Abstract
We have previously shown that an adverse perinatal environment significantly alters lung growth and development and results in persistently altered cardiopulmonary physiology in adulthood. Our model of maternal LPS treatment followed by 14 days of neonatal hyperoxia exposure causes severe pulmonary disease characterized by permanent decreases in alveolarization and diffuse interstitial fibrosis. The current investigations tested the hypothesis that dysregulation of Notch signaling pathways contributes to the permanently altered lung phenotype in our model and that the improvements we have observed previously with maternal docosahexaenoic acid (DHA) supplementation are mediated through normalization of Notch-related protein expression. Results indicated that inflammation (IL-6 levels) and oxidation (F2a-isoprostanes) persisted through 8 wk of life in mice exposed to LPS/O2 perinatally. These changes were attenuated by maternal DHA supplementation. Modest but inconsistent differences were observed in Notch-pathway proteins Jagged 1, DLL 1, PEN2, and presenilin-2. We detected substantial increases in markers of apoptosis including PARP-1, APAF-1, caspase-9, BCL2, and HMGB1, and these increases were attenuated in mice that were nursed by DHA-supplemented dams during the perinatal period. Although Notch signaling is not significantly altered at 8 wk of age in mice with perinatal exposure to LPS/O2, our findings indicate that persistent apoptosis continues to occur at 8 wk of age. We speculate that ongoing apoptosis may contribute to persistently altered lung development and may further enhance susceptibility to additional pulmonary disease. Finally, we found that maternal DHA supplementation prevented sustained inflammation, oxidation, and apoptosis in our model.
Keywords: maternal inflammation; neonatal hyperoxia; docosahexaenoic acid, apoptosis
the life-long effects of an adverse maternal environment on the developing fetus and the contributions of this perinatal milieu to adult disease pathogenesis are currently being defined (10, 36). We have previously demonstrated in a mouse model that the combination of maternal inflammation and neonatal hyperoxia exposure causes an exacerbated pathophysiology with alterations in lung biology that persists into adulthood (24, 31–33). This critical phenotype is similar to that observed in a preterm infant that develops severe bronchopulmonary dysplasia (BPD) (9, 28, 29). Whereas infants that develop mild or moderate BPD often recover with little to no lingering morbidities, infants with severe BPD often have life-long pathology that significantly impairs their lifestyle. Since this group is a small percentage of all preterm infants compared with those that develop mild or moderate BPD, the unique morbidities of these infants are often overlooked. These infants are at greatest risk for developing adult disease such as asthma and chronic obstructive pulmonary disease (COPD) (28, 29, 38). Although affected infants exhibit decreased alveolarization, as observed in mild and moderate BPD, they also develop restrictive and/or obstructive interstitial lung disease, have permanently altered pulmonary function, and are much more likely to suffer from long-term pulmonary morbidities (6, 28, 29, 41). These infants are likely affected by a “multiple hit” scenario in which maternal inflammation, prematurity, and hyperoxia exposure elicit additive or synergistic effects.
Whereas many studies have focused on intrauterine infections such as chorioamnionitis (1, 12), we have chosen to investigate the additive effects of subtle systemic inflammation, such as that seen in periodontitis, pneumonia, and urinary tract infections, combined with neonatal hyperoxia (9, 36). Although the long-term effects of perinatal inflammation are documented in humans and in animal models, the mechanisms responsible are poorly defined. Previously, we investigated the persistent effects of maternal inflammation (LPS) and neonatal hyperoxia (85%) on lung growth and function. We observed decreased alveolarization, activated TGF-β signaling, and increased collagen deposition. The resulting phenotype is characterized by a diffuse interstitial fibrosis and restrictive lung disease in mouse pups exposed to both perinatal inflammation and hyperoxia (31–33).
The current study tested the hypothesis that persistent deficits in lung growth in mice exposed to maternal inflammation and neonatal hyperoxia are caused by inflammation that, in turn, disrupts key lung developmental pathways and limits proliferation and repair and/or increases apoptosis in lung tissues. Notch signaling is responsible for the regulation of both proliferation and apoptosis and is essential for lung development. Consequently, components of the Notch pathway were analyzed. Although disruption of Notch signaling was not evident at 8 wk of age, we observed increased evidence of ongoing apoptosis: specifically, increased numbers of 2TdT-positive cells and greater levels of proapoptotic proteins. We speculate that this persistent upregulation of cell death pathways prevents efficient repair and resolution of injury even after a significant recovery period.
Natural products have been effective in attenuating inflammation in many disease states (13). In earlier studies, we have documented improvements in lung growth and function in mice exposed to maternal inflammation and neonatal hyperoxia by supplementing the pregnant and nursing dams with docosahexaenoic acid (DHA) (24, 31). In the present studies we tested the hypothesis that maternal DHA supplementation during the inflammatory period would prevent long-term dysregulation of cell death pathways.
METHODS
Animals and exposure.
Animal study protocols were approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children's Hospital (Columbus, OH). All animals were housed in a “specific pathogen-free” facility for at least 7 days before breeding was started. Pregnancy was time dated by the presence of a vaginal plug (E1). Pregnant C3H/HeN mice were injected on embryonic day 16 (E16) with LPS (80 μg/kg ip; serotype 0111:B4, catalog no. 437627; Calbiochem, Gibbstown, NJ) or an equal volume of saline, as previously reported (32, 33, 35). Also beginning on E16, dams injected with LPS or saline were placed on control diets (CD) or DHA-supplemented diets (DHA) until weaning, as described previously (22, 31). At birth, each litter of newborn mice was paired with a litter born to a dam receiving the same embryonic exposures (LPS or saline, control or DHA diets), and the pups were pooled and redistributed randomly as previously described (33). One of each of the paired litters was exposed to 85% O2 for 2 wk and subsequently returned to room air (RA), whereas the corresponding group was maintained in RA. Nursing dams were rotated between their RA and O2 litters every 24 h to reduce O2 toxicity. Twenty-four hours of RA or O2 exposure was designated as day 1. At 8 wk of age, the mice were administered a ketamine-xylazine overdose and exsanguinated to limit the amount of blood in the lung. A maximum of two pups per litter, from three to six litters, were used at each time point for any given analysis, and equal numbers of males and females were included. For simplicity the groups were designated control diet-saline/room air (CD-saline/RA), DHA diet saline/room air (DHA-saline/RA), control diet-LPS/85% O2 (CD-LPS/O2), and DHA diet-LPS/85% O2 (DHA-LPS/O2).
TdT-mediated dUTP nick end labeling.
Apoptosis was observed by TdT-mediated dUTP nick end labeling (TUNEL) assay in paraffin-embedded lung tissues by using the TACS 2TdT-Fluor in situ apoptosis detection kit (Trevigen, Gaithersburg, MD) per the manufacturer's instructions.
Enzyme-linked immunosorbent assay.
Frozen lungs were homogenized and protein concentrations determined by Bradford assay. IL-6, IL-8, and IL-10 levels were measured using enzyme-linked immunosorbent assay (ELISA) (Duoset ELISA kits; R&D Systems, Minneapolis, MN) according to the manufacturer's protocols. Absorbances were determined spectrophotometrically using a Spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA).
Western immunoblotting.
Equal protein amounts were separated on SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were probed with primary antibodies as indicated: Notch 1 (1:500) and Notch 2 (1:500), Jagged 1 (1:500) and Jagged 2 (1:500), Delta-like (DLL) 1 (1:500) and DLL 3 (1:500), NICD (1:500), NUMB (1:500), presenilin-1 (1:500) and presenilin-2 (1:500), PEN2 (1:500), PARP (1:500), and Hes-1 (1:500) (Cell Signaling Technology, Danvers, MA); HMGB1 (1:500) and caspase-9 (1:500) (Abcam, Cambridge, MA); and BCL2 (1:500) and APAF-1 (1:500) (BD Biosciences, San Jose, CA). Secondary antibodies specific for the species of the secondary antibody were employed. Blots were developed using enhanced chemiluminescence (ECL Western blotting detection; GE Healthcare, Hatfield, UK), and expression levels were quantified using TotalLab software (Nonlinear Dynamics, Newcastle upon Tyne, UK). The density of the band for the protein of interest was normalized to the density of β-actin protein (ab6276; Abcam, Cambridge, MA).
GSH and GSSG.
GSH and GSSG levels were measured by the enzyme recycling method as previously described (2).
F2α-isoprostanes.
F2α-isoprostane levels were measured by liquid chromatography-mass spectrometry using multiple reaction monitoring as previously described (25).
Statistics.
Data were analyzed by two-way ANOVA and Tukey's post hoc test with the variables saline/RA or LPS/O2 as exposures and CD or DHA as diets using GraphPad PRISM 6 (La Jolla, CA). All data were analyzed for equal variances, and if indicated the data were log-transformed before the ANOVA was performed. P < 0.05 was considered statistically significant, and data are as means ± SE.
RESULTS
Chronic inflammation and oxidative stress are evident at 8 wk of life and are attenuated by neonatal DHA supplementation.
Cytokines, including IL-6, IL-8, and IL-10, were measured as markers of inflammation. Oxidation was assessed by measuring glutathione GSH and GSSG levels and F2α-isoprostane levels. Even after 6 wk of recovery, IL-6 levels remained elevated and IL-10 levels were decreased in the CD-LPS/O2-exposed mice compared with all other groups, whereas IL-8 was not different (Table 1). DHA supplementation attenuated the increased and decreased expression of IL-6 and IL-10, respectively. At 8 wk, we observed no differences in GSH or GSSG levels in the LPS/O2-exposed mice compared with saline/RA controls fed either diet. However, an effect of diet was observed in the ratios of GSH to GSSG (Table 2). F2α-isoprostane levels were substantially elevated in the CD-LPS/O2-exposed mice compared with RA groups, and DHA supplementation lowered the F2α-isoprostane levels in the supplemented groups (Table 2).
Table 1.
Inflammatory cytokines in lung tissues
| IL-6 | IL-8 | IL-10 | |
|---|---|---|---|
| CD-saline/RA | 1.00 ± 0.05 | 1.00 ± 0.07 | 1.00 ± 0.10 |
| DHA-saline/RA | 0.91 ± 0.07 | 0.92 ± 0.08 | 0.97 ± 0.02 |
| CD-LPS/O2 | 1.77 ± 0.51 | 0.96 ± 0.09 | 0.74 ± 0.07 |
| DHA-LPS/O2 | 0.86 ± 0.07# | 1.04 ± 0.09 | 1.05 ± 0.08# |
Cytokines were measured by ELISA as described in methods. Data were normalized to the control diet-saline/room air (CD-saline/RA)-exposed group and analyzed by 2-way ANOVA with Tukey's multiple comparisons test post hoc. Values are means ± SE; n = 8–12 per group. An effect of diet is indicated with IL-6 levels elevated and IL-10 levels decreased in the CD-LPS/85% O2 (CD-LPS/O2)-exposed group.
P < 0.05, different from CD-LPS/O2. DHA, docosahexaenoic acid diet.
Table 2.
Parameters of oxidation in lung tissues
| GSH, μmol/g tissue | GSSG, nmol/g tissue | GSH/GSSG | F2α-Isoprostanes, pmol/g tissue | |
|---|---|---|---|---|
| CD-saline/RA | 1.09 ± 0.10 | 23.20 ± 6.79 | 72.92 ± 16.09 | 228.3 ± 30.6 |
| DHA-saline/RA | 1.13 ± 0.08 | 16.64 ± 7.31 | 165.59 ± 36.22 | 149.2 ± 32.8 |
| CD-LPS/O2 | 1.31 ± 0.13 | 23.76 ± 5.48 | 72.65 ± 12.59 | 292.4 ± 30.7 |
| DHA-LPS/O2 | 1.24 ± 0.15 | 17.52 ± 7.48 | 184.52 ± 41.34*# | 109.1 ± 28.8# |
Glutathione (GSH) and glutathione disulfide (GSSG) were measured by enzyme recycling and 8-isoprostane by liquid chromatography-mass spectrometry as described in methods. Data were analyzed by 2-way ANOVA with Fisher's least significant difference test post hoc. Values are means ± SE; n = 8–12 per group for GSH and GSSG; n = 4 for F2α-isoprostanes. An effect of diet is indicated with the GSH/GSSG ratio and F2α-isoprostanes levels substantially elevated in the CD-LPS/O2-exposed group compared with the RA groups, and DHA lowered F2α-isoprostane levels in the supplemented groups.
P < 0.05, different from CD-saline/RA.
P < 0.05, different from CD-LPS/O2.
Notch pathways regulate both proliferation and apoptosis.
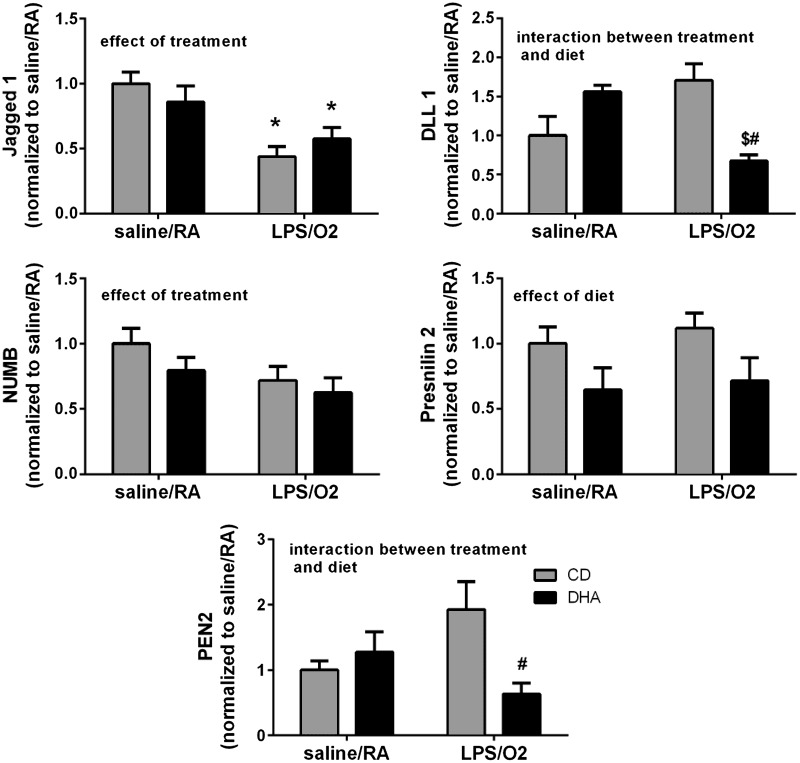
To determine the role of Notch-mediated signaling in this model, we examined representative members of the Notch pathway. Notch 1 and Notch 2 protein levels were not different between groups, nor were the protein levels of the ligands Jagged 2 and DLL 3 (data not shown). Jagged 1 levels were lower in both of the LPS/O2-exposed groups than in the saline/RA groups with an effect of treatment, and DLL 1 levels were greater in the CD-LPS/O2-exposed group than in the DHA-LPS/O2 group with an interaction between treatment and diet (Fig. 1). Members of the Notch signaling pathways involved in intracellular cleavage of the Notch receptor including NUMB, presenilin-1, presenilin-2, and PEN2 were further evaluated. Protein levels of NUMB, the enzyme responsible for blocking the intracellular cleavage of Notch, were decreased, and an effect of treatment was indicated (Fig. 1). Conversely, members of the Notch cleavage complex, PEN2 and presenilin-2, were also affected with PEN2 levels increasing by LPS/O2 treatment, and this increase was prevented by maternal DHA supplementation (an interaction between treatment and diet) (Fig. 1). Furthermore, presenilin-2 levels were suppressed by diet in both treatment groups (an effect of diet). No differences between treatments or exposure groups were observed in the protein levels of NICD, the intracellular Notch cleavage product, or in the transcription factor Hes-1, a canonical Notch-regulated transcription factor (data not shown).
Fig. 1.
Notch pathway proteins. Protein levels of Notch ligands Jagged 1 and Delta-like 1 (DLL 1) and Notch cleavage enzymes NUMB, presenilin-2, and PEN2 were measured by Western blot in whole lung homogenates as described in methods. A total of n = 4–12 mice were assessed in each group with no more than 1 mouse per litter from each treatment group. The data were quantified by densitometry and analyzed by 2-way ANOVA with Tukey's post hoc test, and the results are indicated on each individual graph. Data are means ± SE and were analyzed for the effects of LPS/O2 exposure and diet. *P < 0.05, different from control diet-saline/room air exposure (CD-saline/RA). $P < 0.05, different from docosahexaenoic acid (DHA) diet-LPS/85% O2 exposure (DHA-LPS/O2). #P < 0.05, different from control diet-LPS/85% O2 exposure (CD-LPS/O2).
Apoptosis is evident in the lung tissue of CD-LPS/O2-exposed mice at 8 wk.
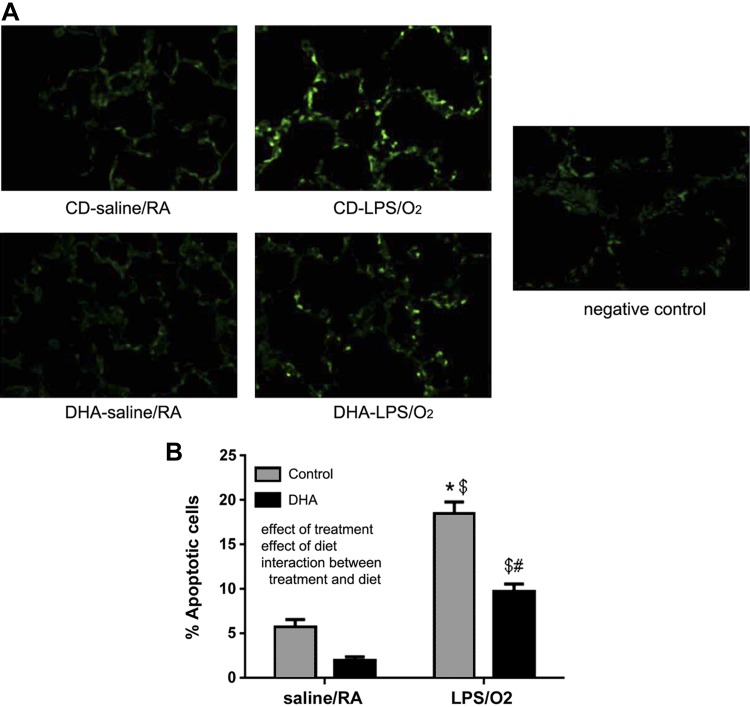
Apoptosis occurs during the acute course of hyperoxic lung injury, but resolution of apoptotic pathways has not been extensively evaluated. Evaluation of TUNEL-stained tissue sections revealed that the number of apoptotic cells was more than three times greater in the tissues of CD-LPS/O2-exposed mice than in the room air-exposed groups (Fig. 2). The numbers of apoptotic cells were substantially less in the DHA-LPS/O2-exposed group than in the CD-LPS/O2 group.
Fig. 2.
TdT-mediated dUTP nick end labeling (TUNEL) immunofluorescence. Lung tissues were formalin fixed and paraffin embedded. Tissue sections were treated as described in methods, and photomicrographs were taken using fluorescence imaging (A). The green fluorescent cells (indicative of cell death) were counted and the data graphed (B). A total of n = 4 mice were assessed in each group with no more than 1 mouse per litter from each treatment group. The data were analyzed by 2-way ANOVA with Tukey's post hoc test. An effect of treatment, an effect of diet, and an interaction were indicated. Data are means ± SE and were analyzed for the effects of LPS/O2 exposure and diet. *P < 0.05, different from CD-saline/RA. $P < 0.05, different from DHA-LPS/O2; #P < 0.05, different from CD-LPS/O2.
Apoptosis-related pathways are elevated.
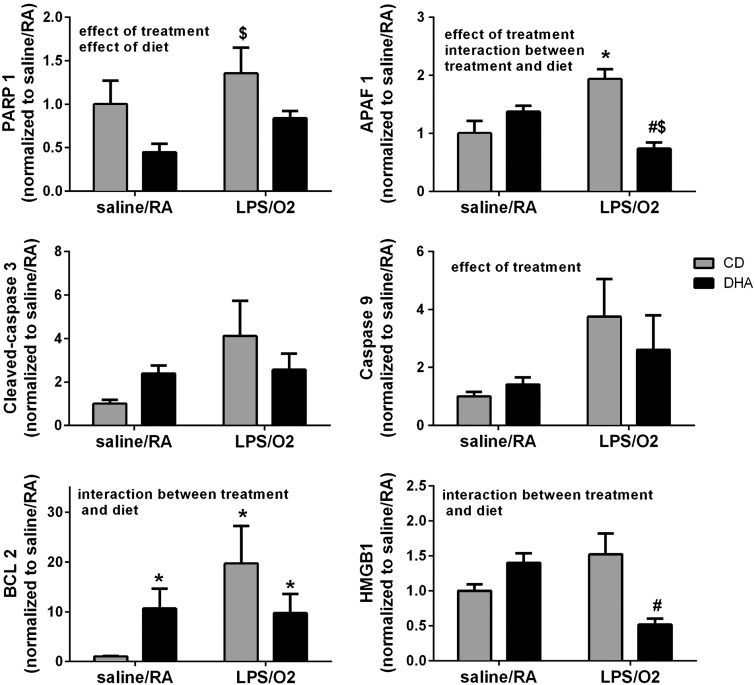
Poly (ADP-ribose) polymerase-1 (PARP-1) protein levels were modestly elevated in the CD-LPS/O2-exposed mice compared with saline/RA controls (Fig. 3). DHA supplementation decreased PARP-1 expression in both saline/RA- and LPS/O2-exposed groups, and independent effects of treatment and diet were detected. Likewise, apoptotic protease activating factor (APAF)-1 levels were elevated in CD-LPS/O2-exposed mice, and this elevation was attenuated by perinatal DHA supplementation (Fig. 3) as indicated by an effect of treatment and an interaction between diet and treatment. There were no significant differences in cleaved caspase-3; however, a trend toward an effect of treatment (P = 0.1) was detected. Although our data indicated an effect of LPS/O2 treatment on caspase-9 levels, there was no effect of diet (Fig. 3). The antiapoptotic protein BCL2 was elevated in the CD-LPS/O2-exposed group, and an interaction between diet and treatment was identified. Levels of HMGB1, a marker of cell death, were greater in CD-LPS/O2-exposed mice than in DHA-LPS/O2-exposed mice, and an interaction between treatment and diet indicated that HMGB1 expression was attenuated by neonatal DHA supplementation.
Fig. 3.
Protein markers of apoptosis. Protein levels of Poly (ADP-ribose) polymerase-1 (PARP-1), apoptotic protease activating factor (APAF-1), cleaved caspase-3, caspase-9, BCL2, and HMGB1 were measured by Western blot in whole lung homogenates as described in methods. A total of n = 4–12 mice were assessed in each group with no more than 1 mouse per litter from each treatment group. The data were quantified by densitometry and analyzed by 2-way ANOVA with Tukey's post hoc test, and the results are indicated on each individual graph. Data are means ± SE and were analyzed for the effects of LPS/O2 exposure and diet. *P < 0.05, different from CD-saline/RA. $P < 0.05, different from DHA-LPS/O2; #P < 0.05, different from CD-LPS/O2.
DISCUSSION
Deficits in alveolarization and lung growth are hallmarks of neonatal hyperoxic lung injury (23, 39). Hyperoxia exposure alone decreases lung development and alveolar septation; however, our model of combined systemic maternal inflammation and neonatal hyperoxia results in much more severe pulmonary pathology (32, 33). This current investigation sought to determine the mechanisms responsible for the persistent severe pulmonary pathophysiology observed in our combined LPS and hyperoxia exposure model (32).
Inflammation and oxidation of macromolecules are well-defined consequences of neonatal hyperoxic lung injury (8, 14, 21, 40). We and others have detected elevations in cytokines, chemokines, and antioxidants in lung tissues during the acute phase of injury (23). In the present study, increased levels of IL-6 and decreased levels of IL-10 in the lungs of mice with perinatal exposure to LPS/O2 indicate an ongoing state of chronic pulmonary inflammation 6 wk after the primary and secondary exposures (Table 1). Interestingly, differences due to perinatal exposures were not observed in GSH and GSSG levels but were evident in the levels of F2α-isoprostanes (Table 2). This observation may be due to the acute nature of GSH and GSSG responses, which are more reflective of the current redox status of the lung, whereas F2α-isoprostane levels may represent a more chronic oxidant stress. Alternatively, chronic oxidant stress may be better reflected in lipid oxidation than in antioxidant status. Nonetheless, maternal DHA supplementation prevented the sustained increases in IL-6 and F2α-isoprostane levels.
Notch signaling pathways have been extensively studied in the context of lung development and repair (5, 43). Notch signaling directs terminal differentiation in specific lung cell types such as club cells and macrophages (20, 42). Previous studies have suggested that Notch may be involved in upregulation of antioxidant or inflammatory genes in response to stressors (37). Furthermore, Notch interacts with TGF-β, which is increased during acute injury and remains elevated at 8 wk in this model (7, 32). To investigate the role of the Notch pathway in our model, we measured the levels of the Notch receptors, common ligands, Notch cleavage regulators, and Notch-regulated gene products. At 8 wk, Notch pathway proteins in a whole lung homogenates were not dramatically altered by neonatal exposure to LPS/O2 (Fig. 1). Our findings do not preclude the possibility that Notch-related pathways might be altered in specific cell types that are difficult to detect in whole homogenates. Moreover, it is also possible that Notch pathways were altered early in the exposure period and that these changes are resolved by 8 wk of age. Apoptosis occurs through cell signaling processes that are regulated by multiple pathways including Notch. Apoptosis and necrosis both occur following neonatal hyperoxia exposure (15, 26). Notch directly regulates the BCL family proteins, and LPS has been shown to induce Notch ligands (18, 19).
Apoptosis is a normal component of mammalian lung development and occurs as the embryonic lung prepares for extrauterine life (3, 4, 27). Disruption of the temporal sequence of apoptotic events is likely to lead to altered lung growth, potentially due to aberrant expression of apoptotic pathways with persistent cell death. During hyperoxic lung injury, the number of apoptotic cells found in the lung directly correlates with degree of lung injury (15). Even after 6 wk of recovery, we observed significantly more TUNEL-positive cells in the mice exposed to LPS/O2. Maternal DHA supplementation during pregnancy and lactation significantly attenuated sustained increases in cell death (Fig. 2).
Examination of proteins representative of specific apoptotic pathways revealed sustained upregulation of these pathways in lung tissues from mice exposed to perinatal LPS/O2 (Fig. 4). PARP-1 is activated by DNA damage, specifically strand breaks, and aids in the recruitment of DNA repair mechanisms. PARP-1 is induced by hyperoxia exposure and plays an essential role in cell repair after hyperoxic injury (16, 17). PARP-1 protein levels were increased in the mice exposed to LPS/O2, suggesting higher levels of ongoing DNA repair. APAF-1 recruits and activates caspase-9 to further propagate the apoptotic process; however, APAF-1 has not been routinely measured in response to hyperoxia exposure. Levels of APAF-1 and caspase-9 were elevated in adult mice previously exposed to LPS/O2 (Fig. 3). Maternal DHA supplementation attenuated these increases. Caspase-3 levels tended to be increased in the CD-LPS/O2-exposed group; however, the differences failed to reach statistical significance. Although this finding suggests ongoing apoptosis in the lungs of LPS/O2-exposed mice, the effect might be occurring in specific cell types, making it difficult to detect in whole tissue homogenate.
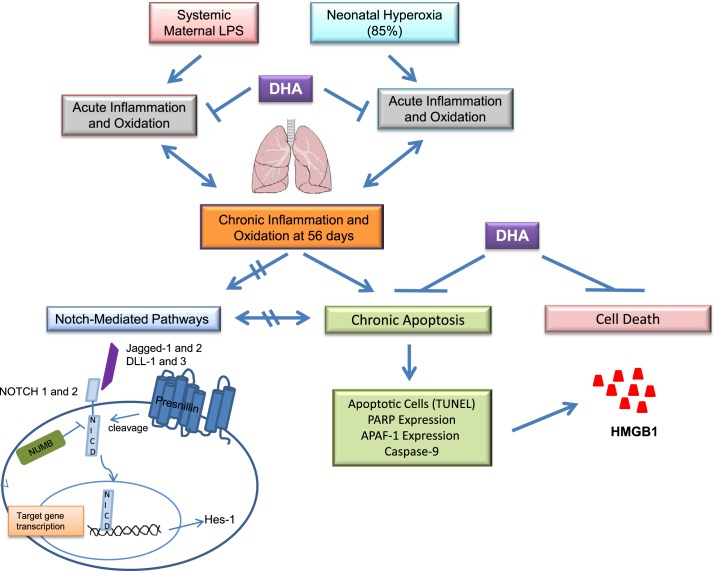
Fig. 4.
Proposed scheme for the pathways analyzed in this study.
The BCL family of proteins differentially possesses both pro- and antiapoptotic properties, which in turn regulate mitochondrial permeability (11, 30). Specifically, BCL2 is antiapoptotic and serves to counteract the apoptotic properties of other proapoptotic proteins. We observed a modest elevation of BCL2 in the mice previously exposed to LPS/O2; however, BCL2 levels were increased in both groups of maternal DHA supplemented mice compared with the CD-saline/RA group.
HMGB1 is a danger-associated molecule pattern (DAMP) molecule. HMGB1 is released from cells that are dying from either necrosis or excess apoptosis and can be used as a marker to identify cell death due to a specific treatment. There was a modest increase in HMGB1 levels in the CD-LPS/O2 group, and this increase was suppressed beyond the CD-saline/RA group in the DHA-LPS/O2 group (Fig. 4). Increased levels of HMGB1 in these lung tissues support the presence of ongoing cell death in mice exposed to perinatal inflammation.
Collectively, our data support the hypothesis that early life exposure to inflammation causes alterations in apoptotic signaling pathways that continue to alter cell fate long after the initial inflammatory exposure has ended (Fig. 4). We interpret these findings to suggest that persistent apoptosis is a mechanism that contributes to the permanent decreases in lung alveolarization and deficits in lung function observed in our model. Although Notch is essential for normal lung growth and is involved in regulating apoptosis, this pathway does not appear to be responsible for the persistent apoptosis we observed. The implications of these finding are unknown at this time but may be the result of changes in cell type-specific signaling rather than an effect of the entire lung.
The most impressive component of the current work is that levels of ongoing apoptosis remain elevated in adulthood following perinatal exposures and suggest that early life supplementation with DHA, which is relatively inexpensive and straightforward to implement clinically, may counteract the adverse effects of perinatal inflammation and improve outcomes in highly vulnerable patient populations.
GRANTS
This work was supported by Deutsche Forschungsgemeinschaft Grant VE 614/1-1 and National Institutes of Health Grant R01AT006880 (to L. K. Rogers).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A., M.V., T.E.T., and L.K.R. conception and design of research; M.A. and K.M.H. performed experiments; M.A., K.M.H., and L.K.R. analyzed data; M.A. drafted manuscript; K.M.H. prepared figures; M.V., T.E.T., and L.K.R. interpreted results of experiments; M.V., T.E.T., and L.K.R. edited and revised manuscript; M.V., T.E.T., and L.K.R. approved final version of manuscript.
REFERENCES
- 1.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci 15: 121–127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal G, Singh R, Saini B, Bansal Y. ESI-MSn and LC-ESI-MS studies to characterize forced degradation products of bosentan and a validated stability-indicating LC-UV method. J Pharm Biomed Anal 72: 186–197, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Ivy D, Dingemanse J, Widlitz A, Schmitt K, Doran A, Bingaman D, Nguyen N, Gaitonde M, van Giersbergen PL. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther 73: 372–382, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. Am J Respir Cell Mol Biol 20: 228–236, 1999. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene 27: 5148–5167, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 182: 237–245, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira AC, Suriano G, Mendes N, Gomes B, Wen X, Carneiro F, Seruca R, Machado JC. E-cadherin impairment increases cell survival through Notch-dependent upregulation of Bcl-2. Hum Mol Genet 21: 334–343, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Frank L. Developmental aspects of experimental pulmonary oxygen toxicity. Free Radic Biol Med 11: 463–494, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Greenough A. Long term respiratory outcomes of very premature birth (<32 weeks). Semin Fetal Neonatal Med 17: 73–76, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Harding R, Maritz G. Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med 17: 67–72, 2012. [DOI] [PubMed] [Google Scholar]
- 11.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, Nakayama KI, Homer R, Elias JA. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 115: 1039–1048, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res 2: 27–32, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornfeld JW, Baitzel C, Konner AC, Nicholls HT, Vogt MC, Herrmanns K, Scheja L, Haumaitre C, Wolf AM, Knippschild U, Seibler J, Cereghini S, Heeren J, Stoffel M, Bruning JC. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494: 111–115, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 16: 557–567, 1997. [DOI] [PubMed] [Google Scholar]
- 15.McGrath-Morrow SA, Stahl J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol Biol 25: 150–155, 2001. [DOI] [PubMed] [Google Scholar]
- 16.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells 27: 623–633, 2009. [DOI] [PubMed] [Google Scholar]
- 17.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA 107: 1414–1419, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monsalve E, Perez MA, Rubio A, Ruiz-Hidalgo MJ, Baladron V, Garcia-Ramirez JJ, Gomez JC, Laborda J, Diaz-Guerra MJ. Notch-1 up-regulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen-presenting capacity and cytotoxic activity. J Immunol 176: 5362–5373, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto M, Takeda K, Lucas JJ, Joetham A, Yasutomo K, Gelfand EW. Low-dose lipopolysaccharide affects lung allergic responses by regulating Jagged1 expression on antigen-pulsed dendritic cells. Int Arch Allergy Immunol 157: 65–72, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Outtz HH, Wu JK, Wang X, Kitajewski J. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. J Immunol 185: 4363–4373, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piedboeuf B, Horowitz S, Johnston CJ, Gamache M, Belanger S, Poubelle PE, Welty SE, Watkins RH. Interleukin-1 expression during hyperoxic lung injury in the mouse. Free Radic Biol Med 24: 1446–1454, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Rogers L, Valentine C, Pennell M, Velten M, Britt R, Dingess K, Zhao X, Welty S, Tipple T. Maternal DHA supplementation decreases lung inflammation in hyperoxia-exposed newborn mice. J Nutr 141: 214–222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res 65: 33–38, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers LK, Valentine CJ, Pennell M, Velten M, Britt RD, Dingess K, Zhao X, Welty SE, Tipple TE. Maternal docosahexaenoic acid supplementation decreases lung inflammation in hyperoxia-exposed newborn mice. J Nutr 141: 214–222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers LK, Young CM, Pennell ML, Tipple TE, Leonhart KL, Welty SE. Plasma lipid metabolites are associated with gestational age but not bronchopulmonary dysplasia. Acta Paediatr 101: e321–e326, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenzweig EB, Ivy DD, Widlitz A, Doran A, Claussen LR, Yung D, Abman SH, Morganti A, Nguyen N, Barst RJ. Effects of long-term bosentan in children with pulmonary arterial hypertension. J Am Coll Cardiol 46: 697–704, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Schittny JC, Djonov V, Fine A, Burri PH. Programmed cell death contributes to postnatal lung development. Am J Respir Cell Mol Biol 18: 786–793, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest 132: 651–656, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Shi W, Warburton D. Is COPD in adulthood really so far removed from early development? Eur Respir J 35: 12–13, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Tai DS, Hu C, Kim EH, Lipshutz GS. Augmentation of transgene-encoded protein after neonatal injection of adeno-associated virus improves hepatic copy number without immune responses. Pediatr Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velten M, Britt RD Jr, Heyob KM, Tipple TE, Rogers LK. Maternal dietary docosahexaenoic acid supplementation attenuates fetal growth restriction and enhances pulmonary function in a newborn mouse model of perinatal inflammation. J Nutr 144: 258–266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velten M, Britt RD Jr, Heyob KM, Welty SE, Eiberger B, Tipple TE, Rogers LK. Prenatal inflammation exacerbates hyperoxia-induced functional and structural changes in adult mice. Am J Physiol Regul Integr Comp Physiol 303: R279–R290, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol 108: 1347–1356, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velten M, Hutchinson KR, Gorr MW, Wold LE, Lucchesi PA, Rogers LK. Systemic maternal inflammation and neonatal hyperoxia induces remodeling and left ventricular dysfunction in mice. PLoS One 6: e24544, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viscardi RM. Perinatal inflammation and lung injury. Semin Fetal Neonatal Med 17: 30–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi N, Skoko JJ, Chartoumpekis DV, Kimura S, Slocum SL, Noda K, Palliyaguru DL, Fujimuro M, Boley PA, Tanaka Y, Shigemura N, Biswal S, Yamamoto M, Kensler TW. Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol Cell Biol 34: 653–663, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warburton D, Gauldie J, Bellusci S, Shi W. Lung development and susceptibility to chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 668–672, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Welty SE, Rivera JL, Wu B. Hyperoxic increases in lung ICAM-1 mRNA are independent of TNF-alpha and IL-1 beta mRNA. Free Radic Biol Med 23: 898–908, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 32: 321–328, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Xing Y, Li A, Borok Z, Li C, Minoo P. NOTCH1 is required for regeneration of Clara cells during repair of airway injury. Stem Cells 30: 946–955, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol 727: 89–98, 2012. [DOI] [PubMed] [Google Scholar]