at week 7. These data correlate with the appearance of low levels of CFTR message and a stabilized tight junction barrier characteristic of NuLi-1 cells. CFTR currents were nearly undetectable in CuFi-5 cells at all time points tested, which is to be expected in cells expressing mutant forms of CFTR. As shown by lectin (Fig. 1), mRNA, and protein (Fig. 4) analysis, CuFi-5 cells are similar to NuLi-1 cells in all aspects tested here, except the expected deficit in CFTR function. Thus, together, NuLi-1 and CuFi-5 cells represent an airway cell line model that can be used to study the effects of low CFTR expression in a population of age- and sex-matched airway cells that are mostly serous in nature.
We found that CuFi-5 cells, once stabilized, had significantly lower TER than NuLi-1 cells (Fig. 2). This is consistent with previous reports associating impaired airway barrier function in CF with CFTR-null models or in cells expressing mutant CFTR (7, 34, 44, 66). Although the mRNA expression profile of some tight junction proteins was lower for NuLi-1 and CuFi-5 cells than primary airway cells, these corresponding proteins were readily detectable. Taken together, these data validate the use of NuLi-1 and CuFi-5 cells for further studies on the effects of mutant CFTR on airway tight junctions.
Gap junctions are important for Ca2+ signaling and metabolite sharing in tissues. Additionally, they act as voltage-gated ion channel systems that are regulated by charge potentials and cell membrane polarizations. In the airway, specifically, gap junctions have proven important for coordinated epithelial action in response to bitter substances (32) and activation of the innate immune system in epithelial cells (22) and have further implications in CFTR-related cell functions (21, 36, 38, 42). Not all connexins are compatible, although they can be grouped by observed compatibility (31). However, the importance of the heteromeric and heterotypic gap junction compatibility to tissue physiology is not known and remains an enigma in the field of gap junction biology. CuFi-5 cells display an irregular connexin trafficking pattern similar to that found in the CFTRW1282X/ΔF508 heterozygous IB3-1 cells for the α-type Cx43 but display a normal trafficking and gap junction plaque formation for the β-type Cx26 (Figs. 6 and 7). Of interest with regard to connexin trafficking is the fact that CFTRW1282X encodes only 86% of the residues in the full CFTR protein, yet there remains a strong interaction with Cx43, but not Cx26. These data are consistent with differential processing and trafficking of α- vs. β-type connexins (29) and underscore the hypothesis that mistrafficked F508del CFTR at physiological expression levels can have downstream effects on specific proteins, including gap junction proteins, as shown in this study. For a complete understanding of the pathological consequences of mutant CFTR that lead to CF, downstream, off-target effects beyond CFTR deficiency need to be considered.
Interestingly, we found that 4-PBA rescued Cx43 trafficking and function in CuFi-5 and IB3-1 cells (Figs. 6 and 7), and it is also known to partially rescue mutant CFTR trafficking in other cell types (53, 56). This process could occur through a shared mechanism where trafficking of Cx43 and CFTR is regulated by ERp29, an ER luminal resident quality-control chaperone upregulated by 4-PBA that acts on CFTR (51, 55, 56) and Cx43 (9, 10). This mechanism could potentially explain the differences in cell function that are observed when F508del CFTR-expressing cells are only partially rescued by functional CFTR expression in terms of Cl− conduction but are not completely rescued in terms of cell responses to stress, such as in wound repair (57), mucus clearance (33, 48), and bicarbonate secretion (18). Use of fully differentiated, age- and sex-matched NuLi-1 and CuFi-5 cells will aid in understanding the off-target effects that F508del CFTR expression can have on other cell functions.
NuLi-1 and CuFi-5 airway epithelial cells offer an attractive alternative model system to either primary hBE cells or cancer-derived cell lines that have numerous restrictions on use. Primary cells are difficult to obtain because of the lack of routine access to human lungs, high development costs of cell acquisition and culture supplies, technical ability to isolate and culture, genetic variation between donors, and inability to propagate without rich media or feeder layers. Cell models derived from tumors are useful but may obscure normal physiological processes. Therefore, our data demonstrate that NuLi-1 and CuFi-5 cell lines offer an advantageous system for the study of nonciliated cell types found in the airway and can be used to provide insights into normal and pathological airway epithelial physiology, particularly in the study of intercellular junctions.
GRANTS
This work was supported by Cystic Fibrosis Foundation Grants MCCART13I0 and MCCART13P0 and National Institutes of Health Grants RO1-HL-116958 (M. Koval) and T32-AA-013528 (S. A. Molina) and, in part, by the Emory University Integrated Cellular Imaging Microscopy Core of the Emory+Children's Pediatric Research Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.M., N.A.M., and M.K. developed the concept and designed the research; S.A.M., B.S., H.K.M., A.H.K., and M.K. performed the experiments; S.A.M., B.S., H.K.M., A.H.K., and M.K. analyzed the data; S.A.M., N.A.M., and M.K. interpreted the results of the experiments; S.A.M. and B.S. prepared the figures; S.A.M. drafted the manuscript; S.A.M., B.S., H.K.M., A.H.K., N.A.M., and M.K. edited and revised the manuscript; S.A.M., B.S., H.K.M., A.H.K., N.A.M., and M.K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Barbara Schlingmann for assistance with editing. CFTR antibodies were provided by John Riordan (University of North Carolina-Chapel Hill) and Cystic Fibrosis Foundation Therapeutics.
REFERENCES
- 1.Al-Alawi M, Buchanan P, Verriere V, Higgins G, McCabe O, Costello RW, McNally P, Urbach V, Harvey BJ. Physiological levels of lipoxin A4 inhibit ENaC and restore airway surface liquid height in cystic fibrosis bronchial epithelium. Physiol Rep 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349–352, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol 279: L623–L630, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bou Saab J, Losa D, Chanson M, Ruez R. Connexins in respiratory and gastrointestinal mucosal immunity. FEBS Lett 588: 1288–1296, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan PJ, McNally P, Harvey BJ, Urbach V. Lipoxin A4-mediated KATP potassium channel activation results in cystic fibrosis airway epithelial repair. Am J Physiol Lung Cell Mol Physiol 305: L193–L201, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Chanson M, Kotsias BA, Peracchia C, O'Grady SM. Interactions of connexins with other membrane channels and transporters. Prog Biophys Mol Biol 94: 233–244, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 13: 3218–3234, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dannhoffer L, Billet A, Jollivet M, Melin-Heschel P, Faveau C, Becq F. Stimulation of wild-type, F508del- and G551D-CFTR chloride channels by non-toxic modified pyrrolo[2,3-b]pyrazine derivatives. Front Pharmacol 2: 48, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das S, Smith TD, Sarma JD, Ritzenthaler JD, Maza J, Kaplan BE, Cunningham LA, Suaud L, Hubbard MJ, Rubenstein RC, Koval M. ERp29 restricts connexin43 oligomerization in the endoplasmic reticulum. Mol Biol Cell 20: 2593–2604, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das Sarma J, Kaplan BE, Willemsen D, Koval M. Identification of rab20 as a potential regulator of connexin 43 trafficking. Cell Commun Adhes 15: 65–74, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Dorscheid DR, Conforti AE, Hamann KJ, Rabe KF, White SR. Characterization of cell surface lectin-binding patterns of human airway epithelium. Histochem J 31: 145–151, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Eisele NA, Anderson DM. Host defense and the airway epithelium: frontline responses that protect against bacterial invasion and pneumonia. J Pathogens 2011: 249802, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbri E, Borgatti M, Montagner G, Bianchi N, Finotti A, Lampronti I, Bezzerri V, Dechecchi MC, Cabrini G, Gambari R. Expression of microRNA-93 and interleukin-8 during Pseudomonas aeruginosa-mediated induction of proinflammatory responses. Am J Respir Cell Mol Biol 50: 1144–1155, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Farinha CM, King-Underwood J, Sousa M, Correia AR, Henriques BJ, Roxo-Rosa M, Da Paula AC, Williams J, Hirst S, Gomes CM, Amaral MD. Revertants, low temperature, and correctors reveal the mechanism of F508del-CFTR rescue by VX-809 and suggest multiple agents for full correction. Chem Biol 20: 943–955, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Fischer H, Illek B, Sachs L, Finkbeiner WE, Widdicombe JH. CFTR and calcium-activated chloride channels in primary cultures of human airway gland cells of serous or mucous phenotype. Am J Physiol Lung Cell Mol Physiol 299: L585–L594, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnett JP, Baker EH, Baines DL. Sweet talk: insights into the nature and importance of glucose transport in lung epithelium. Eur Respir J 40: 1269–1276, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Gruenert DC, Willems M, Cassiman JJ, Frizzell RA. Established cell lines used in cystic fibrosis research. J Cystic Fibrosis 3 Suppl 2: 191–196, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins G, Buchanan P, Perriere M, Al-Alawi M, Costello RW, Verriere V, McNally P, Harvey BJ, Urbach V. Activation of P2RY11 and ATP release by lipoxin A4 restores the airway surface liquid layer and epithelial repair in cystic fibrosis. Am J Respir Cell Mol Biol 51: 178–190, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. Cystic fibrosis. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345: 818–822, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Dudez T, Scerri I, Thomas MA, Giepmans BN, Suter S, Chanson M. Defective activation of c-Src in cystic fibrosis airway epithelial cells results in loss of tumor necrosis factor-α-induced gap junction regulation. J Biol Chem 278: 8326–8332, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol 588: 2343–2350, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito T, Nagahara N, Ogawa T, Inayama Y, Kanisawa M. Lectin binding to the luminal surface of distal airway epithelial cells of rodents. J Electron Microsc (Tokyo) 34: 381–388, 1985. [PubMed] [Google Scholar]
- 24.Ito T, Newkirk C, Strum JM, McDowell EM. Changes in glycoconjugates revealed by lectin staining in the developing airways of Syrian golden hamsters. Anat Rec 228: 151–162, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, Lillehoj EP, Kai H, Kim KC. MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosci 2: 68–77, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman J, Gordon C, Bergamaschi R, Wang HZ, Cohen IS, Valiunas V, Brink PR. The effects of the histone deacetylase inhibitor 4-phenylbutyrate on gap junction conductance and permeability. Front Pharmacol 4: 111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein H, Garneau L, Trinh NT, Prive A, Dionne F, Goupil E, Thuringer D, Parent L, Brochiero E, Sauve R. Inhibition of the KCa3.1 channels by AMP-activated protein kinase in human airway epithelial cells. Am J Physiol Cell Physiol 296: C285–C295, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol 75: 551–567, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol 16: 159–166, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH. Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol 130: 987–995, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koval M, Molina SA, Burt JM. Mix and match: investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett 588: 1193–1204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, Kreindler JL, Margolskee RF, Cohen NA. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124: 1393–1405, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeSimple P, Goepp J, Palmer ML, Fahrenkrug SC, O'Grady SM, Ferraro P, Robert R, Hanrahan JW. Cystic fibrosis transmembrane conductance regulator is expressed in mucin granules from Calu-3 and primary human airway epithelial cells. Am J Respir Cell Mol Biol 49: 511–516, 2013. [DOI] [PubMed] [Google Scholar]
- 34.LeSimple P, Liao J, Robert R, Gruenert DC, Hanrahan JW. Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol 588: 1195–1209, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losa D, Chanson M, Crespin S. Connexins as therapeutic targets in lung disease. Expert Opin Ther Targets 15: 989–1002, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Losa D, Kohler T, Bellec J, Dudez T, Crespin S, Bacchetta M, Boulanger P, Hong SS, Morel S, Nguyen TH, van Delden C, Chanson M. Pseudomonas aeruginosa-induced apoptosis in airway epithelial cells is mediated by gap junctional communication in a JNK-dependent manner. J Immunol 192: 4804–4812, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Maille E, Trinh NT, Prive A, Bilodeau C, Bissonnette E, Grandvaux N, Brochiero E. Regulation of normal and cystic fibrosis airway epithelial repair processes by TNF-α after injury. Am J Physiol Lung Cell Mol Physiol 301: L945–L955, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Martin FJ, Prince AS. TLR2 regulates gap junction intercellular communication in airway cells. J Immunol 180: 4986–4993, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer ML, Sheridan JA, Blohmke CJ, Turvey SE, Hancock RE. The Pseudomonas aeruginosa autoinducer 3O-C12 homoserine lactone provokes hyperinflammatory responses from cystic fibrosis airway epithelial cells. PLos One 6: e16246, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molenda N, Urbanova K, Weiser N, Kusche-Vihrog K, Gunzel D, Schillers H. Paracellular transport through healthy and cystic fibrosis bronchial epithelial cell lines—do we have a proper model? PLos One 9: e100621, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet 21: 115–118, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Patel SJ, King KR, Casali M, Yarmush ML. DNA-triggered innate immune responses are propagated by gap junction communication. Proc Natl Acad Sci USA 106: 12867–12872, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezaee F, Georas SN. Breaking barriers. New insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol 50: 857–869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, Hill E, Brown D, Chan HC, Breton S. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci 127: 4396–4408, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saint-Criq V, Kim SH, Katzenellenbogen JA, Harvey BJ. Non-genomic estrogen regulation of ion transport and airway surface liquid dynamics in cystic fibrosis bronchial epithelium. PLos One 8: e78593, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheckenbach KE, Losa D, Dudez T, Bacchetta M, O'Grady S, Crespin S, Chanson M. Prostaglandin E2 regulation of cystic fibrosis transmembrane conductance regulator activity and airway surface liquid volume requires gap junctional communication. Am J Respir Cell Mol Biol 44: 74–82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlingmann B, Molina SA, Koval M. Claudins: gatekeepers of lung epithelial function. Semin Cell Dev Biol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schutte A, Ermund A, Becker-Pauly C, Johansson ME, Rodriguez-Pineiro AM, Backhed F, Muller S, Lottaz D, Bond JS, Hansson GC. Microbial-induced meprin β-cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc Natl Acad Sci USA 111: 12396–12401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaukat Z, Liu D, Choo A, Hussain R, O'Keefe L, Richards R, Saint R, Gregory SL. Chromosomal instability causes sensitivity to metabolic stress. Oncogene. In press. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu T, Nettesheim P, Mahler JF, Randell SH. Cell type-specific lectin staining of the tracheobronchial epithelium of the rat: quantitative studies with Griffonia simplicifolia I isolectin B4. J Histochem Cytochem 39: 7–14, 1991. [DOI] [PubMed] [Google Scholar]
- 51.Singh OV, Pollard HB, Zeitlin PL. Chemical rescue of ΔF508-CFTR mimics genetic repair in cystic fibrosis bronchial epithelial cells. Mol Cell Proteomics 7: 1099–1110, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, Ramalho AS, Amaral MD, Dorfman R, Zielenski J, Masica DL, Karchin R, Millen L, Thomas PJ, Patrinos GP, Corey M, Lewis MH, Rommens JM, Castellani C, Penland CM, Cutting GR. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet 45: 1160–1167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatia A, Suaud L, Rubenstein RC. Regulation of the ERp29 promoter by the F508del-CFTR corrector 4-phenylbutyrate and the E2F-1 transcription factor (Abstract). Am J Respir Crit Care Med 189: A5510, 2014. [Google Scholar]
- 54.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 372: 351–362, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suaud L, Miller K, Alvey L, Yan W, Robay A, Kebler C, Kreindler JL, Guttentag S, Hubbard MJ, Rubenstein RC. ERp29 regulates ΔF508 and wild-type cystic fibrosis transmembrane conductance regulator (CFTR) trafficking to the plasma membrane in cystic fibrosis (CF) and non-CF epithelial cells. J Biol Chem 286: 21239–21253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suaud L, Miller K, Panichelli AE, Randell RL, Marando CM, Rubenstein RC. 4-Phenylbutyrate stimulates Hsp70 expression through the Elp2 component of elongator and STAT-3 in cystic fibrosis epithelial cells. J Biol Chem 286: 45083–45092, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trinh NT, Bardou O, Prive A, Maille E, Adam D, Lingee S, Ferraro P, Desrosiers MY, Coraux C, Brochiero E. Improvement of defective cystic fibrosis airway epithelial wound repair after CFTR rescue. Eur Respir J 40: 1390–1400, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Trinh NT, Prive A, Maille E, Noel J, Brochiero E. EGF and K+ channel activity control normal and cystic fibrosis bronchial epithelia repair. Am J Physiol Lung Cell Mol Physiol 295: L866–L880, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Urdiales-Viedma M, De Haro-Munoz T, Martos-Padilla S, Abad-Ortega JM, Varela-Duran J, Granda-Paez R. Jacalin, another marker for histiocytes in paraffin-embedded tissues. Histol Histopathol 10: 597–602, 1995. [PubMed] [Google Scholar]
- 60.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA 108: 18843–18848, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 36: 157–165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev 24: 210–229, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verriere V, Higgins G, Al-Alawi M, Costello RW, McNally P, Chiron R, Harvey BJ, Urbach V. Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia. PLos One 7: e37746, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voisin G, Bouvet GF, Legendre P, Dagenais A, Masse C, Berthiaume Y. Oxidative stress modulates the expression of genes involved in cell survival in ΔF508 cystic fibrosis airway epithelial cells. Physiol Genomics 46: 634–646, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Wang HZ, Rosati B, Gordon C, Valiunas V, McKinnon D, Cohen IS, Brink PR. Inhibition of histone deacetylase (HDAC) by 4-phenylbutyrate results in increased junctional conductance between rat corpora smooth muscle cells. Front Pharmacol 6: 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiser N, Molenda N, Urbanova K, Bahler M, Pieper U, Oberleithner H, Schillers H. Paracellular permeability of bronchial epithelium is controlled by CFTR. Cell Physiol Biochem 28: 289–296, 2011. [DOI] [PubMed] [Google Scholar]
- 67.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol 16: 27–35, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yi SM, Harson RE, Zabner J, Welsh MJ. Lectin binding and endocytosis at the apical surface of human airway epithelia. Gene Ther 8: 1826–1832, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, Welsh M, Klingelhutz AJ. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am J Physiol Lung Cell Mol Physiol 284: L844–L854, 2003. [DOI] [PubMed] [Google Scholar]