Abstract
Patients with chronic heart failure (CHF) have dyspnea and exercise intolerance, which are caused in part by diaphragm abnormalities. Oxidants impair diaphragm contractile function, and CHF increases diaphragm oxidants. However, the specific source of oxidants and its relevance to diaphragm abnormalities in CHF is unclear. The p47phox-dependent Nox2 isoform of NAD(P)H oxidase is a putative source of diaphragm oxidants. Thus, we conducted our study with the goal of determining the effects of CHF on the diaphragm levels of Nox2 complex subunits and test the hypothesis that p47phox knockout prevents diaphragm contractile dysfunction elicited by CHF. CHF caused a two- to sixfold increase (P < 0.05) in diaphragm mRNA and protein levels of several Nox2 subunits, with p47phox being upregulated and hyperphosphorylated. CHF increased diaphragm extracellular oxidant emission in wild-type but not p47phox knockout mice. Diaphragm isometric force, shortening velocity, and peak power were decreased by 20–50% in CHF wild-type mice (P < 0.05), whereas p47phox knockout mice were protected from impairments in diaphragm contractile function elicited by CHF. Our experiments show that p47phox is upregulated and involved in the increased oxidants and contractile dysfunction in CHF diaphragm. These findings suggest that a p47phox-dependent NAD(P)H oxidase mediates the increase in diaphragm oxidants and contractile dysfunction in CHF.
Keywords: oxidative stress, respiratory muscle, myocardial infarction
abnormalities of the diaphragm muscle contribute to the pathophysiology of chronic heart failure (CHF) (10, 46). The degree of diaphragm dysfunction depends on the stage of the disease, where patients with severe CHF (class III or IV) are weaker than patients with mild CHF (class I), as shown by studies using volitional tests (3, 24, 28) or direct measurement of diaphragm strength with phrenic nerve stimulation (31). Importantly, left ventricular ejection fraction is not correlated with maximal inspiratory pressure (43), and decreased inspiratory muscle endurance is not reversed by heart transplant (41).
Diaphragm biopsies from patients with severe CHF undergoing heart transplant show ultrastructural abnormalities (38), and studies in animal models have shown that CHF leads to impairments in diaphragm contractile function (12, 29, 55, 59). Oxidants impair diaphragm contractile function (11, 49), and CHF increases oxidants in the diaphragm (12, 55). In CHF rats, systemic antioxidant administration increases submaximal diaphragm force to values seen in control animals (55). However, the specific source of oxidants and its relevance to diaphragm weakness in CHF remain to be elucidated.
NAD(P)H oxidases (Nox) have emerged as an important source of oxidants in skeletal muscle (47, 51) and diaphragm (6, 48). The Nox2 isoform of NAD(P)H oxidase is present in the diaphragm and, like in heart and vessels, the functionally active enzyme consists of phagocyte oxidase (phox) subunits gp91phox (Nox2), p22phox, p67phox, p40phox, and p47phox (33, 36). Nox2 is activated by factors that are increased in the serum of CHF patients such as angiotensin II and cytokines (19, 45), and indirect evidence supports a role for NAD(P)H oxidase in the pathophysiology of limb muscle abnormalities in CHF (5, 52). It is conceivable that CHF triggers an endocrine-mediated activation of Nox2 in the diaphragm that increases oxidants and impairs contractile function. Receptor-mediated activation of Nox2 involves phosphorylation of p47phox (34, 36). Mice deficient in p47phox lack NAD(P)H oxidase activity in skeletal muscle (47, 52), are protected from increases in oxidants and diaphragm weakness in vitro (6), and have reduced pathophysiological left ventricular remodeling and dysfunction after myocardial infarction (MI) (18).
Our goal in the current study was to examine the role of NAD(P)H oxidase on diaphragm abnormalities in CHF. Initially, we characterized the effects of CHF on the mRNA and protein level profile of Nox2-related subunits in the diaphragm. Based on our initial findings, we tested the hypothesis that p47phox knockout prevents the increases in diaphragm oxidants and contractile dysfunction in CHF.
METHODS
Animals.
Our study involved a total of 65 male adult wild-type (WT; C57BL6) and 59 B6(Cg)-Ncf1m1J/J mice (Jackson Laboratories) that are deficient in p47phox (p47phox−/−) due to a spontaneous mutation in the Ncf1 gene (30). Eighteen WT and 14 p47phox−/− mice underwent sham surgery. The remainder (WT, n = 47; p47phox−/−, n = 45) underwent MI surgery, as described below. Breeding pairs of p47phox−/− mice were purchased from Jackson Laboratories, and a colony was established at the University of Florida and maintained by institutional animal care services breeding personnel. Mice were individually caged and exposed to standard dark-light cycles with free access to food and water throughout the study. We confirmed deficiency of p47phox in the diaphragm using immunoblotting, as described below. The Institutional Animal Care and Use Committee of the University of Florida approved all procedures performed in our study.
Surgical preparation and coronary artery ligation.
We caused MI via ligation of the left anterior descending coronary artery to induce CHF (56). We shaved the left side of the thorax and cleaned the surgical area with 4% chlorhexidine and sterile saline. Thereafter, we performed orotracheal intubation using a PE tube (outer diameter 1.27 mm) and connected the animal to a mechanical ventilator (Model 683; Harvard Apparatus). While the animal was in the surgical plane of anesthesia (2% isoflurane), we exposed the heart via a left-sided thoracotomy in the fourth or fifth intercostal space, removed the pericardium, and ligated the left anterior descending coronary artery near the left atrium using a 6-0 PGA suture (Demesorb; Demetech). After the ligation, we hyperinflated the lungs, approximated the ribs using a 6-0 PGA suture, and closed the skin incision with 3-0 suture (Demelon; Demetech). Once extubated, the animals were transferred to a heated pad for recovery. Sham surgeries were similar to the MI procedure, except that we skipped the ligation of the coronary artery. All surgical procedures were performed maintaining aseptic conditions. The animals received topical bupivacaine injection immediately after the skin was closed. In addition, we injected buprenorphine (20–40 μg/kg sc) during surgery and every 8–12 h for 3 days postsurgery. Experiments were performed ∼14 wk post-MI surgeries, as a study in rats showed decreases in maximal and submaximal diaphragm force at this time (59). A small subgroup of WT mice (n = 5 sham and 4 CHF) was studied 4 wk postsurgery for measurements of Nox2 subunit mRNA levels, as increases in diaphragm oxidants with CHF have been shown within 6 wk postsurgery (55).
Echocardiography.
We used transthoracic echocardiography (Vevo 770; Visual Sonics, Toronto, ON, Canada) with a 30-MHz probe to obtain parasternal two-dimensional views of the left ventricle (LV). We placed the anesthetized mouse (1–2% isoflurane delivered via a nose cone) in the supine position on a heated pad. Heart rates of the animals ranged from 400 to 500 beats/min during the imaging. We used M-mode tracings of the parasternal short-axis view to determine LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) at approximately the midpapillary level over three cardiac cycles to calculate LV fractional shortening (%) as (LVEDD − LVESD)/LVEDD × 100. Our measurements were consistent with the recommendations of the American Society of Echocardiography (35).
Tissue harvesting and infarct size.
We isolated the diaphragm and heart with the animals in the surgical plane of anesthesia. Fresh diaphragms were used for contractile functions and Amplex Red Assay, while portions of the costal diaphragm were snap-frozen in liquid nitrogen and later processed for measurement of mRNA and protein expressions. We further dissected the right (RV) and left ventricles (LV) for measurements of weight and infarct area. To determine infarct area, we cut the interventricular septum from the base to the apex of the LV and acquired a digital photograph using a stereozoom microscope. The transmural infarct area was determined by planimetry (25). Based on previous studies in rodents (25, 59, 60) and the more pronounced diaphragm weakness in patients with severe CHF (24), we included only mice with infarct area >20% of LV + septum in the study. Sixteen (out of 27) WT MI mice that survived met the inclusion criteria for infarct area. Among knockouts, 12 (out of 19) survivors met the inclusion criteria for infarct area. Thus, the number of animals used for data analysis in the 14-wk studies was 18 sham and 16 CHF for WT and 14 sham and 12 CHF forp47phox−/−.
Diaphragm contractile properties in vitro.
The assessment of diaphragm isometric and isotonic contractile properties was consistent with previous studies (2, 50). We dissected a diaphragm strip from the left hemidiaphragm along with the rib and central tendon in bicarbonate-buffered solution (in mmol/l: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 2 CaCl2) gassed with a mixture of 95% O2 and 5% CO2 at room temperature. We used a 4.0 braided silk suture to tie the rib to a glass rod and attach the central tendon to a Dual-Mode Muscle Lever System (300C-LR; Aurora Scientific, Aurora, ON, Canada). We placed the diaphragm strip in an organ bath containing bicarbonate-buffered solution at room temperature and determined the length that elicited maximal twitch force [optimal length (Lo)]. To find Lo, we preloaded the muscle with 25–30 mN and stimulated at 1 Hz (600-mA current, 0.25-ms pulse). The stimulations were repeated in 0.2- to 0.3-mm shortening steps until reaching maximal twitch force. We then placed the muscle at L0, increased the temperature of the organ bath to 37°C, and added d-tubocurarine (25 μM) to the buffer. After 20 min of thermoequilibration, we started our force-frequency protocol. The isometric force-frequency protocol consisted of stimulus frequencies of 1–300 Hz interspersed by 1-min intervals. The stimulation protocol consisted of supramaximal electrical current (600 mA) with pulse duration of 0.25- and 300-ms train duration delivered through platinum electrodes using a biphasic high-power stimulator (701C; Aurora Scientific). Isometric force was normalized for bundle cross-sectional area (CSA; N/cm2). After the protocol, we measured the bundle's Lo and weight. To estimate the bundle CSA, we divided the diaphragm bundle weight (g) by L0 and multiplied to muscle-specific density (1.056 g/cm3). We used the sigmoidal Hill equation to analyze the force-frequency relationship and determine the frequency that elicits 50% maximal force and the slope of the relationship.
To test isotonic properties of the diaphragm, we used afterloaded contractions employing a protocol similar to previous studies (9, 59). The bundle was dissected and placed at Lo, as described above. After 20 min of thermoequilibration, we stimulated the muscle supramaximally (600 mA, 300 Hz, 0.25-pulse, 200-ms train) and allowed it to shorten against an external load corresponding to 2–80% of the maximal isometric tension. Each step of the protocol was done with 2-min intervals between stimulations. Force and length data were sampled at 1,000 Hz. After the protocol, we measured Lo and bundle weight to estimate bundle CSA. We analyzed shortening velocity ≥10 ms after the initial change in length and within the linear portion of the tracing (DMA software; Aurora Scientific). The force-velocity curve was plotted and fitted to the hyperbolic Hill equation. We determined maximal shortening velocity (Vmax) as the velocity at zero force in the force-velocity relationship. We multiplied force and velocity to calculate power and used the curve fit of the force-velocity relationship to determine peak power (W). Shortening velocity was normalized to Lo, and peak power was normalized to bundle weight (kg).
Diaphragm oxidants.
We used an Amplex Red assay to measure extracellular oxidants from intact tissue, following established procedures (16) with slight adaptation to the diaphragm muscle. Specifically, we dissected a diaphragm bundle and clamped the muscle and central tendon using tissue ring supports (Radnoti). We then placed the muscle in an organ bath containing bicarbonate-buffered solution (see above) at room temperature, attached the bundle to a glass rod and lever system, adjusted muscle length to ∼10 mm (average Lo in our preparation), and increased the temperature of the organ bath to 37°C, allowing 10 min for thermoequilibration. After thermoequilibration, we exposed the muscle to the buffer solution with 20 μM Amplex Red, 0.4 U/ml horseradish peroxidase, and 35 U/ml superoxide dismutase (SOD) at 37°C for 30 min under quiescent (unstimulated) conditions. We measured Amplex Red fluorescence (excitation = 530 nm, emission = 590 nm) in a standard cuvette using a spectrofluorometer (SpectraMax M5; Molecular Devices) and normalized the signal to diaphragm bundle wet weight. The assay reagents were prepared fresh daily from frozen or refrigerated stock solutions. Amplex Red is a membrane-impermeable probe specific for measurement of hydrogen peroxide. We included SOD in our preparation to convert superoxide to hydrogen peroxide and obtain a global measure of extracellular oxidants.
To avoid problems introduced by day-to-day and time-of-day variability in the preparation and fluorescence measurements, we performed experiments involving Amplex Red fluorescence in matched pairs of sham and CHF mice within strains. The assay reagents were prepared fresh from frozen or refrigerated stock solutions for each paired set of experiments. This approach is consistent with that used by other groups in fluorescence assays of oxidants in the diaphragm (37, 53).
Gel electrophoresis and immunoblotting.
We homogenized diaphragm samples on ice in a protein extraction buffer consisting of 20 mmol/l HEPES, 2 mmol/l EGTA, 1% Triton X-100, and 50 mmol/l β-glycerophosphate, pH 7.4, with protease and phosphatase inhibitor cocktails. We rotated the homogenates end over end for 1 h at ∼4°C, sonicated once for ∼3 s, and centrifuged at 1,500 g for 2 min at room temperature. We isolated the supernatant and determined its protein contents using the DC protein assay (Bio-Rad Laboratories). Homogenates were mixed with Laemmli sample buffer and heat-denatured for SDS-PAGE.
We loaded 10–30 μg of proteins into 4–20% stain-free TGX gels (Bio-Rad Laboratories) and performed electrophoresis at 200 V for 50 min on ice. We scanned the gel to quantify total proteins (Gel Doc EZ Imager; Bio-Rad Laboratories) and transferred the proteins to a nitrocellulose membrane at 100 mA overnight at 4°C. We blocked the membrane using Li-COR Blocking Buffer (Li-COR, Lincoln, NE) for 1 h at room temperature and subsequently probed with primary antibodies. We used primary antibodies targeting the following proteins: p47phox (SAB2500674; Sigma-Aldrich), Rac1 (05-389; Millipore), gp91phox (611414; BD Transduction Laboratories), p22phox (FL-195; Santa Cruz Biotechnology), p67phox (07-502; Millipore), and 4-hydroxynonenol (4-HNE; ab46544; Abcam). We diluted the primary antibodies in Li-COR Blocking Buffer at a 1:1,000 ratio, except for p22phox (1:250). Primary antibody incubations were done at room temperature for either 1 (p22phox, 4-HNE) or 4 h (p47phox, Rac1, gp91phox, p67phox). After primary antibody incubation, we washed the membranes in TBS-T (4 × 5 min), incubated in secondary antibody (IR Dye, 1:10,000; LI-COR) for 1 h at room temperature, washed again (TBS-T, 4 × 5 min), and rinsed in 1× TBS. We dried the membranes (37°C, 15 min) and scanned using an Odyssey Infrared Imaging system (LI-COR). The immunoblot signal of each target protein was normalized to the total protein signal measured in the corresponding stain-free gel lanes, as described in our recent study (1). Stain-free gels provide a total protein signal that is conceptually similar to gel staining with Comassie Blue (57). These procedures are consistent with recent recommendations for data analysis of Western blots using fluorescence methods and stain-free gels (20, 44).
Oxyblot.
We measured protein carbonyls in whole diaphragm homogenates using the OxyBlot Protein Oxidation Detection Kit (S7150; Millipore), following the manufacturer's instructions with minor modifications. Briefly, we denatured 10 μg of proteins using 6% SDS and derivatized the sample by adding 10 μl of 2,4 dinitrophenylhydrazine or derivatization-control solution (negative control). We incubated the samples at room temperature for 15 min before adding 15 μl of neutralization solution to stop the derivatization reaction. We immunoblotted (see above) using anti-DNP primary antibody (diluted at 1:150 ratio) at room temperatuere for 1 h. We then incubated the membrane for 1 h at room temperature in anti-rabbit secondary antibody (IRDye 800CW; 1:10,000). We scanned the membrane, quantified integrated intensity in each lane, and determined total protein in the gels, as explained above.
Immunoprecipitation.
We homogenized diaphragm bundles in 1× RIPA buffer (in mmol/l: 20 Tris·HCl, 150 NaCl, 1 Na2EDTA, 1 EGTA, 2.5 Na4O7P2, 1 C3H7Na2O6P, 1 Na3VO4, 1 μg/ml leupeptin, 1% NP-40, and 1% C24H39NaO4; Cell Signaling Technology) using a Kontes Duall Homogenizer centrifuged for 2 min at 1,500 g (4°C), saved the supernatant, and measured protein content using DC protein assay (Bio-Rad Laboratories). We diluted each sample as needed to obtain a final protein content of 2.5 μg/μl and used a commercial kit for immunoprecipitation (Catch-and-Release version 2.0 kit, cat. no. 17-500; Milipore), following the manufacturer's recommendations with optimizations for our experiment. Specifically, we centrifuged spin columns for 30 s at 2,000 g to remove resin buffer and washed twice in 1× wash buffer (400 μl). For immunoprecipitation reaction, we added to the spin column 1× wash buffer (370 μl), tissue lysate (250 μg of protein), anti-p47phox monoclonal antibody (Ab; 4 μg of Ab, sc-17845; Santa Cruz Biotechnology), and Ab capture affinity ligand (10 μl). We then incubated samples using a 360° rotator for ∼20 h at 4°C. After the incubation, we centrifuged the spin columns for 30 s at 2,000 g, followed by three washes with 1× wash buffer (2,000 g, 30 s each time), added 70 μl of 1 × denaturing elution buffer with 5% vol/vol β-mercaptoethanol, incubated on a vortex shaker for 45 min, and centrifuged for 1 min at 5,000 g to collect the eluent. We heat-denatured (5 min, 98°C) the eluent and stored at −20°C until use for gel electrophoresis and immunoblotting. We used a phosphoserine antibody (Clone 7F12; Invitrogen) at 1:1,000 and total p47phox antibody (SAB2500674; Sigma-Aldrich) to calculate the phosphorylated-to-total p47phox ratio. This is a standard approach to examine p47phox phosphorylation in animal tissue (32).
Quantitative PCR.
We isolated total RNA from diaphragm tissue with Trizol reagent. We then used an Ambion RETROscript First Strand Synthesis Kit (Life Technologies, Carlsbad, CA) to generate cDNA from 1 μg of RNA. The cDNA was then used as template for qRT-PCR (7300 real-time PCR system; Applied Biosystems, Austin, TX). We used TaqMan PCR assay primers (Life Technologies) targeting the following genes: p47phox (Ncf1; GeneBank NM_001286037.1), Rac1 (GeneBank NM_009007.2), gp91phox (Cybb; GeneBank NM_007807.5), p22phox (Cyba; GeneBank NM_007806.3), p67phox (Ncf2; GeneBank NM_010877.4), and p40phox (Ncf4; GeneBank NM_008677.2). Gene expression quantification was performed using the relative standard curve method, and all data were normalized to the absolute control group and subsequently normalized to the gene expression of 18S rRNA.
Statistical analysis.
We performed statistical analysis using SigmaPlot version 12.5 (Systat Software, San Jose, CA). For specific comparisons, we used paired and unpaired Student's t-test, one-way ANOVA, and repeated-measures two-way ANOVA. Post hoc comparisons were done with Dunnett's test. These data are given as means ± SE. Data that failed the normality (Shapiro-Wilk) or equal variance tests were compared using the Mann-Whitney rank sum test. Nonparametric data are presented as median (interquartile range) and shown in box and whisker plots. We declared statistical significance when P < 0.05.
RESULTS
Mouse model of CHF.
Echocardiography and morphological cardiac measurements showed signs of LV dilation and hypertrophy and decreased fractional shortening that are consistent with CHF postinfarct (Table 1).
Table 1.
Animal characteristics
| Wild Type |
p47phox−/− |
|||
|---|---|---|---|---|
| Sham | CHF | Sham | CHF | |
| Body weight presurgery, g | 30 ± 1 | 28 ± 1 | 22 ± 1 | 24 ± 1 |
| Body weight postsurgery, g | 35 ± 1 | 35 ± 1 | 25 ± 1 | 26 ± 1 |
| LV weight, mg | 112 ± 2 | 143 ± 4* | 92 ± 4 | 148 ± 7* |
| RV weight, mg | 25 ± 1 | 32 ± 1* | 18 ± 1 | 26 ± 1* |
| LV weight/tibial length, mg/mm | 5.8 ± 0.4 | 7.8 ± 0.2* | 5.3 ± 0.3 | 8.3 ± 1.2* |
| RV weight/tibial length, mg/mm | 1.4 ± 0.4 | 1.8 ± 0.1* | 1.0 ± 0.04 | 1.5 ± 0.2* |
| Infarcted area, % | 26 ± 1 | 40 ± 5† | ||
| Heart rate, beats/min | 441 ± 18 | 418 ± 33 | 490 ± 15 | 475 ± 15 |
| LVEDD, mm | 3.76 ± 0.15 | 4.41 ± 0.11* | 3.25 ± 0.12 | 4.60 ± 0.39* |
| LVESD, mm | 2.29 ± 0.07 | 3.45 ± 0.08* | 1.97 ± 0.20 | 3.47 ± 0.38* |
| Fractional shortening, % | 39 ± 2 | 21.6 ± 4* | 40 ± 10 | 25 ± 6* |
Data are means ± SE; n = 16–18/group (wild type) and 8–10/group (p47phox−/−). CHF, chronic heart failure; LV, left ventricle; RV, right ventricle; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter. Heart rate was measured during echocardiography
P < 0.05 vs. sham within strain
P < 0.05 vs. WT CHF
Nox2 subunit mRNA, protein level, and p47phox phosphorylation.
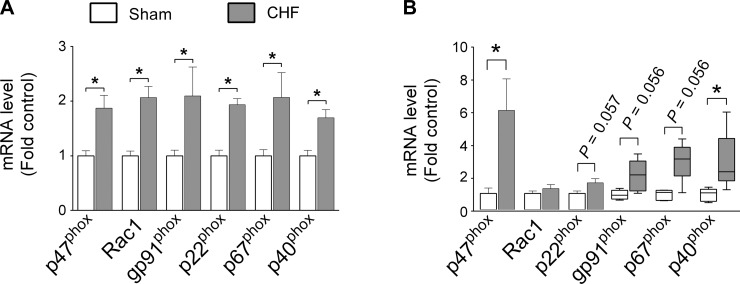
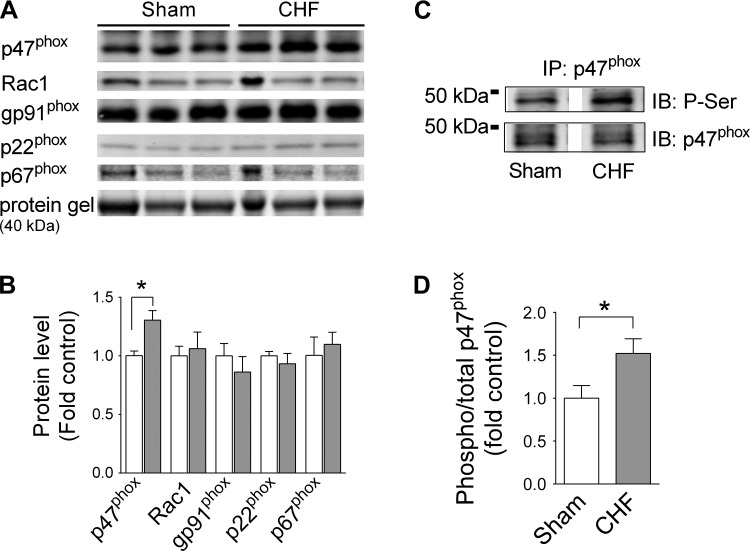
CHF increased diaphragm mRNA levels of all Nox2-related subunits within 4 wk (Fig. 1A). At ∼14 wk postsurgery, the mRNA levels of p47phox and p40phox remained significantly elevated in CHF (Fig. 1B). Similarly, CHF increased the protein level of p47phox, with no effect on other Nox2-related subunits (Fig. 2, A and B). Considering that p47phox phosphorylation regulates activation of Nox2, we immunoprecipitated p47phox and immunoblotted for serine phosphorylation. This approach revealed that CHF increased p47phox phosphorylation in the mouse diaphragm (Fig. 2, C and D). Immunoblotting confirmed the absence of p47phox in the diaphragm of p47phox−/− mice (data not shown). Based on these observations, we examined the role of p47phox on diaphragm oxidants and contractile dysfunction in CHF.
Fig. 1.
Diaphragm mRNA levels of NAD(P)H oxidase isoform 2 (Nox2) subunits 4 (A) and ∼14 wk postsurgery (B). Note that each of the graphs has a different scale on its respective y-axis. *P < 0.05 by unpaired t-test or Mann-Whitney rank sum test. Nonparametric data are shown by box and whisker plots. CHF, chronic heart failure.
Fig. 2.
Mouse diaphragm Nox2 subunit protein level and serine phosphorylation of p47phox. A and C: representative images of immunoblots (IB). IP, immunoprecipitation. B and D: quantification of protein level and serine phosphorylation (p-Ser) in sham (open bars; n = 4–5) and CHF mice (gray bars; n = 4–6). *P < 0.05 by t-test.
Extracellular oxidants and Nox activity.
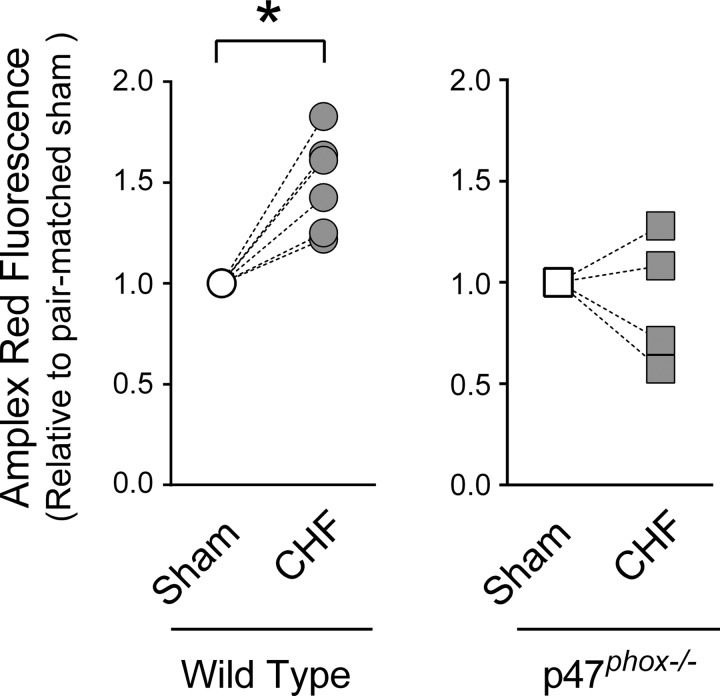
Nox is localized in cell membranes and can produce oxidants in the extracellular space (36). Our Amplex Red assay included SOD to convert superoxide to hydrogen peroxide and provide a global measure of extracellular oxidants. We observed that CHF increased diaphragm extracellular oxidants in WT mice, whereas deficiency in p47phox prevented the increase in extracellular oxidants elicited by CHF (Fig. 3). However, we were not able to detect changes in Nox activity measured by NADH consumption from cytosolic plus membrane fractions (in μM·min−1·mg protein−1: sham 4.42 ± 0.7, CHF 5.4 ± 0.50; n = 4/group).
Fig. 3.
Extracellular oxidants in diaphragm bundles. Amplex Red fluorescence was divided by diaphragm bundle weight, and value shown is relative to pair-matched sham within strains. *P < 0.05 by paired Student's t-test. See methods for rationale on paired experiments and statistical analysis.
Diaphragm contractile function and markers of protein oxidation.
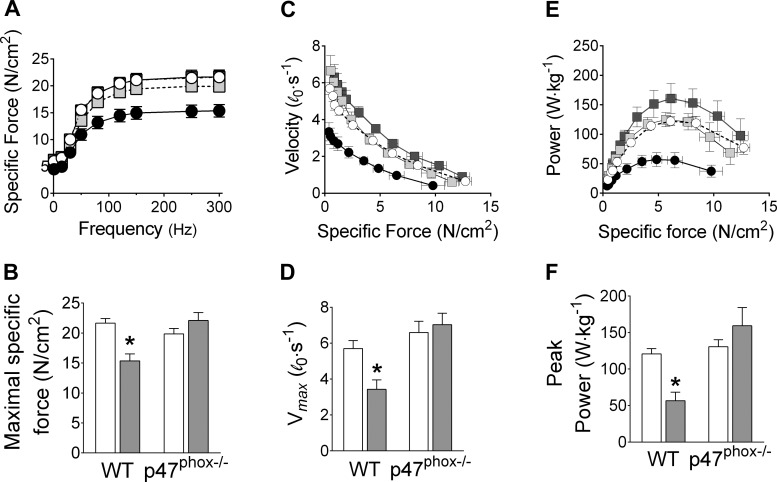
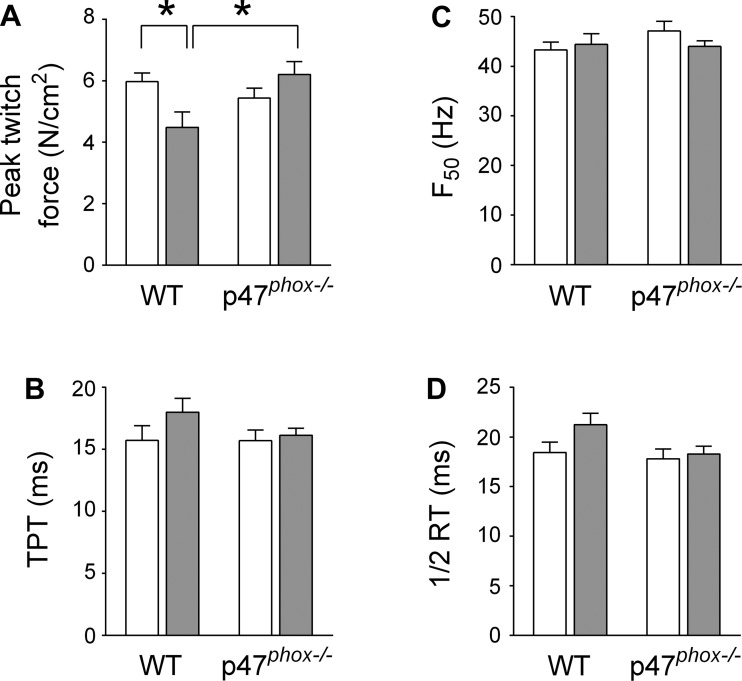
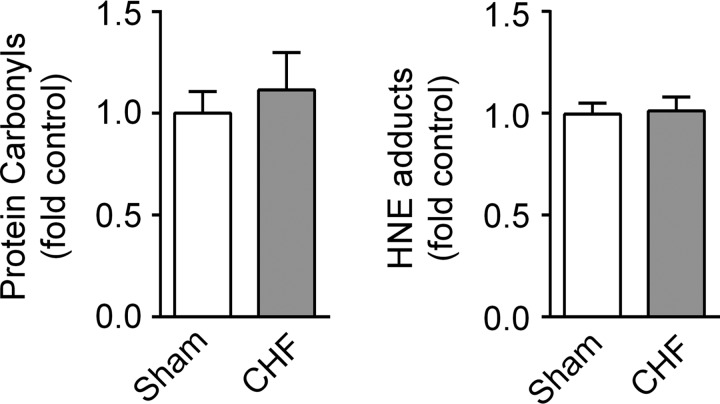
We examined isometric and isotonic contractile properties of mouse diaphragm bundles in vitro. CHF decreased maximal isometric specific force and peak twitch force (Figs. 4, A and B, and 5). These data are consistent with a decrease in diaphragm force in all stimulus frequencies. Isotonic contractile properties of the diaphragm were also impaired by CHF. Specifically, CHF slowed Vmax by 40% (Fig. 4, C and D) and diminished peak power by 50% compared with sham (Fig. 4, E and F). To our knowledge, these are the first data to show diaphragm contractile dysfunction in a mouse model of CHF. However, our most important findings were that p47phox−/− mice were fully protected from isometric and isotonic contractile dysfunction induced by CHF (Fig. 4, A–F). Twitch kinetics and the frequency that elicits 50% maximal force were unchanged in CHF compared with sham WT mice (Fig. 5). Abnormalities in contractile function are typically associated with markers of protein oxidation such as carbonyls and 4-HNE adducts (7, 12). However, protein carbonyls and 4-HNE adducts were not increased in diaphragm of WT mice, as shown in Fig. 6.
Fig. 4.
Diaphragm isometric (A and B) and isotonic (C–F) contractile properties. A and B: absolute force (N) is normalized for bundle cross-sectional area (cm2) and shown as specific force in N/cm2 (n = 7–10 mice/group). Wild type (WT): sham (○) and CHF (●). p47phox−/−: sham (light gray squares) and CHF (dark gray squares). Maximal shortening velocity (Vmax; D) and peak power (F) are determined from force-velocity (C) and force-power relationship (E) (n = 5–7 mice/group). *P < 0.05 vs. other groups by Dunnett's test.
Fig. 5.
Diaphragm twitch and force-frequency characteristics. A: peak twitch force. B: time to peak tension (TPT). C: stimulus frequency that elicits 50% of maximal force (F50). D: one-half (½) relaxation time (RT). *P < 0.05 vs. other groups by Dunnett's test.
Fig. 6.
Protein carbonyls and 4-hydroxynonenal (HNE) adducts in mouse diaphragm. Western blot data are from n = 5–6 mice/group. The total sum of carbonyls (A) or HNE (B) signal in each lane was normalized to total protein of respective lanes.
DISCUSSION
Ventilatory abnormalities play an important role in the prognosis, diminished physical capacity, and dyspnea of CHF patients (10, 14, 40). The diaphragm is the main inspiratory muscle, and our study suggests that the p47phox subunit of NAD(P)H oxidase is involved in the diaphragm dysfunction elicited by CHF. The diaphragm of CHF mice showed increases in gene and protein levels of Nox2 subunits and phosphorylation of p47phox. In this setting, deficiency in p47phox prevented the increase in diaphragm oxidants and contractile dysfunction induced by CHF.
Mouse model of CHF.
As expected (18), MI caused CHF in mice at 14 wk postsurgery. Infarct area was greater in p47phox−/− mice, likely because WT mice with infarcts as large as those of p47phox−/− had lower survival rates (18). The changes in RV and LV weights were greater in p47phox−/− compared with WT, with similar decreases in fractional shortening in both strains. These data suggest that the p47phox−/− mice included in our study had similar or even worse degrees of CHF than the WT group due to the larger infarct area in p47phox knockout mice. Thus, it is unlikely that our results from p47phox−/− mice would be explained by attenuated LV dysfunction post-MI (18). This is important because diaphragm weakness in CHF depends on the severity of the disease (3, 24, 31).
Diaphragm Nox2 mRNA and protein levels.
Diaphragm Nox2 has a subunit composition and subcellular localization generally similar to the isoform found in heart and vasculature (33, 36), where p47phox is required for enzyme activation. CHF increased diaphragm mRNA levels of p47phox and p40phox, whereas the increase in other subunits was close (P < 0.06) to the α-level of 0.05 declared a priori for significance at the 14-wk time point. There was also a less prominent but uniform increase in mRNA levels of all Nox2-related subunits in the diaphragm at an earlier stage of the disease (4 wk; Fig. 1A). At this time point, the increase in diaphragm mRNA level of Nox2 subunits is similar to that seen in the heart (18, 39). Overall, our data also suggest that CHF's effect on the Nox2 subunit mRNA level is time/disease dependent, being increased in the more severe and advanced stage of the disease.
Phosphorylation of p47phox.
Receptor-mediated activation of Nox2 by endocrine mediators such as angiotensin II, cytokines, and adrenergic agonists that are elevated in CHF occurs via phosphorylation of p47phox (13, 32, 34, 36). CHF increased p47phox phosphorylation in the diaphragm. We did not determine the specific residues, as site-specific mouse antibodies are not available, and immunoprecipitation followed by immunoblot with phosphoserine specific antibody is the standard approach to detect p47phox phosphorylation in animals (e.g., see Ref. 32). However, site-specific modification studies in white blood cells have established that phosphorylation of serine residues between amino acids 303 and 379 releases autoinhibition and is required for full enzyme activation (21, 26). Thus, the increase in p47phox phosphorylation in CHF suggests elevated Nox2 activity and oxidant production in the diaphragm.
Diaphragm oxidants.
CHF increased NAD(P)H oxidase activity and extracellular oxidants in the diaphragm, which is in agreement with previous studies (7, 12, 55). To date, the specific source of CHF-induced heightened oxidants in diaphragm has been unclear. A recent study showed increased diaphragm Nox activity during early-stage (72 h) post-MI (7). We used a similar approach but observed no significant change in Nox activity, which may reflect a limitation of the assay or the number of animals per group that we tested. However, we found that deficiency in p47phox prevented the increase in diaphragm extracellular oxidants stimulated by CHF. This is evidence in support of the involvement of p47phox, presumably via Nox2 action, on heightened oxidants in the CHF diaphragm. Mitochondria isolated from the diaphragms of CHF rats also show excess oxidant emission in the CHF diaphragm (55), and xanthine oxidase could also be involved, as seen in the heart (18). At first glance, our findings and those of Supinski and Callahan (55) may appear conflicting, but mitochondrial oxidants could be upstream or downstream of p47phox signaling. Indeed, a cross-talk between Nox2 and mitochondria oxidants has been reported in vascular smooth muscle (15, 17). Overall, our data show that whole body p47phox knockout modulates CHF-induced accumulation of oxidants in the diaphragm.
Diaphragm contractile function.
Patients with CHF have decreased inspiratory muscle pressure during static and dynamic maneuvers (31, 40), which suggests impairments in diaphragm function that worsen as the disease progresses. Consistent with this notion, diaphragm contractile dysfunction has been shown in several animal models of CHF (8, 12, 54, 59). Assessment of isometric function gives insights into the force-generating capacity of the diaphragm. As the diaphragm exerts inspiratory function primarily by shortening, isotonic contractile properties are the most relevant for ventilation. We show herein that diaphragms from WT mice with CHF induced by MI have isometric and isotonic contractile dysfunction, as seen in rats (12, 22, 59). Our findings in mice set the stage for specific hypothesis testing using genetically modified animals. In this regard, the absence of the p47phox subunit of NAD(P)H oxidase prevented both isometric and isotonic diaphragm contractile impairments caused by CHF (see Fig. 5).
The increase in oxidants that we observed in CHF is a putative mechanism for impaired diaphragm contractile function. Direct exposure of skeletal muscle cells to excess oxidants impairs contractile function, with decreases in maximal force (11), shortening velocity (12), actomyosin ATPase activity (49), and calcium sensitivity of the contractile apparatus (4). Importantly, systemic treatment of CHF rats with a membrane-permeable SOD prevents the decrease in submaximal diaphragm isometric force (55). Thus, we reason that the lack of increase in diaphragm oxidants in p47phox−/− mice conferred protection against depression of force, slowing of maximal shortening velocity, and reduction in peak power induced by CHF.
Protein oxidation.
The exact molecular mechanisms underlying the depression in diaphragm contractile function in CHF is less clear. Our data cannot distinguish between impairments in excitation-contraction coupling or myofibrillar proteins, both of which could be impaired by protein oxidation (12, 58). In an acute model of heart failure in mice (72 h post-MI), protein carbonyls were increased in the diaphragm (7). In our study, carbonyls were not changed in a chronic model of heart failure (∼14 wk post-MI). The discrepancy between our findings and those of Bowen et al. (7) may be related to the time course or the severity of the disease. A potential cause of loss of diaphragm force, shortening velocity, and power that we observed in CHF mice is the oxidation of thiol groups in the ryanodine-receptor channel (58), myosin heavy chain (27, 49), or actin (23). The resolution of specific thiol modifications underlying the CHF-induced diaphragm contractile dysfunction requires the use of more sophisticated and sensitive techniques (e.g., see Refs. 23 and 42). Alternatively, posttranslational modifications such as ubiquitination and (de)phosphorylation could be triggered by oxidants and impair contractile function.
Methodological considerations.
We cannot attribute our findings to p47phox or NAD(P)H oxidase within diaphragm muscle cells per se. In addition to muscle fibers, several other cell types within the diaphragm express p47phox, e.g., endothelium, smooth muscle, and macrophages. Experiments using cell type-specific interventions and inducible knockouts will be required to resolve the source of p47phox and NAD(P)H oxidase responsible for diaphragm abnormalities in CHF.
Conclusion.
To sum up, oxidants are known to cause abnormalities in diaphragm muscles during chronic diseases. However, the specific sources of oxidants and their clinical relevance are not well defined. Our study establishes a critical role for the p47phox subunit of NAD(P)H oxidase on the pathophysiology of diaphragm dysfunction in CHF. In this setting, Nox2 is presumably an important source of increased diaphragm oxidants that causes weakness in heart failure. Thus, targeting p47phox signaling should prevent the increase in diaphragm oxidants that causes weakness and loss of power that contributes to dyspnea and exercise intolerance in CHF.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grant R00-HL-098453 to L. F. Ferreira. A. R. Judge was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01-AR-060209.
DISCLOSURES
No conflicts of interest, financial otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S.A., A.W.B., G.S.F., and L.F.F. performed experiments; B.S.A., A.W.B., G.S.F., A.R.J., and L.F.F. analyzed data; B.S.A., A.W.B., G.S.F., and A.R.J. interpreted results of experiments; B.S.A., A.W.B., G.S.F., and L.F.F. prepared figures; B.S.A., A.W.B., A.R.J., and L.F.F. edited and revised manuscript; B.S.A., A.W.B., G.S.F., A.R.J., and L.F.F. approved final version of manuscript; L.F.F. conception and design of research; L.F.F. drafted manuscript.
REFERENCES
- 1.Ahn B, Beaver T, Martin T, Hess P, Brumback BA, Ahmed S, Smith BK, Leeuwenburgh C, Martin AD, Ferreira LF. Phrenic nerve stimulation increases human diaphragm fiber force after cardiothoracic surgery. Am J Respir Crit Care Med 190: 837–839, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn B, Empinado HM, Al-Rajhi M, Judge AR, Ferreira LF. Diaphragm atrophy and contractile dysfunction in a murine model of pulmonary hypertension. PLoS One 8: e62702, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosino N, Opasich C, Crotti P, Cobelli F, Tavazzi L, Rampulla C. Breathing pattern, ventilatory drive and respiratory muscle strength in patients with chronic heart failure. Eur Respir J 7: 17–22, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechara LR, Moreira JB, Jannig PR, Voltarelli VA, Dourado PM, Vasconcelos AR, Scavone C, Ramires PR, Brum PC. NADPH oxidase hyperactivity induces plantaris atrophy in heart failure rats. Int J Cardiol 175: 499–507, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Bost ER, Frye GS, Ahn B, Ferreira LF. Diaphragm dysfunction caused by sphingomyelinase requires the p47(phox) subunit of NADPH oxidase. Respir Physiol Neurobiol 205: 47–52, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen TS, Mangner N, Werner S, Glaser S, Kullnick Y, Schrepper A, Doenst T, Oberbach A, Linke A, Steil L, Schuler G, Adams V. Diaphragm muscle weakness in mice is early-onset post-myocardial infarction and associated with elevated protein oxidation. J Appl Physiol (1985) 118: 11–19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen TS, Rolim NP, Fischer T, Baekkerud FH, Medeiros A, Werner S, Bronstad E, Rognmo O, Mangner N, Linke A, Schuler G, Silva GJ, Wisloff U, Adams V. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail 17: 263–272, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Bullimore SR, Saunders TJ, Herzog W, MacIntosh BR. Calculation of muscle maximal shortening velocity by extrapolation of the force-velocity relationship: afterloaded versus isotonic release contractions. Can J Physiol Pharmacol 88: 937–948, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Cahalin LP, Arena R, Guazzi M, Myers J, Cipriano G, Chiappa G, Lavie CJ, Forman DE. Inspiratory muscle training in heart disease and heart failure: a review of the literature with a focus on method of training and outcomes. Expert Rev Cardiovasc Ther 11: 161–177, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol 90: 45–54, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Coirault C, Guellich A, Barbry T, Samuel JL, Riou B, Lecarpentier Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 292: H1009–H1017, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest 116: 2033–2043, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62: 2422–2430, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 100: 894–903, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Drexler H, Banhardt U, Meinertz T, Wollschlager H, Lehmann M, Just H. Contrasting peripheral short-term and long-term effects of converting enzyme inhibition in patients with congestive heart failure. A double-blind, placebo-controlled trial. Circulation 79: 491–502, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One 8: e72457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med 41: 217–225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Empinado HM, Deevska GM, Nikolova-Karakashian M, Yoo JK, Christou DD, Ferreira LF. Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. Eur J Heart Fail 16: 519–525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedorova M, Kuleva N, Hoffmann R. Reversible and irreversible modifications of skeletal muscle proteins in a rat model of acute oxidative stress. Biochim Biophys Acta 1792: 1185–1193, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Filusch A, Ewert R, Altesellmeier M, Zugck C, Hetzer R, Borst MM, Katus HA, Meyer FJ. Respiratory muscle dysfunction in congestive heart failure—the role of pulmonary hypertension. Int J Cardiol 150: 182–185, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Finsen AV, Christensen G, Sjaastad I. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J Appl Physiol (1985) 98: 680–689, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113: 343–355, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Gross SM, Lehman SL. Accessibility of myofilament cysteines and effects on ATPase depend on the activation state during exposure to oxidants. PLoS One 8: e69110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond MD, Bauer KA, Sharp JT, Rocha RD. Respiratory muscle strength in congestive heart failure. Chest 98: 1091–1094, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Howell S, Maarek JM, Fournier M, Sullivan K, Zhan WZ, Sieck GC. Congestive heart failure: differential adaptation of the diaphragm and latissimus dorsi. J Appl Physiol 79: 389–397, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Huang CK, Zhan L, Hannigan MO, Ai Y, Leto TL. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+. J Leukoc Biol 67: 210–215, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Hughes PD, Polkey MI, Harrus ML, Coats AJ, Moxham J, Green M. Diaphragm strength in chronic heart failure. Am J Respir Crit Care Med 160: 529–534, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Isabelle M, Monteil C, Moritz F, Dautreaux B, Henry JP, Richard V, Mulder P, Thuillez C. Role of alpha1-adrenoreceptors in cocaine-induced NADPH oxidase expression and cardiac dysfunction. Cardiovasc Res 67: 699–704, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med 165: 412–418, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102: 1690–1696, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Lindsay DC, Lovegrove CA, Dunn MJ, Bennett JG, Pepper JR, Yacoub MH, Poole-Wilson PA. Histological abnormalities of muscle from limb, thorax and diaphragm in chronic heart failure. Eur Heart J 17: 1239–1250, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51: 319–325, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Mancini DM, Henson D, La Manca J, Donchez L, Levine S. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation 91: 320–329, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Mancini DM, LaManca JJ, Donchez LJ, Levine S, Henson DJ. Diminished respiratory muscle endurance persists after cardiac transplantation. Am J Cardiol 75: 418–421, 1995. [DOI] [PubMed] [Google Scholar]
- 42.McDonagh B, Sakellariou GK, Smith NT, Brownridge P, Jackson MJ. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J Proteome Res 13: 5008–5021, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, Haass M. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 103: 2153–2158, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Murphy RM, Lamb GD. Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol 591: 5823–5831, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nymo SH, Hulthe J, Ueland T, McMurray J, Wikstrand J, Askevold ET, Yndestad A, Gullestad L, Aukrust P. Inflammatory cytokines in chronic heart failure: interleukin-8 is associated with adverse outcome. Results from CORONA. Eur J Heart Fail 16: 68–75, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pal R, Basu Thakur P, Li S, Minard C, Rodney GG. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PLoS One 8: e63989, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal R, Palmieri M, Loehr JA, Li S, Abo-Zahrah R, Monroe TO, Thakur PB, Sardiello M, Rodney GG. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun 5: 4425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perkins WJ, Han YS, Sieck GC. Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J Appl Physiol 83: 1326–1332, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Roberts BM, Ahn B, Smuder AJ, Al-Rajhi M, Gill LC, Beharry AW, Powers SK, Fuller DD, Ferreira LF, Judge AR. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J 27: 2600–2610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18: 603–621, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, Michael Tabony A, Delafontaine P. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun 409: 217–221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stasko SA, Hardin BJ, Smith JD, Moylan JS, Reid MB. TNF signals via neuronal-type nitric oxide synthase and reactive oxygen species to depress specific force of skeletal muscle. J Appl Physiol (1985) 114: 1629–1636, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supinski G, DiMarco A, Dibner-Dunlap M. Alterations in diaphragm strength and fatiguability in congestive heart failure. J Appl Physiol 76: 2707–2713, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Supinski GS, Callahan LA. Diaphragmatic free radical generation increases in an animal model of heart failure. J Appl Physiol 99: 1078–1084, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16: 349–360, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int 2014: 361590, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umanskaya A, Santulli G, Xie W, Andersson DC, Reiken SR, Marks AR. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci USA 111: 15250–15255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Hees HW, van der Heijden HF, Hafmans T, Ennen L, Heunks LM, Verheugt FW, Dekhuijzen PN. Impaired isotonic contractility and structural abnormalities in the diaphragm of congestive heart failure rats. Int J Cardiol 128: 326–335, 2008. [DOI] [PubMed] [Google Scholar]
- 60.van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, Brouwer RM, Dekhuijzen PN. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol 293: H819–H828, 2007. [DOI] [PubMed] [Google Scholar]