Abstract
Low expression of vitamin D receptor (VDR) and dysfunction of vitamin D/VDR signaling are reported in patients with inflammatory bowel disease (IBD); therefore, restoration of VDR function to control inflammation in IBD is desirable. Probiotics have been used in the treatment of IBD. However, the role of probiotics in the modulation of VDR signaling to effectively reduce inflammation is unknown. We identified a novel role of probiotics in activating VDR activity, thus inhibiting inflammation, using cell models and VDR knockout mice. We found that the probiotics Lactobacillus rhamnosus strain GG (LGG) and Lactobacillus plantarum (LP) increased VDR protein expression in both mouse and human intestinal epithelial cells. Using the VDR luciferase reporter vector, we detected increased transcriptional activity of VDR after probiotic treatment. Probiotics increased the expression of the VDR target genes, such as antimicrobial peptide cathelicidin, at the transcriptional level. Furthermore, the role of probiotics in regulating VDR signaling was tested in vivo using a Salmonella-colitis model in VDR knockout mice. Probiotic treatment conferred physiological and histologic protection from Salmonella-induced colitis in VDR+/+ mice, whereas probiotics had no effects in the VDR−/− mice. Probiotic treatment also enhanced numbers of Paneth cells, which secrete AMPs for host defense. These data indicate that the VDR pathway is required for probiotic protection in colitis. Understanding how probiotics enhance VDR signaling and inhibit inflammation will allow probiotics to be used effectively, resulting in innovative approaches to the prevention and treatment of chronic inflammation.
Keywords: antimicrobial peptide, AMP, inflammatory bowel disease, IBD, vitamin D, vitamin D receptor, VDR, probiotics, Lactobacillus plantarum, Lactobacillus rhamnosus strain GG, Salmonella
inflammatory bowel disease (IBD) affects 1–1.5 million Americans and is currently incurable, resulting in substantial public health burden (10, 17, 18). Currently available therapy aims to slow the progression of disease by controlling inflammation. Vitamin D deficiency has been implicated in patients with IBD (1, 14, 20, 27, 37). The vitamin D receptor (VDR) is a nuclear receptor and transcription factor that mediates most functions of vitamin D (7). In addition to its anti-inflammatory actions, VDR serves multiple critical functions in cell differentiation and growth (16, 42). VDR also plays many key roles in the regulation of intestinal homeostasis, tight junction structure, response to invasive pathogens, and commensal bacterial colonization (6, 9, 12, 13, 15, 39). In experimental models, VDR−/− knockout mice were more susceptible to infection and invasion by pathogenic bacteria (40). Paneth cells are specialized intestinal epithelial cells that play an important role in innate immune responses and in shaping the gut microbiota (34). We have recently reported that the conditional deletion of intestinal epithelial VDR leads to dysbiosis and abnormal Paneth cells (42).
Probiotics have been used in clinical trial for the treatment of IBD. However, the responses to treatment and clinical outcomes are inconsistent (4, 5, 22, 28). Studies demonstrate that specific probiotic strains exert specific effects in IBD therapy; however, the anti-inflammatory role of probiotics remains unclear (36). Elucidating how probiotics specifically regulate VDR signaling will advance our understanding of bacterial-host interactions under conditions of inflammation. As dysregulation of bacterial-host interactions can result in chronic inflammation and VDR expression is significantly decreased in IBD patients (1, 35), strategies to restore VDR expression in inflamed mucosa may be important for the prevention and treatment of IBD.
We postulate that probiotics increase VDR expression, thereby inhibiting the inflammatory pathway. The purpose of this study was twofold: first, to determine whether probiotics enhance VDR expression. Second, if found, we sought to define the mechanism by which these probiotics promote VDR signaling and exert their anti-inflammatory effects. We found that probiotics, such as Lactobacillus rhamnosus strain GG (LGG) and Lactobacillus plantarum (LP), increased VDR expression. The transcriptional activity of VDR is also enhanced by probiotics treatment. Moreover, using VDR−/− mouse models, we further confirmed that the effects of probiotics to inhibit intestinal inflammation depended on the VDR pathway in vivo.
MATERIALS AND METHODS
Ethics statement.
All animal work was approved by Rush University Animal Resources Committee (12-016). If a mouse showed that it had aspirated fluid or had experienced significant loss of body weight (15% or more), the mouse was humanely euthanized.
Bacterial strains and growth condition.
LGG (ATCC No. 53103) and LP were propagated in deMann Rogosa and Sharpe broth (Weber Scientific) overnight at 37°C and centrifuged at 5,000 rpm for 10 min, and the supernatant was collected as conditioned media, after filtering through a 0.22-um filter, aliquoted, and stored at −80°C until use. Salmonella typhimurium wild-type ATCC14028 (19) were used in this study. Nonagitated microaerophilic bacterial cultures were grown as follows: nonagitated microaerophilic bacterial cultures were prepared by inoculation of 10 ml of Luria-Bertani (LB) broth with 0.01 ml of a stationary phase culture, followed by overnight incubation (∼18 h) at 37°C, as previously described (31). Bacterial overnight cultures were concentrated 33-fold in Hank's balanced salt solution (HBSS) supplemented with 10 mM HEPES, pH 7.4.
Cell culture.
Mouse embryonic fibroblasts (MEF) were isolated from embryonic day 13.5 embryos generated from VDR+/− x VDR+/− mouse breeding as previously described (32). VDR+/− and VDR−/− MEFs were used in experiments after more than 15 passages when they had been immortalized. MEFs, mouse rectum epithelial CMT-93 cells, and human colon carcinoma HCT116 cells were grown in DMEM (high glucose, 4.5 g/l) containing 5% (vol/vol) fetal bovine serum, 50 μg/ml streptomycin, and 50 U/ml penicillin.
Protection of probiotics on Salmonella invasion in cultured cells in vitro.
HCT116 cells (3 × 105) were seeded to sixwell plate. When cells were at 90% confluence, cells were colonized with 0.75 × 107 colony-forming units (CFU) of Salmonella with or without same amount LP for 30 min, washed with HBSS, and incubated in DMEM containing gentamicin (500 μg/ml) for 30 min (30, 31). In the first 30-min incubation, Salmonella adhered to and/or internalized into the cells. After being extensively washed in HBSS, the extracellular Salmonella were washed away. Incubation with gentamicin inhibited the growth of extracellular Salmonella. Internalized Salmonella were those obtained from lysis of the epithelial cells with 1% Triton X-100 for 30 min. LB broth (0.9 ml) was added, and each sample was vigorously mixed and counted by plating for CFU on MacConkey agar medium.
MEF cells (3 × 105) were seeded on sixwell plate. When cells were at 90% confluence, the cells were treated with 10% LP conditioned media in DMEM for 2 h and washed with HBSS. After LP conditioned media pretreatment, cells were infected with 0.75 × 107 CFU of Salmonella for 30 min, washed with HBSS, and incubated in DMEM containing gentamicin (500 μg/ml) for 30 min. The internalized Salmonella were obtained from lysis of the MEF cells with 1% Triton X-100 for 30 min, as described in the HCT116 group.
Salmonella mouse model and probiotics treatment in vivo.
Animal experiments were performed using specific pathogen-free female C57BL/6 mice (Taconic, Hudson, NY) that were 7–8 wk old and VDR knockout C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), as previously described. The protocol (No. 12-016) was approved by the Animal Resources Committee at Rush University. Water and food were withdrawn 4 h before oral gavage with 7.5 mg/mouse of streptomycin (100 μl of sterile solution or 100 μl of sterile water as control). Afterward, animals were supplied with water and food. Twenty hours after streptomycin treatment, water and food were withdrawn again for 4 h before the mice were infected with 1 × 107 CFU of S. typhimurium (100 μl suspension in HBSS). Twenty-four hours after Salmonella infection, the LP treatment group mice were gavaged with 1 × 107 CFU of LP (100 μl suspension in HBSS) daily, for 3 days, and then mice were killed. Tissue samples from the intestinal tracts were removed for analysis.
Salmonella burden in spleen.
The spleen was dissected from each mouse, cut into pieces with scissors, put into a 14-ml tube with 5 ml sterile PBS, and then homogenized using a homogenizer (Polytron PT2100, Kinematica, Switzerland). Homogenate was diluted at 1,000 to 10,000 with LB broth. The diluted homogenate was plated out on MacConkey agar plates and incubated at 37°C overnight. CFU were quantified.
Western blotting.
Cells were rinsed twice in ice-cold HBSS, lysed in protein loading buffer (50 mM Tris, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, and 10% glycerol). Immunoblot was performed with primary antibodies: anti-VDR or anti-β-actin (Sigma-Aldrich) antibodies and visualized by enhanced chemiluminescence (ECL) (32, 33).
Salmonella burden in intestine.
The cecum was dissected from each mouse, cut into pieces, put into a 14-ml tube with 5 ml sterile PBS, and then homogenized using a homogenizer (Polytron PT2100). The homogenate was diluted at 1,000 to 10,000 with LB. One-hundred microliters of diluted homogenate were plated out on MacConkey agar plates and incubated at 37° overnight. CFU were quantified.
Histology of mouse colon.
Mouse colons were harvested, fixed in 10% formalin (pH 7.4), processed, and paraffin embedded. Sections (5 μm) were stained with hematoxylin and eosin. Histologic inflammatory scores were performed by a validated scoring system (26).
Immunofluorescence.
Ileal tissues from the distal portion of the ileum and ceca were freshly isolated and paraffin embedded after fixation with 10% neutral buffered formalin. Immunofluorescence was performed on paraffin-embedded sections (5 μm). After preparation of the slides as described previously, ileal tissue samples were incubated with antilysozyme (sc27958; Santa Cruz) and ceca samples with anti Salmonella (sc-52224; Santa Cruz) at 4°C overnight. Samples were then incubated with sheep anti-goat Alexa Fluor 594 (A11058; Life Technologies) or goat anti-mouse Alexa Fluor 488 (A-11029; Life Technologies) and DAPI (D1306; Life Technologies) for 1 h at room temperature. Tissues were mounted with SlowFade (s2828; Life Technologies) and covered by a coverslip, and the edges were sealed to prevent drying. Specimens were examined with a Zeiss laser scanning microscope (LSM) 710.
Paneth cells counting.
Paneth cells in mouse ileal cells were counted after anti-lysozyme immunofluorescence staining. Paneth cells were counted according to published methods (2, 42).
Salmonella-induced mouse cytokines.
Mouse blood samples were collected by cardiac puncture and placed in tubes containing EDTA (10 mg/ml). Mouse cytokines were measured using a mouse cytokine 10-Plex Panel kit (LMC0001; Life Technologies) according to the manufacturer's instructions. The cytokines included IL-12, IL4, IFNγ, and TNFα. Cytokines were analyzed with the Luminex detection system (PerkinElmer CS1000 Autoplex Analyzer).
VDR protein expression transcriptional activity.
Cells were grown in triplicate and transfected with Cignal Vitamin D Reporter (luc) Kit (SABiosciences, Frederick, MD) using Surefect reagent (SABiosciences). The plasmid for the VDR Reporter is a combination of an inducible Vitamin D-responsive firefly luciferase construct and a constitutively expressing Renilla luciferase construct (40:1). The negative control is a combination of a noninducible firefly luciferase construct and a constitutively expressing Renilla luciferase construct (40:1). After transfection for 24 h, cells were colonized with Salmonella for 30 min, washed, and incubated in DMEM with gentamicin (500 μg/ml) for 12 h or 16 h. Luciferase activity was determined using the Dual Luciferase Reporter Assay System (Promega) with a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA).
Quantitative real-time PCR analysis.
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA). The RT cDNA reaction products were subjected to quantitative real-time PCR using the MyiQ single-color real-time PCR detection system (Bio-Rad) and iQ SYBR green supermix (Bio-Rad) according to the manufacturer's directions (43). All expression levels were normalized to the β-actin levels of the same sample. Percent expression was calculated as the ratio of the normalized value of each sample to that of the corresponding untreated control cells. All real-time PCR reactions were performed in triplicate, as previous described (40, 42). PCR primers are listed in Table 1.
Table 1.
Real-time PCR primers
| Gene Name | Primer (5′→3′) |
|---|---|
| hCathelicidin F | TGCCCAGGTCCTCAGCTAC |
| hCathelicidin R | GTGACTGCTGTGTCGTCCT |
| hCyp24 F | GCCTGGCAGAGCTTGAATT |
| hCyp24 R | ACAGTCCGGGTCTTGGGT |
| hβ-Actin F | AGAGCAAGAGAGGCATCCTC |
| hβ-Actin R | CTCAAACATGATCTGGGTCA |
| mlyz1 F | GAGACCGAAGCACCGACTATG |
| mlyz1 R | CGGTTTTGACATTGTGTTCGC |
| mlyz2 F | ATGGAATGGCTGGCTACTATGG |
| mlyz2 R | ACCAGTATCGGCTATTGATCTGA |
| mDEFars1 F | AGCAGCCATTGTGCGAAGAA |
| mDEFars1 R | TGCTGTGTATTTGGAGCTTGG |
| mDEFα5 F | AGGCTGATCCTATCCACAAAACAG |
| mDEFα5 R | TGAAGAGCAGACCCTTCTTGGC |
| mDEFα22 F | ACCAGGCTGTGTCTGTCTCCTT |
| mDEFα22 R | TGGCCTCAGAGCTGATGGTTGT |
| mRIP3 g F | GGTGAGGAGCATTAGTAACAGC |
| mRIP3 g R | CCAGGGTTTAAGATGGTGGAGG |
| mVDR F | GAATGTGCCTCGGATCTGTGG |
| mVDR R | ATGCGGCAATCTCCATTGAAG |
| mβ-Actin F | TGTTACCAACTGGGACGACA |
| mβ-Actin R | CTGGGTCATCTTTTCACGGT |
F, forward; R, reverse.
Statistical analysis.
Data are expressed as means ± SD. Differences between two samples were analyzed by Student's t-test. P ≤ 0.05 was considered significant. Differences among three or more groups were analyzed using ANOVA (SAS 9.3 version; SAS Institute, Cary, NC).
RESULTS
VDR level is elevated by LGG and LP in vitro.
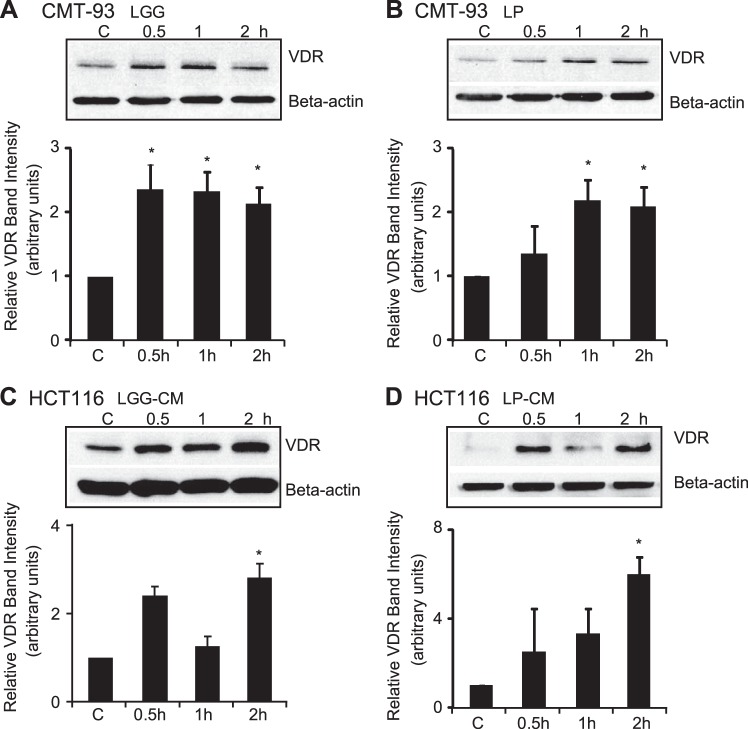
We assessed the effect of probiotics on VDR expression in various epithelial cell models. We chose the probiotic strains LGG and LP as they have been utilized in the treatment of IBD patients (4, 5, 22, 28). VDR protein expression was increased in probiotics-treated cells in vitro (Fig. 1). We found that VDR expression was significantly increased in LGG- and LP-treated mouse rectum epithelial CMT-93 cells (Fig. 1, A and B). Human epithelial HCT116 cells have a low expression of VDR at the basal level. In HCT 116 cells, we can clearly see the probiotics-induced VDR after probiotic treatment. We also observed consistently increased VDR protein induced by the culture medium of LGG and LP in a time-dependent manner (Fig. 1, C and D).
Fig. 1.
Probiotics Lactobacillus rhamnosus strain GG (LGG) and Lactobacillus plantarum (LP) increased vitamin D receptor (VDR) protein expression in vitro. A: LGG increases VDR protein expression in mouse epithelia CMT-93 cells. B: LP increases VDR protein expression in CMT-93 cells. C: LGG conditioned medium (CM) increases VDR protein expression in human epithelial HCT116 cells. D: LP CM increases VDR protein expression in HCT116 cells. Cells were treated with indicated time course. Three separate experiments with 3 replicates each, *P < 0.05, compared with control (C).
VDR signaling is elevated by LGG and LP at the transcriptional level.
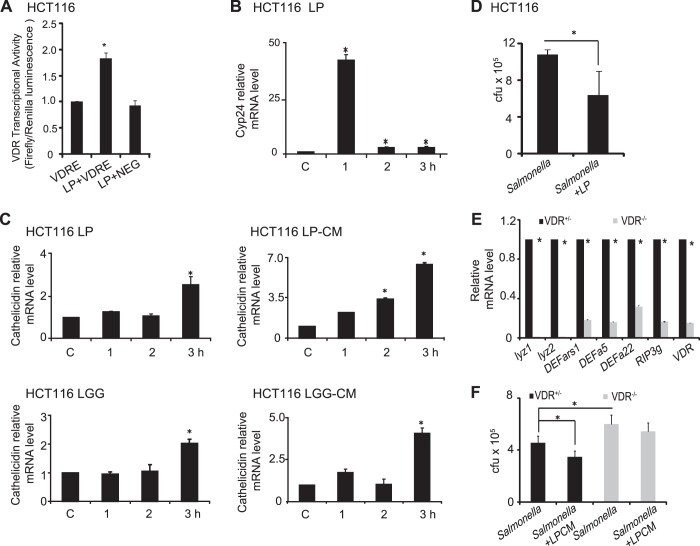
VDR functions as a transcription factor (11). We next examined whether the probiotic-induced VDR protein increase is at the transcriptional level. Using the VDR luciferase reporter vector, we detected increased transcriptional activity of VDR after only 1 h of probiotic treatment (Fig. 2A). VDR target genes included Cyp24 and antimicrobial peptides (AMPs) cathelicidin precursor (6) (also called LL 37). Cyp24 regulates the level of vitamin D3. Our data of real-time PCR showed LP treatment for 1 h significantly increased the expression of Cyp24 at the mRNA level. At the 2- and 3-h time courses, the expression level were lower than the 1 h-treatment with LP but still remained higher level, compared with the control cells without LP treatment (Fig. 2B). We also found that LP and LGG bacteria and conditioned media all significantly enhanced expression of cathelicidin at the transcriptional level (Fig. 2C). To detect the protective effects of probiotics, we used LP to treat HCT116 cells after Salmonella infection. We found that LP treatment significantly decreased Salmonella invasion in HCT116 cells (Fig. 2D).
Fig. 2.
Probiotics increased VDR transcription activity and defensin. A: LP increased VDR transcriptional activity in HCT116 cells. Three separate experiments with 3 replicates each, *P < 0.05. B: LP increased Cyp24 mRNA level in HCT116 cells. C: LP, LP CM, LGG, and LGG CM increased mRNA levels of cathelicidin in HCT116 cells. D: counting of invaded Salmonella with/without LP in HCT116 cells. Cells were infected by Salmonella for 30 min with/without LP and then cultured 30 min with gentamycin. E: mRNA levels of antimicrobial peptides in mouse embryonic fibroblasts (MEF) VDR−/− and VDR+/− cells. F: counting of invaded Salmonella with/without LP CM in MEF VDR+/− and MEF VDR−/− cells. Cells were treated with LP CM for 2 h, then infected by Salmonella for 30 min, and then cultured 30 min with gentamycin. Cells were treated with indicated time course. CFU, colony-forming units. Three separate experiments with 3 replicates each, *P < 0.05.
Probiotic-induced AMPs depend on the VDR status.
We further examined the effect of one allele of the VDR gene on the expression of AMPs, using VDR−/− and VDR+/− MEF cells (41). We chose to use MEF cells because 1) MEF cells isolated from mice are easy to be established as immortalized cells and contain highly inducible VDR activity; and 2) we established MEF VDR+/− and VDR−/− cell lines, which allow us to focus on the role of one vdr allele in regulating host responses to both pathogenic Salmonella and probiotic strains. We found that the VDR knockout in MEF cells showed a significant decrease of AMPS, including Lyz 1, lyz2, Defa-rs1, defensins α5 and α22, and RIP3γ (Fig. 2E). In contrast, one allele of the VDR gene in the VDR+/− MEF cells was able to significantly increase the expression of AMPs (Lyz 1, Lyz2, Defa-rs1, defensins α5 and α22, and RIP3γ,), compared with the MEF VDR−/− cells (Fig. 2E). Because cathelicidin is only expressed in cells of human origin (6), we could not test their expression in MEF cells. Furthermore, our data (Fig. 2F) showed that Salmonella invasion was inhibited by LP conditioned media in MEF VDR+/− cells, whereas probiotic treatment was not able to protect MEF VDR−/− cells against Salmonella invasion. These data indicate that probiotic induction of AMPs depends on VDR.
Mice lacking VDR have worse outcomes after probiotic treatment compared with the VDR+/+ mice in Salmonella-colitis.
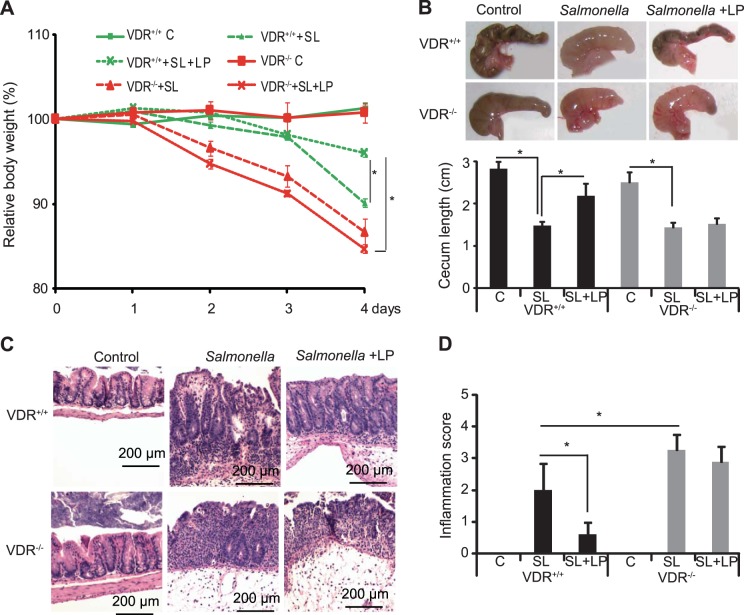
We further hypothesized that VDR is a cytoprotective factor for the intestine and that mice lacking VDR would not respond to probiotic treatment. With the use of a Salmonella-colitis model in VDR knockout and wild-type mice, the role of probiotics in regulating VDR signaling was tested. VDR−/− mice lost significantly more body weight than VDR+/+ mice after Salmonella infection. Probiotic treatment did not protect VDR−/− from losing body weight. (Fig. 3A). Cecum shortening is one of the pathological features in the Salmonella-colitis model. LP inhibited cecum shortening in the VDR+/+ mice but not in the mice lacking VDR (Fig. 3B). Probiotic treatment conferred physiologic and histologic protection from Salmonella infection in VDR+/+ mice, whereas probiotics had no protective effect in the VDR−/− mice (Fig. 3, C and D). Inflammation scores also indicated that LP inhibited inflammation in VDR+/+ mice but not in the mice lacking VDR.
Fig. 3.
Probiotics LP protected VDR+/+ mice from Salmonella (SL)-induced colitis. A: LP protects body weight decrease induced by SL in VDR+/+ mice but not in VDR−/− mice; *P < 0.05 VDP+/+ +SL +LP vs. VDP−/− +SL +LP (4 days postinfection). B: LP protects cecum shortening induced by SL in VDR+/+ mice (n = 4; *P < 0.05). Hematoxylin and eosin staining (C) and scores (D) of the mouse intestine with or without Salmonella infection and LP treatment (n = 4; *P < 0.05).
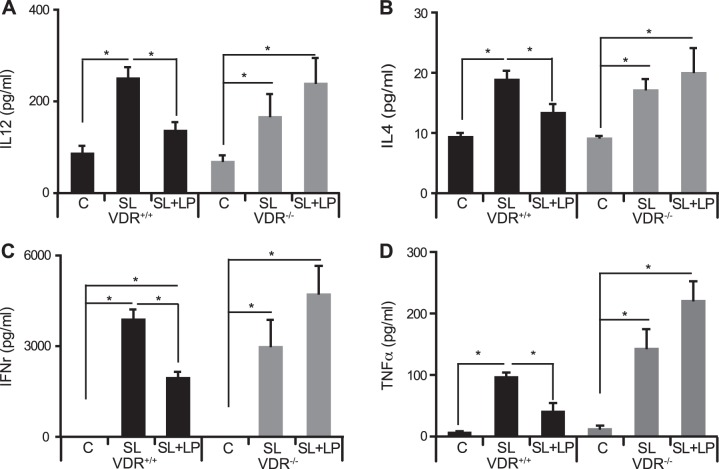
To investigate the effects on systemic inflammation, we analyzed cytokines from mouse serum. We found that LP inhibited Salmonella-induced cytokines IL-12, IL-4, and IFNγ in VDR+/+ mice but not in the mice lacking VDR (Fig. 4).
Fig. 4.
Probiotics LP inhibited inflammatory cytokines induced by Salmonella in VDR+/+ mice but not in VDR−/− mice. A: LP decreased IL-12 induced by SL in VDR+/+ mice but not in VDR−/− mice. B: LP decreased IL-4 induced by SL in VDR+/+ mice but not in VDR−/− mice. C: LP decreased IFNr induced by SL in VDR+/+ mice but not in VDR−/− mice. D: LP decreased TNFα induced by SL in VDR+/+ mice but not in VDR−/− mice (n = 4; *P < 0.05).
Less Salmonella invasion occurred in the VDR+/+ mice compared with the VDR−/− mice.
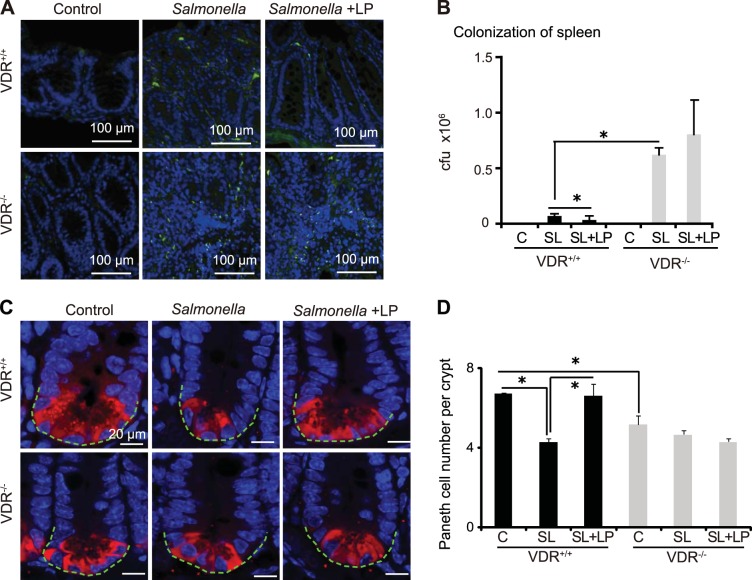
Immunofluorescence results showed that there were more Salmonella in the intestine of infected VDR−/− mice compared with VDR+/+ mice and that there was no difference in VDR−/− mice with or without LP treatment (Fig. 5A). To further investigate the protective effects of LP in Salmonella-infected mice, we also determined Salmonella translocation to the spleen. In the VDR+/+ mice, 75% of the mice had Salmonella translocation to the spleen without LP treatment and 43% with LP treatment. In VDR−/− mice, 75% were Salmonella positive in the spleen without LP treatment and 83% with LP treatment. Quantification of the transferred Salmonella showed there was significantly more Salmonella in the spleen of VDR−/− mice compared with the VDR+/+ mice. With LP treatment, the amount of Salmonella in spleen decreased in VDR+/+ mice, however, there was no changes in VDR−/− mice (Fig. 5B).
Fig. 5.
Probiotics prevent Salmonella invasion and preserve Paneth cells in infected mice. A: LP protects VDR+/+ mice from Salmonella invasion in cecum. Immunofluorescence staining of Salmonella. Green: Salmonella; blue: DAPI. B: counting of Salmonella invaded in spleen. C: antilysozyme staining indicates Paneth cells in small intestine. Red, lysozyme; blue, DAPI. LP increases the number of Paneth cell in VDR+/+ mice (n = 4; *P < 0.05). D: working model for the VDR-based probiotic protection in inflammation and infection.
Probiotics recovered functions of Paneth cells in VDR+/− mice.
Paneth cells are specialized intestinal epithelial that secrete AMPs and play a key role in innate immune responses and in shaping the gut microbiota (34). In VDR+/+ mice, Salmonella infection reduced the amount of Paneth cells. In contrast, the amount of Paneth cells was significantly increased by LP. However, there was no significant change in VDR−/− mice with Salmonella infection or with LP treatment (Fig. 5C). We have demonstrated that intestinal VDR deletion leads to abnormal Paneth cells (43). The probiotics data further indicate that their protective role is VDR dependent.
DISCUSSION
In the current study, we identified that the VDR pathway is required for probiotic protection in colitis, using an experimental Salmonella-colitis model. We investigated the effects of probiotics in increasing VDR expression and determined the mechanism by which probiotics enhance VDR signaling at the transcriptional level. Because VDR is a transcriptional factor, our data further showed that the transcriptional activity of VDR was increased and VDR target genes were enhanced after probiotic treatment.
We report that probiotics increase the expression level of intestinal VDR and enhance the number of Paneth cells, thus inhibiting pathogenic bacterial invasion and inflammation.
Our study showed that probiotic treatment increases VDR signaling pathway activation. We found that the decreased AMPs in VDR−/− cells may be due to decreased expression of VDR directly downregulating expression of defensins (38). Highly expressed defensins and lysozyme in VDR+/− MEF cells compared with the VDR−/− MEF cells (Fig. 4) indicate that one vdr allele is able to regulate its target gene defensin and other related antimicrobial peptides at the mRNA level. These data also help to explain that highly expressed defensins and lysozyme contribute to the clearance of bacteria, therefore, less Salmonella invasion in the VDR+/− MEF cells compared with the VDR−/− MEF cells. In vivo, one of its consequences is may be associated with Paneth cells. Our recent Gut article (42) demonstrated that VDR deletion leads to abnormal Paneth cells and decreased autophagy, which could alter the ability to clear bacterial infection and alter mucosal defense. Thus our findings indicate the effects of VDR on numbers and patterns of Paneth cells, expression of AMPs, and clearance of pathogenic bacteria.
We recognized that mice with different VDR status have microbiota difference and will impact their response to the probiotics. Our previous studies have demonstrated that VDR−/− mice and intestinal epithelial VDR conditional knockout mice had intestinal dysbiosis (8, 40, 42), which may make them susceptible to bacterial infection. Interestingly, in the fecal stool from the whole body VDR−/− mice, lactic acid bacteria Lactobacillus was depleted. For the future study, we will cohouse VDR+/+ and VDR−/− mice for 4 wk and see if their response to probiotics is transmissible.
Probiotics are not equal. They may use various strategies to interact with host cells. In IBD patients, the responses to probiotic treatments and clinic outcomes are inconsistent (4, 5, 22, 28). Our data indicate that the probiotic function of LP and LGG involves VDR signaling. Our current study showed that probiotic-induced VDR happened via transcriptional pathway. Using purified human VDR protein, we pulled down bacterial proteins from probiotics LGG and LP. The pulled down proteins were subjected to SDS-PAGE using silver staining. We analyzed the VDR-associated bands by mass spectrometry. Our unpublished data identified two bacterial surface proteins binding with VDR. Hence, we will further determine the interactions among bacterial factors and VDR. The probiotic-induced VDR may be ligand-stimulated transcription, similar as the vitamin-induced VDR at the transcriptional level. We also believe that the effect of the probiotics-induced VDR is not generalized genome wide. It only affects a specific set of genes. However, at the current stage, we do not know what in the culture supernatant seems to be working. For the future study, we plan to characterize the supernatants using different size filtering, heating, or change in pH. Those studies can give us an idea about what the factor(s) might be.
It is reported that VSL#3, a mixture of eight probiotic bacteria, produces soluble factors that inhibit the chymotrypsin-like activity of the proteasome in gut epithelial cells (21). Our unpublished data showed that VDR protein was degraded with a half-life of ∼8 h and this rate of degradation was completely blocked by the proteasome inhibitor MG262. Thus we speculate that VDR stabilization could also via ubiquitination/proteasome inhibition by probiotics. On the other hand, our previous study indicates that wild-type Salmonella increased VDR expression after infection for 6 h (40). Pathogenic bacteria and probiotics regulate VDR expression differently.
What remains unknown is how probiotics specifically work on VDR signaling and effectively play an anti-inflammatory role. Our previous studies have shown that VDR negatively regulates the bacteria-induced NF-κB activity and attenuates response to infection. (41). NF-κB p65 formed a complex with VDR in noninfected wild-type mouse intestine. In contrast, deletion of VDR abolished VDR/P65 binding. The process of NF-κB activation involves degradation of the inhibitory molecule IκBα. VDR also acts as a transcription factor that binds with the IκBα promoter. (41) A study showed that probiotic VSL#3 resulted in upregulation of antagonists of NF-κB inflammatory pathways, including VDR signaling (23). Therefore, lacking VDR in intestine may lead to downregulation of antagonists of NF-κB inflammatory pathways, thus abolishing the protective role of probiotics.
Our findings that probiotic function depends on VDR status may provide an explanation for the inconsistent clinical response of some patients with IBD. These data further indicate the importance of bacteria and VDR interaction in inflammation. There are different groups with IBD: those with 1) dysfunctional VDR signaling; 2) vitamin D deficiency; and 3) dysbiosis (1, 14, 24, 25). However, the current usage of probiotics is based on a generic, nonspecific approach. Thus a more personalized approach to the use of probiotics is needed. Strategies to restore VDR expression in inflamed mucosa may be important for preventing and treating IBD. In the current article, we explored the molecular mechanisms behind probiotic therapy through VDR signaling. These results may provide an explanation for the inconsistent results of probiotics in certain groups and provide insights into individual therapy. We hope to integrate our findings with other studies and, more importantly, to understand how probiotics coordinate the effects of vitamin D/VDR.
In summary, we report that probiotic treatment is able to enhance VDR expression and activity in the host. In addition to fundamentally advancing the field of nuclear receptor and bacterial-host interaction, we will enter the era that clinical interventions can be custom-tailored to individual patients to achieve better outcomes (29). Understanding how probiotics enhance VDR signaling and inhibit inflammation will allow probiotics to be used effectively. Our studies will provide a new target for therapeutic interventions for bacterial infection and chronic inflammation.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants KO1-DK-075386 and R03-DK-089010-01, Swim Across America Cancer Award, and Brain Piccolo Cancer Award (to J. Sun).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W., S.Y., Y.-G.Z., and R.L. performed experiments; S.W., S.Y., Y.-G.Z., R.L., Y.X., J.W., E.O.P., E.C.C., and J.S. analyzed data; S.W., S.Y., Y.X., J.W., E.O.P., E.C.C., D.C., and J.S. interpreted results of experiments; S.W. and J.S. prepared figures; S.W. and J.S. drafted manuscript; S.W., S.Y., Y.-G.Z., R.L., Y.X., J.W., E.O.P., E.C.C., D.C., and J.S. edited and revised manuscript; D.C. and J.S. approved final version of manuscript; J.S. conception and design of research.
ACKNOWLEDGMENTS
Part of this work was awarded the Third Place Prize in the Probiotics Challenge 2011, American Gastroenterological Association Institute and Institute Rosell-Lallemand.
REFERENCES
- 1.Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 53: 1129–1136, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demay MB. Mechanism of vitamin D receptor action. Ann NY Acad Sci 1068: 204–213, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Fujimori S, Tatsuguchi A, Gudis K, Kishida T, Mitsui K, Ehara A, Kobayashi T, Sekita Y, Seo T, Sakamoto C. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn's disease. J Gastroenterol Hepatol 22: 1199–1204, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, Guerra Pinto A., Carolina Carneiro Aguirre A, Paiva Martins F, Marcos Andrade Goulart E, Sales Da Cunha A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol 43: 842–848, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19: 1067–1077, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13: 325–349, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Jin D, Wu S, Zhang Yg Lu R, Xia Y, Dong H, Sun J. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther 996–1009, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med 88: 441–450.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, Finkelstein JA. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology 135: 1907–1913, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato S. The function of vitamin D receptor in vitamin D action. J Biochem 127: 717–722, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin d deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 151: 2423–2432, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol 2: 308–315, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med 13: 117–124, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Chen Y, Golan MA, Annunziata ML, Du J, Dougherty U, Kong J, Musch M, Huang Y, Pekow J, Zheng C, Bissonnette M, Hanauer SB, Li YC. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 123: 3983–3996, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loftus EV., Jr The burden of inflammatory bowel disease in the United States: a moving target? Clin Gastroenterol Hepatol 5: 1383–1384, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am 31: 1–20, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Miller SI, Mekalanos JJ. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol 172: 2485–2490, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis 12: 1162–1174, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127: 1474–1487, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, Abdollahi M. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn's disease. Dig Dis Sci 53: 2524–2531, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Reiff C, Delday M, Rucklidge G, Reid M, Duncan G, Wohlgemuth S, Hormannsperger G, Loh G, Blaut M, Collie-Duguid E, Haller D, Kelly D. Balancing inflammatory, lipid, and xenobiotic signaling pathways by VSL#3, a biotherapeutic agent, in the treatment of inflammatory bowel disease. Inflamm Bowel Dis 15: 1721–1736, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3: 390–407, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66: 5224–5231, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr 76: 1077–1081, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 7: 1202–1209, 1209.e1, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Chang EB. Exploring gut microbes in human health and disease: pushing the envelope. Genes Dis 1: 132–139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Hobert ME, Duan Y, Rao AS, He TC, Chang EB, Madara JL. Crosstalk between NF-κB and β-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 289: G129–G137, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Hobert ME, Rao AS, Neish AS, Madara JL. Bacterial activation of β-catenin signaling in human epithelia. Am J Physiol Gastrointest Liver Physiol 287: G220–G227, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC. Increased NF-κB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab 291: E315–E322, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Mustafi R, Cerda S, Chumsangsri A, Xia YR, Li YC, Bissonnette M. Lithocholic acid down-regulation of NF-kappaB activity through vitamin D receptor in colonic cancer cells. J Steroid Biochem Mol Biol 111: 37–40, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA 105: 20858–20863, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M, Sawada T, Nakata B, Ohira M, Hirakawa K. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep 22: 1021–1025, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Walker A. Genome watch: probiotics stick it to the man. Nat Rev Microbiol 7: 843, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem 285: 2227–2231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173: 2909–2912, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Waterhouse JC, Perez TH, Albert PJ. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann NY Acad Sci 1173: 757–765, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol 177: 686–697, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S, Xia Y, Liu X, Sun J. Vitamin D receptor deletion leads to reduced level of IkappaBalpha protein through protein translation, protein-protein interaction, and post-translational modification. Int J Biochem Cell Biol 42: 329–336, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S, Zhang YG, Lu R, Xia Y, Zhou D, Petrof EO, Claud EC, Chen D, Chang EB, Carmeliet G, Sun J. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 64: 1082–1094, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye Z, Petrof EO, Boone D, Claud EC, Sun J. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol 171: 882–892, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]