Abstract
While a high-cholesterol diet induces hepatic steatosis, the role of intracellular sterol carrier protein-2/sterol carrier protein-x (SCP-2/SCP-x) proteins is unknown. We hypothesized that ablating SCP-2/SCP-x [double knockout (DKO)] would impact hepatic lipids (cholesterol and cholesteryl ester), especially in high-cholesterol-fed mice. DKO did not alter food consumption, and body weight (BW) gain decreased especially in females, concomitant with hepatic steatosis in females and less so in males. DKO-induced steatosis in control-fed wild-type (WT) mice was associated with 1) loss of SCP-2; 2) upregulation of liver fatty acid binding protein (L-FABP); 3) increased mRNA and/or protein levels of sterol regulatory element binding proteins (SREBP1 and SREBP2) as well as increased expression of target genes of cholesterol synthesis (Hmgcs1 and Hmgcr) and fatty acid synthesis (Acc1 and Fas); and 4) cholesteryl ester accumulation was also associated with increased acyl-CoA cholesterol acyltransferase-2 (ACAT2) in males. DKO exacerbated the high-cholesterol diet-induced hepatic cholesterol and glyceride accumulation, without further increasing SREBP1, SREBP2, or target genes. This exacerbation was associated both with loss of SCP-2 and concomitant downregulation of Ceh/Hsl, apolipoprotein B (ApoB), MTP, and/or L-FABP protein expression. DKO diminished the ability to secrete excess cholesterol into bile and oxidize cholesterol to bile acid for biliary excretion, especially in females. This suggested that SCP-2/SCP-x affects cholesterol transport to particular intracellular compartments, with ablation resulting in less to the endoplasmic reticulum for SREBP regulation, making more available for cholesteryl ester synthesis, for cholesteryl-ester storage in lipid droplets, and for bile salt synthesis and/or secretion. These alterations are significant findings, since they affect key processes in regulation of sterol metabolism.
Keywords: mouse, sterol carrier protein, gene ablation, liver, cholesterol
despite increased understanding of cholesterol homeostasis, the role of cytosolic cholesterol transport proteins in intracellular targeting of cholesterol is not well understood. Cholesterol arriving at the endoplasmic reticulum (ER) is not only esterified via acyl-CoA cholesterol acyl transferase (ACAT2) but also regulates release of sterol regulatory element binding proteins (SREBP1 and SREBP2), inducing transcription of target genes in cholesterol and fatty acid metabolism (39, 80, 82, 93, 105). High-density lipoprotein (HDL)-derived cholesterol traffics to peroxisomes for oxidation to bile acids and to bile canaliculus for biliary elimination (24, 70, 83).
Studies in vitro and with cultured cells show that the 13-kDa sterol carrier protein-2 (SCP-2) binds cholesterol (15, 44, 61, 68, 85, 87, 96, 110), markedly enhancing cholesterol transfer from plasma membranes and lysosomes to plasma membranes, ER, and mitochondria (3, 26, 27, 29, 30, 32, 33, 43, 65, 84, 98, 109). SCP-2 overexpression increases cholesterol uptake as well as transfer from plasma membranes to ER for esterification and cholesteryl ester accumulation in L-cell fibroblasts (11, 67, 95). SCP-2 is an intracellular binding partner with caveolin-1 and SR-B1 and preferentially enhances cholesterol trafficking from cholesterol-rich plasma membrane microdomains in which these receptors are abundantly distributed (3, 8, 88, 97, 98, 115). SCP-2 and sterol carrier protein-x (SCP-x) proteins also contribute to rapid clearance of HDL-cholesterol by facilitating biliary bile acid synthesis (97, 100). By binding and transferring cholesterol to ER, SCP-2 stimulates hepatic cholesterol 7α-hydroxylase, the rate-limiting enzyme in hepatic bile acid synthesis (52, 92). Through an alternate transcription site, the SCP-2/SCP-x gene also encodes a larger protein, SCP-x (31, 90), which catalyzes the peroxisomal oxidation of cholesterol's branched side chain to form bile acids (31, 90). Canalicular secretion of bile acid drives biliary cholesterol secretion (77, 81, 104). These findings suggest a role for SCP-2 in rapid cholesterol intracellular trafficking of HDL-derived cholesterol for biliary excretion, lipoprotein-derived cholesterol to ER for esterification, and potentially regulation of SREBP release.
In vivo support for roles of the SCP-2/SCP-x gene in hepatic cholesterol metabolism and biliary cholesterol secretion comes from studies in both humans and mice. A human SCP-2/SCP-x genetic variation inhibits cholesterol metabolism (23). However, the human studies were performed with only a single patient (23). In mice, SCP-2 overexpression (2, 5, 112), SCP-2 antisense treatment (76), and SCP-2/SCP-x gene ablation (28, 51, 91) significantly impact hepatic cholesterol metabolism and biliary excretion. Despite the fact that hepatic SCP-x expression is severalfold lower in female mice and female humans (6, 9), almost all rodent studies have been conducted with male mice (2, 5, 28, 51, 76, 91, 112). Studies of the impact of SCP-2 in the face of a high-cholesterol diet are even more limited. A single study has examined the effect of SCP-2 overexpression on high-cholesterol-fed mice but only in males (5). The impact of SCP-2/SCP-x gene ablation [double knockout (DKO)] in the context of a high-cholesterol diet is unknown in either sex. Thus the current study examined the impact of the DKO on hepatic cholesterol and biliary phenotype of male and female mice fed a high-cholesterol vs. control diet.
EXPERIMENTAL PROCEDURES
Materials.
Rabbit polyclonal antibodies to the following proteins were obtained as follows: anti-peroxisome proliferator-activated receptor-α (PPARα; PA1-822A) from ThermoFisher Scientific (Rockford, IL), anti-ATP-binding cassette transporter (ABCA1; NB400-105), anti-HMG-CoA synthase (cHMGCS; sc-33829), anti-small heterodimer protein (SHP; sc-30169), anti-sterol regulatory element-binding protein 1 (SREBP1; recognizing p68 and p125, sc-367), and anti-sterol regulatory binding protein-2 (SREBP2; recognizing mature p68 and ER precursor p125; sc-8151) from Santa Cruz Biotechnology (Santa Cruz, CA) (10); anti-sterol carrier protein 2 (recognizing 58 kDa SCP-x, 15 kDa pro-SCP-2, and 13.2 kDa SCP-2) (10), anti-ACAT2 (ab66259), anti-apolipoprotein B (ApoB; ab31992); and anti-cytochrome c oxidase subunit 4 (COX4; ab16056) from Abcam (Cambridge, MA). Goat polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA) as follows: anti-cholesterol-7-alpha-hydroxylase (CYP7A1; sc-14426); anti-sterol-27-hydroxylase (CYP27A1; sc-14835); anti-fatty acid transport protein 2 (FATP-2; sc-161311); anti-fatty acid transport protein 4 (FATP-4; sc-5834), anti-apolipoprotein AI (ApoA1; sc-23606); anti-carnitine palmitoyltransferase I (CPT1; sc-31128); anti-phosphatidylcholine transfer protein (PCTP; sc-23672); anti-sterol regulatory element binding protein 2 (SREBP2; sc-8151); anti-microsomal triglyceride transfer protein (MTP; sc-33116); anti-liver-type fatty acid binding protein (L-FABP; sc-16064); anti-liver X receptor (LXR; sc-1201); and anti-farnesoid X receptor (FXR; sc-1205). All reagents and solvents used for each test method were highest grade available.
Animal care.
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Male and female inbred C57BL/6NCr mice were from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). SCP-2/SCP-x null (DKO) mice on the same C57BL/6NCr background were generated and backcrossed to C57BL/6NCr to the N6 generation (8). Mice were fed a standard rodent chow mix (5% calories from fat; D8604 Teklad Rodent Diet; Teklad Diets, Madison, WI) and were maintained in barrier cages on ventilated racks under a 12:12-h light-dark cycle in a temperature controlled facility (25°C) with access to food and water ad libitum until study initiation. All animals were sentinel monitored quarterly and confirmed free of all known rodent pathogens.
Dietary cholesterol study.
Seven-week-old male and female wild-type (WT) and SCP-2/SCP-x null (DKO) mice on the C57BL/6NCr background were transferred to a control modified AIN-76A phytoestrogen-free, phytol-free control diet (5% calories from fat; D11243; Research Diets, New Brunswick, NJ) 1 wk before beginning the 29-day dietary study. The phytoestrogen-free, phytol-free diet was chosen to minimize any potential complicating effect in sex comparisons due to phytoestrogens, which exert estrogenic effects in mice (101, 102) and from phytol metabolites (e.g., phytanic acid), potent ligand inducers of PPARα (20, 36, 108). After 1 wk, a total of 56 male and female mice were randomized into 8 groups, with half remaining on the control (CO) modified AIN-76A diet while the other half were placed on a high-cholesterol (CH) diet (D01091702; Research Diets) composed of the modified AIN-76A control diet supplemented with 1.25% cholesterol and isocaloric to the control diet. Seven mice were assigned to each group: male WT on CO, male DKO on CO, male WT on CH, male DKO on CH, female WT on CO, female DKO on CO, female WT on CH, and female DKO on CH. Animals were provided with ad libitum food and water throughout the study and maintained singly housed so that individual food intake and body weight could be monitored every other day. Body weights and food intake were measured at approximately the same time of day at each recording. To measure food intake, the bedding was strained for any remaining pellets within the cage as well as any food in the receptacle and the total was weighed. To more clearly visualize any small feed particles in the cage and improve food consumption accuracy, diets were color coded (yellow, CO; blue, CH).
In vivo whole body composition.
Mouse total body lean tissue mass (LTM) and fat tissue mass (FTM) were determined in vivo by dual-energy X-ray absorptiometry (DEXA) using a Lunar PIXImus densitometer (Lunar, Madison, WI) as previously described (9). The DEXA instrument was calibrated using a phantom mouse with known bone mineral density and fat tissue mass as described previously (9, 69). DEXA was performed on individual mice at the beginning of the dietary study after mice were fasted overnight (∼12 h) and anesthetized [100 m/kg ketamine and 10 mg/kg xylazine ip as described previously (9)]. At the end of the study (day 30), DEXA was similarly performed, except mice were euthanized before DEXA. DEXA allowed resolution of bone mass and tissue mass for further resolution into LTM and FTM as described previously (9, 69).
Serum, liver, and bile collection.
On day 29 of the study, mice were fasted overnight (∼12 h) to decrease the influence of recent digestion on serum and liver lipid levels. On day 30, after anesthesia, blood was collected via cardiac puncture, followed by humane euthanasia by cervical dislocation. Blood was stored overnight at 4°C and centrifuged at maximum speed for 20 min, and serum was collected and stored at −80°C. Gall bladders containing bile and livers were separately harvested, and livers were weighed and sectioned. Part of the liver was transferred to a RNA stabilization buffer, RNAlater (Ambion, Austin, TX), and stored at −20°C. All remaining tissue samples were flash frozen on dry ice and stored at −80°C for future analysis.
Liver histopathology and serum markers of liver toxicity.
Liver slices near the porta hepatis were fixed for 24 h in 10% neutral buffered formalin, placed in individual cassettes, stored in 70% alcohol, processed and embedded in paraffin, cut in 4- to 6-μm sections, and hematoxylin and eosin stained for histological evaluation (9, 55). Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and β-hydroxybutyrate (β-HB) were quantified utilizing Stanbio diagnostic kits (Boerne, TX).
Lipid analysis of serum, bile, and liver.
About 0.1-g (wet weight) portions of livers from each mouse were homogenized in 0.5 ml PBS (pH 7.4) with motor-driven pestle (Tekmar, Cincinnati, OH) at 2,000 rpm for 5 min. Liver homogenate and serum protein were determined by a Bradford micro-assay from Bio-Rad Laboratories (cat no. 500-0001; Hercules, CA). Costar 96-well assay plates (Corning, Corning, NY) and a Bio Tek Synergy 2 microplate reader (Bio Tek Instruments, Winooski, VT) were utilized for liver lipid, serum, and bile assays. Liver homogenate and serum lipids were quantified with Wako diagnostic kits (Richmond, VA) for total cholesterol, free cholesterol, triglyceride, phospholipid, and HDL cholesterol (HDL-C). Liver and serum cholesterol ester concentrations were calculated by subtraction of free cholesterol from total cholesterol. Serum non-HDL-C was calculated by subtracting serum HDL-C from serum total cholesterol. Serum ApoA1 and serum ApoB and serum, liver, and bile total bile acids were quantified using the Diazyme diagnostic kit (Ponwy, CA). All commercially available diagnostic kits were utilized according to the manufacturer's instructions, modified for use with 96-well plates and microplate reader as described above.
Hepatic mRNA levels of genes in lipid metabolism.
Total RNA was isolated from liver and purified using the RNeasy mini kit (Qiagen, Valencia, CA) using the manufacturer's standard protocol. Nucleic acid concentration and quality were determined by a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA). Samples were stored at −80°C. Quantitative real-time PCR (QrtPCR) expression patterns were analyzed with an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA) using TaqMan RNA-to-CT 1-Step PCR Master Mix Reagent kit, gene-specific TaqMan PCR probes and primers, and the following thermal cycler protocol: 48°C for 30 min, 95°C for 10min, 95°C for 0.15 min, and 60°C for 1.0 min, repeated a total of 60 cycles. TaqMan gene expression assays for specific probes and primers were obtained from Life Technologies (Carlsbad, CA) to determine hepatic mRNA levels of ATP-binding cassette subfamily G member 5 (Abcg5; Mm01226965), ATP-binding cassette subfamily G member 8 (Abcg8; Mm00445977_m1), acetyl CoA carboxylase-1 (Acc1; Mm01304285_m1), acetyl CoA carboxylase-2 (Acc2; Mm01204657_m1), bile salt export pump (Bsep/Abcb11; Mm00445168_m1), cholesteryl ester hydrolase/hormone-sensitive lipase (Ceh/Hsl; Mm00495359_m1), fatty acid synthase (Fas; Mm00662319_m1), HMC-CoA synthase (Hmgcs1, Mm01304569_ml), HMG-CoA reductase (Hmgcr, Mm01282492_ml), low-density lipoprotein receptor (Ldlr, Mm01177349_ml), Na+-taurocholate cotransporting polypeptide (Ntcp/Slc10a1; Mm01302718), organic anion transporting polypeptide 1 (Oatp1a1/Slco1a1; Mm01267414_m1), organic anion transporting polypeptide 2 (Oatp2/Slco1c1; Mm00460672_m1), scavenger receptor class B type 1 (Scarb1, Mm00450234_ml), sterol regulatory element binding protein-1 (Srebp1; Mm00550338_m1), and sterol regulatory element binding protein-2 (Srebp2; Mm01306289_m1). Two replicates of each sample reaction (20 μl total volume each) were performed on 96-well optical reaction plates (Applied Biosystems, Foster City, CA). ABI Prism 7000 SDS software (Applied Biosystems) established the threshold cycle from each well. QrtPCR data were normalized to the housekeeping gene 18S RNA for mRNA expression of Abcg5, Abcg8, Acc1, Acc2, Bsep, Ceh/Hsl, Fas, Hmgcs1, Hmgcr, Ldlr, Ntcp, Oatp1, Oapt2, Scarb1, Srebp1, and Srebp2 made relative to the control mouse group (male WT mice on control diet) for final calculations.
Hepatic levels of proteins in lipid metabolism.
SDS-PAGE and Western blot analysis was performed on liver postnuclear supernatants (PNS) as described previously (8) to determine relative protein levels of ACAT2, ABCA1, ApoA1, ApoB, COX4, CPTA1, CYP7A1, CYP27A1, FATP-2, FATP-4, HMGCR, L-FABP, PPARα, PCTP, SCP-2, SCP-x, SREBP-1 (68- and 125-kDa forms), SREBP-2 (68- and 125-kDa forms), FXR, and LXR. Images of the blots were captured using an Epson Perfection V700 Photo scanner (Long Beach, CA). Proteins were quantified by densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD) as described earlier (8). Representative cropped Western blots were inserted into figures and presented similarly as in earlier publications wherein individual blots were separated by a white line/space (46, 56, 74, 99, 100).
Statistical analysis.
All result values were stated as the means ± SE. Statistical analysis was performed using one-way ANOVA, followed with the Newman-Keuls multiple comparisons test using either GraphPad software (La Jolla, CA) or Sigma Plot software (Systat, San Jose, CA). Statistical significance was assigned to values with P < 0.05.
RESULTS
Food intake, whole body phenotype, and liver histology.
Neither SCP-2/SCP-x gene ablation (DKO), high-cholesterol diet, nor both altered total food consumption by WT male and female mice (Table 1). Yet, DKO decreased body weight gain and body weight gain/food consumption in female (but not male) mice regardless of diet (Table 1). DEXA showed that, while fat tissue mass was not altered, lean tissue mass was significantly decreased only in high-cholesterol-fed DKO vs. WT mice (Table 1).
Table 1.
Effect of SCP-2/SCP-x gene ablation on total food consumption and body weight gain of mice fed a high-cholesterol diet
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| WT |
DKO |
WT |
DKO |
|||||
| CO | CH | CO | CH | CO | CH | CO | CH | |
| Total food consumption, g | 86 ± 3 | 91 ± 2 | 91 ± 4 | 90 ± 4 | 86 ± 2 | 88 ± 2 | 86 ± 1 | 87 ± 1 |
| Body weight gain, g | 2.5 ± 0.5 | 2.7 ± 0.4 | 2.4 ± 0.6 | 1.6 ± 0.4 | 1.9 ± 0.3 | 2.3 ± 0.2 | 0.6 ± 0.2* | 1.0 ± 0.4* |
| Body weight gain/food consumption, mg/g | 30 ± 5 | 30 ± 3 | 26 ± 5 | 18 ± 4 | 22 ± 3 | 26 ± 3 | 7 ± 2* | 11 ± 4* |
| LTM change, g | 1.4 ± 0.4 | 1.3 ± 0.5 | 0.4 ± 1.2 | 0.1 ± 0.8 | 0.2 ± 0.3 | 0.7 ± 0.4 | −0.7 ± 0.4 | −0.9 ± 0.3* |
| FTM change, g | 0.1 ± 0.2 | 0.5 ± 0.5 | 1.6 ± 0.5 | 1.4 ± 0.4 | −0.2 ± 0.2 | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.3 |
Values represent the mean ± SE, n = 5–7. SCP, sterol carrier protein; WT, wild type; DKO, double knockout; CO, control; CH, cholesterol; LTM, lean tissue mass; FTM, fat tissue mass.
Genotype effect (P < 0.050 for DKO vs. WT within the same diet).
Gross analysis of liver did not detect significant alterations due to genotype, high-cholesterol diet, or both. Neither cholesterol diet, DKO, nor both altered liver weight, liver weight/body weight, or liver total protein (Table 2). Histological analysis revealed that the high-cholesterol diet was associated with increased fatty vacuolation of hepatocytes in WT males and females. The DKO did not significantly exacerbate this effect at the histological level (not shown). There were no other significant changes in liver histopathology. Serum AST and ALT values in all groups were <35 and 60 U/l, respectively, well within the normal range of mouse values [blood chemistry and hematology in 8 inbred strains of mice, MPD:Eumorphia. Mouse Phenome Database web site, The Jackson Laboratory, Bar Harbor, ME: http://phenome.jax.org (cited 29 Oct. 2014)]. Thus the altered whole body phenotype or serum and hepatic changes were not due to toxicity in DKO and high-cholesterol diet groups.
Table 2.
Effect of SCP-2/SCP-x gene ablation, high-cholesterol diet, and both together on liver parameters
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| WT |
DKO |
WT |
DKO |
|||||
| CO | CH | CO | CH | CO | CH | CO | CH | |
| Liver weight, g | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
| Liver weight/body weight, mg/g | 38 ± 2 | 43 ± 1 | 40 ± 1 | 40 ± 1 | 40 ± 1 | 50 ± 2 | 40 ± 2 | 50 ± 2 |
| Protein, mg/g | 135 ± 10 | 126 ± 12 | 122 ± 7 | 130 ± 11 | 126 ± 10 | 141 ± 10 | 121 ± 10 | 107 ± 7 |
| Serum ALT, U/l | 54 ± 4 | 45 ± 4 | 41 ± 5 | 40 ± 5 | 57 ± 5 | 48 ± 7 | 44 ± 5 | 59 ± 6 |
| Serum AST, U/l | 30 ± 2 | 33 ± 4 | 19 ± 2 | 22 ± 3 | 24 ± 1 | 20 ± 2 | 20 ± 2 | 23 ± 2 |
Values represent the means ± SE; n = 6–7. ALT, alanine aminotransferase; AST, aspartate aminotransferase. No significant differences were noted between groups.
Liver lipids.
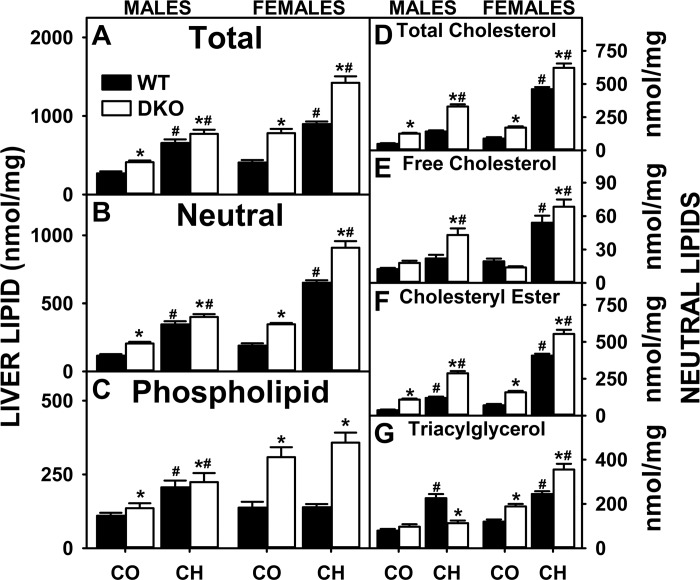
Both the high-cholesterol diet and DKO impacted hepatic lipid accumulation in a sex-dependent manner. The high-cholesterol diet alone increased hepatic accumulation of neutral lipids the most in WT females, without altering phospholipid levels (Fig. 1, B and C), while also increasing both neutral and phospholipid in WT males (Fig. 1, B and C). With neutral lipids, the cholesterol diet alone increased total cholesterol (free and esterified) and triglycerides in both groups (Fig. 1, D–G). These findings were consistent with earlier studies wherein other mouse strains were fed high-cholesterol diets (53, 58, 59).
Fig. 1.
Effects of, sterol carrier protein-2 (SCP-2)/sterol carrier protein-x (SCP-x) gene ablation and cholesterol-rich diet on hepatic lipid concentrations in mice. The total lipid (A), neutral lipid (B), phospholipid (C), total cholesterol (D), free cholesterol (E), cholesteryl ester (F), and triacylglycerol (G) levels from SCP-2/SCP-x −/− [double knockout (DKO)] vs. SCP-2/SCP-x+/+ [wild-type (WT)] mice were quantified as described in experimental materials. CO, control diet; CH, high-cholesterol diet. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 6–7). *P < 0.05 for DKO vs. WT. #P < 0.05, CH vs. CO.
DKO alone modestly increased liver total lipid (Fig. 1A), total neutral lipid (Fig. 1B), and phospholipid (Fig. 1C) in control-fed males and even more so in control-fed females. The increases in hepatic neutral lipid were due to increased total cholesterol (Fig. 1D), primarily cholesteryl ester, in both males and females (Fig. 1F). The DKO mice had significantly increased hepatic triacylglyceride only in control-fed females (Fig. 1G). The high-cholesterol diet induced hepatic lipid accumulation, especially in females. Females had the highest hepatic levels of total lipid (Fig. 1A), total neutral lipid (Fig. 1B), total cholesterol (Fig. 1D), free cholesterol (Fig. 1E), cholesteryl ester (Fig. 1F), phospholipid (Fig. 1C), and triacylglyceride (Fig. 1G). These increases were not due to concomitant upregulation of proteins involved in fatty acid uptake (FATP-2 and FATP-4), downregulation of oxidation (CPT1), or altered serum β-hydroxybutyrate levels (not shown).
In summary, the DKO generally exacerbated the cholesterol diet-induced hepatic accumulation of cholesteryl ester and triglyceride, the major neutral lipid species comprising lipid droplets and the core of secreted VLDL. Accumulation of all forms of cholesterol and triglyceride was highest in the DKO females.
Serum lipids.
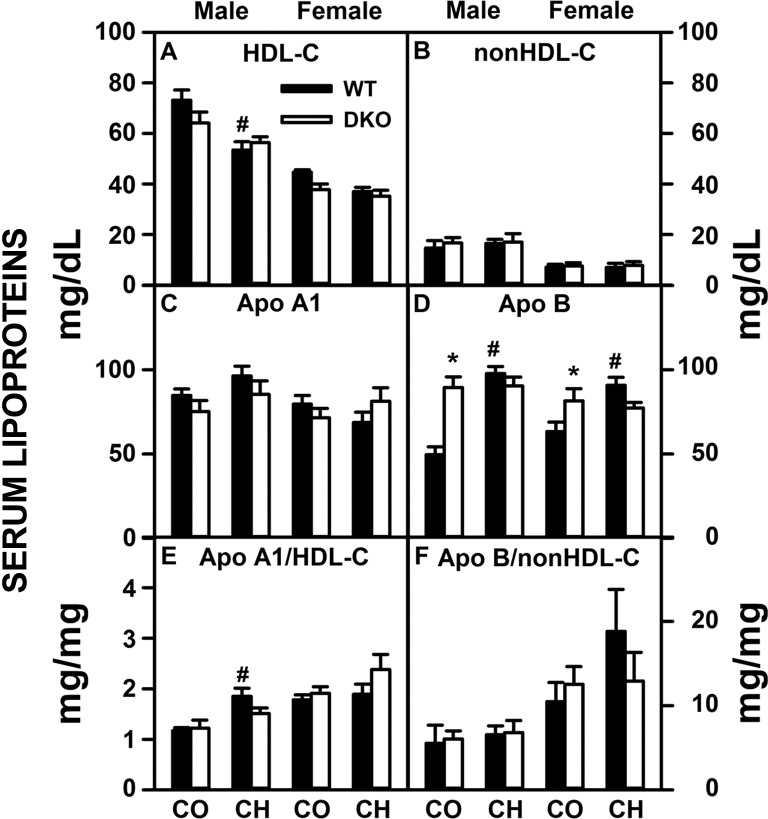
In contrast to the significant lipid accumulation in liver, the high-cholesterol diet, DKO, or both elicited less change in serum lipids in both sexes (Table 3). However, a significant decrease in serum polar/neutral lipid ratio, paralleling a decrease in this ratio in liver lipids, was detected in response to the high-cholesterol diet in WT males but not females (Table 3). The DKO prevented this decrease in ratio in cholesterol-fed males or females (Table 3). Fractionation of serum neutral lipids also showed little or no impact in high-cholesterol diet, DKO, or both on serum total cholesterol, free cholesterol, cholesteryl ester or triacylglycerol (Fig. 2, A–D). Serum cholesterol distribution in the high-density lipoprotein (HDL-C) fraction (Fig. 3A) and non-HDL fraction (Fig. 3B) again showed little impact of high-cholesterol diet, DKO or both. With the exception of increased ApoB in male control-fed DKO mice (Fig. 3D), there were few changes in ApoA1 and ApoB levels or ApoA1/HDL-C and ApoB/non-HDL-C ratios (Fig. 3, C–F).
Table 3.
Effect of SCP-2/SCP-x gene ablation, high-cholesterol diet, and both together on serum lipids
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| WT |
DKO |
WT |
DKO |
|||||
| CO | CH | CO | CH | CO | CH | CO | CH | |
| Total protein, mg/dl | 1,260 ± 30 | 1,140 ± 60 | 1,110 ± 30 | 1,130 ± 40 | 1,190 ± 80 | 1,080 ± 90 | 1,190 ± 110 | 1,080 ± 60 |
| Total lipid, mg/dl | 262 ± 7 | 222 ± 8† | 260 ± 10 | 215 ± 5† | 153 ± 5 | 144 ± 4 | 158 ± 9 | 152 ± 6 |
| Neutral lipid, mg/dl | 160 ± 7 | 143 ± 6 | 157 ± 9 | 124 ± 4† | 89 ± 3 | 94 ± 4 | 98 ± 6 | 91 ± 6 |
| Phospholipid, mg/dl | 99 ± 3 | 77 ± 4† | 98 ± 6 | 87 ± 3 | 62 ± 3 | 48 ± 3† | 59 ± 3 | 58 ± 3* |
| Serum polar/neutral lipid, mg/mg | 0.65 ± 0.04 | 0.56 ± 0.03 | 0.65 ± 0.04 | 0.74 ± 0.03* | 0.73 ± 0.04 | 0.53 ± 0.04† | 0.63 ± 0.02 | 0.69 ± 0.06 |
| Liver polar/neutral lipid, mg/mg | 1.5 ± 0.1 | 1.1 ± 0.1† | 1.1 ± 0.1* | 0.9 ± 0.1 | 1.3 ± 0.2 | 0.5 ± 0.1† | 1.3 ± 0.1 | 0.6 ± 0.1† |
Values represent the mean ± SE; n = 6–7.
Genotype effect (P < 0.05 for DKO vs. WT within the same diet).
Diet effect (P < 0.05 for CO vs. CH diet within the same genotype).
Fig. 2.
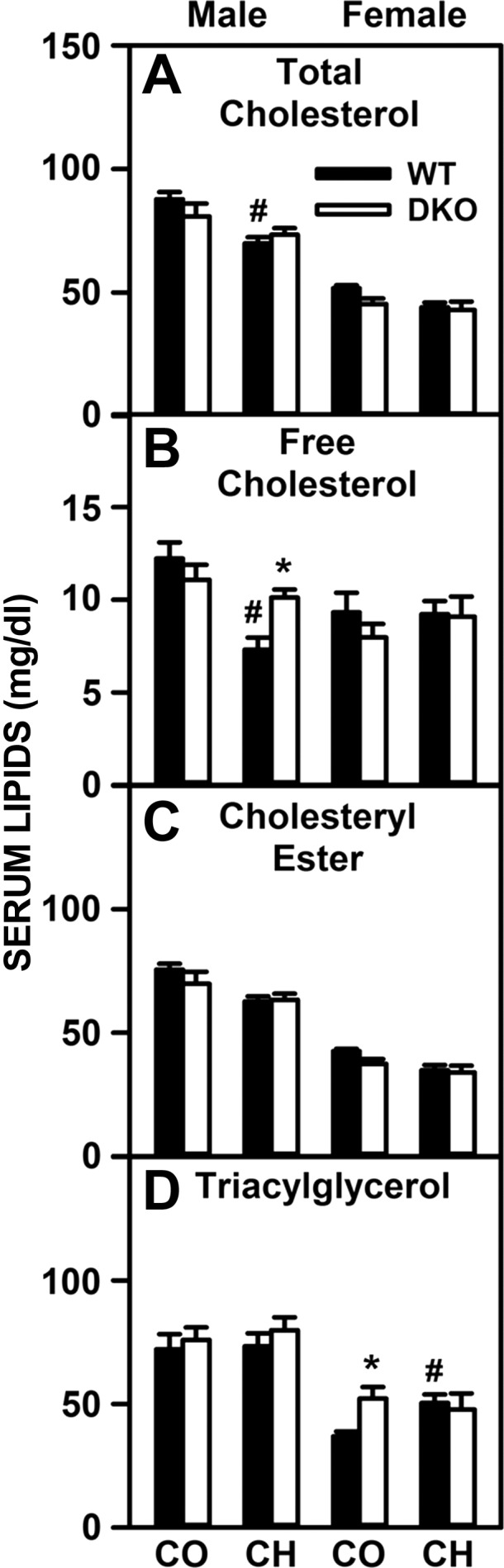
Effects of SCP-2/SCP-x gene ablation and cholesterol-rich diet on serum cholesterol and triacylglycerol composition. Serum levels (mg lipid/dl serum) of total cholesterol (A), free cholesterol (B), cholesteryl ester (C), and triacylglycerol (D) from DKO and WT mice were quantified as described in experimental materials. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 6–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
Fig. 3.
Effects of SCP-2/SCP-x gene ablation and cholesterol-rich diet on serum lipoprotein profiles. Serum levels (mg lipid/dl serum or mg protein/dl serum) of HDL cholesterol (A), non-HDL cholesterol (B), apolipoprotein (Apo)A1 (C), and ApoB (D) were quantified from DKO and WT mice as described in experimental materials. Ratios (mg protein/mg lipid) of ApoA1/HDL-C (E) and ApoB/non-HDL-C (F) were calculated to elucidate particle size, reflecting atherogenicity of the particles present. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 6–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
In summary, high-cholesterol diet or loss of SCP-x/SCP-x or both together did not or only slightly altered serum levels of lipids and key lipoprotein apolipoproteins.
Hepatic proteins in basolateral uptake and intracellular storage of cholesterol from serum lipoproteins.
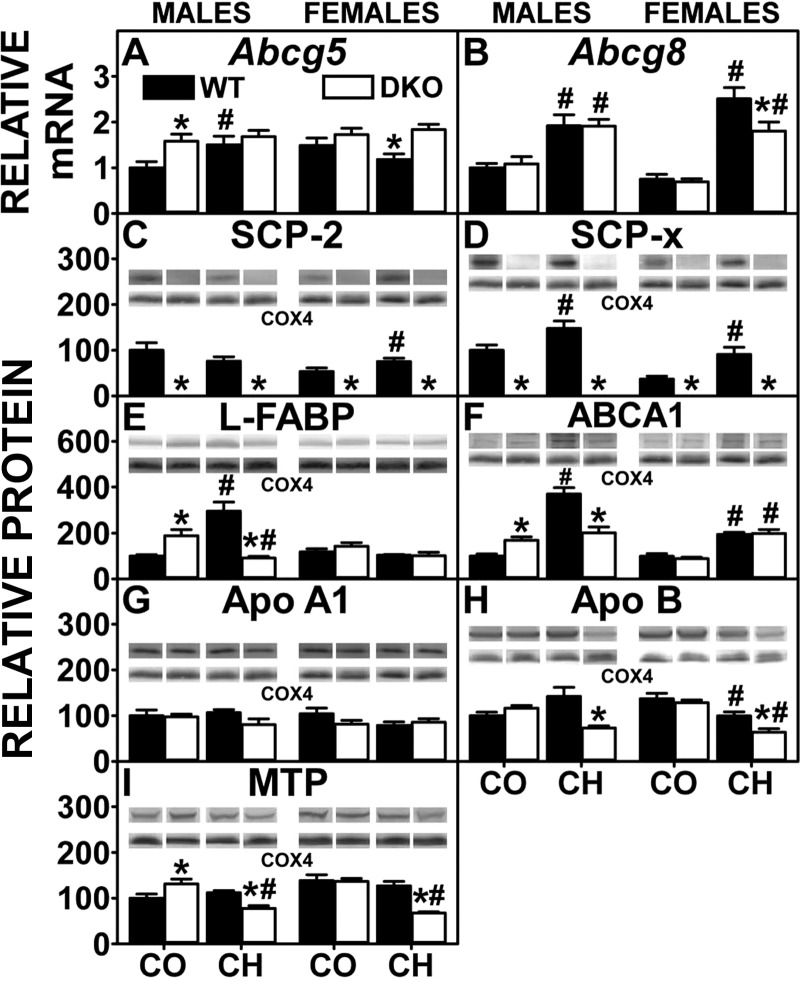
Liver receptors for HDL and LDL facilitate basolateral cholesterol uptake. However, hepatic accumulation of cholesterol in control-fed male DKO mice was associated only in part with concomitant upregulation of Scarb1, while Ldlr tended to decrease (Fig. 4, A and B). Levels in female control-fed DKO mice as well as in both male and female high-cholesterol-fed DKO mice were not increased (Fig. 4, A and B).
Fig. 4.
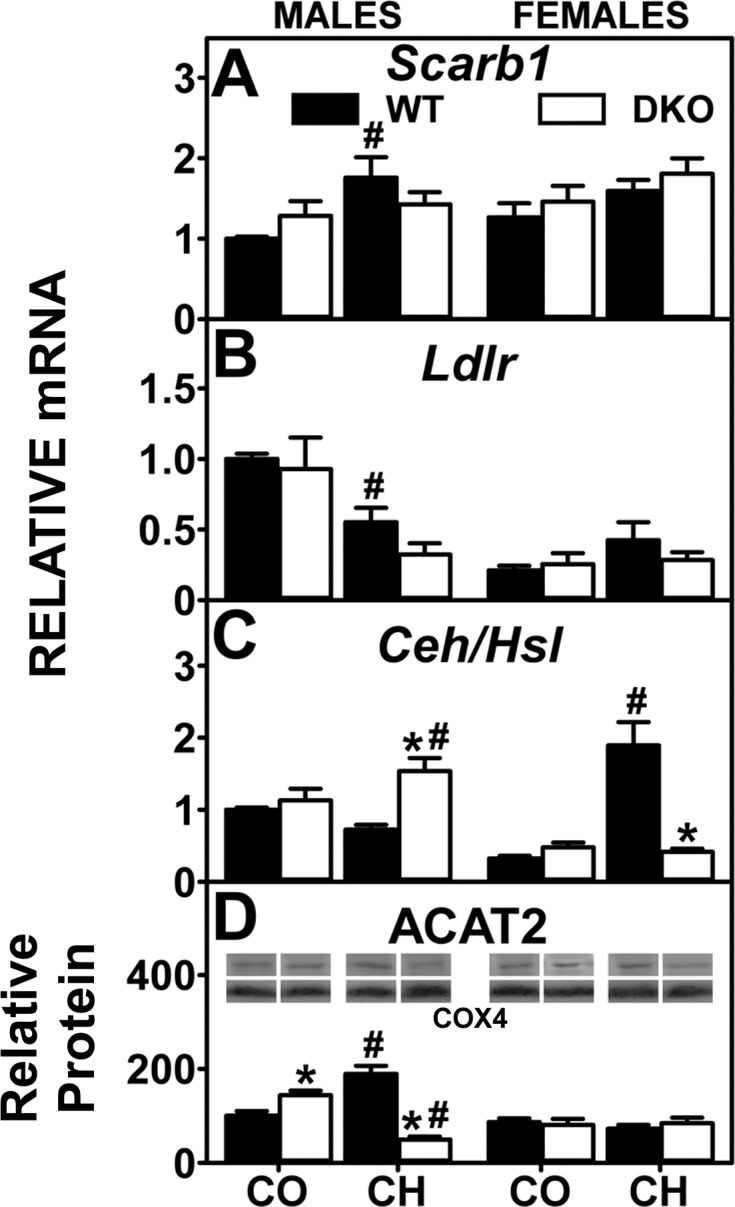
Analysis of key enzymes and receptors involved in the uptake and conversion of cholesterol by quantitative real-time PCR (QrtPCR) and Western blotting. Western blots of liver homogenates isolated from WT and DKO mice were analyzed as described in experimental materials to determine relative protein levels of acyl-CoA cholesterol acyltransferase-2 (ACAT2; D). Cytochrome c oxidase subunit 4 (COX4) was used as a loading control to normalize protein expression. D, inset: representative Western blot for males and females were spliced each from 1 blot, respectively and shows relative protein expression in each mouse group. QrtPCR was used to determine relative mRNA abundance of Scarb1 (A), Ldlr (B), and Ceh/Hsl (C). 18S rRNA was used to normalize mRNA expression levels. mRNA and protein expression levels were quantified as described in experimental materials. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 4–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
Hepatic storage of cholesterol as cholesteryl ester is regulated by the opposing activities of two key proteins: the synthetic enzyme ACAT2 and the degradative Ceh/Hsl. Hepatic accumulation of cholesterol in control-fed male DKO mice was associated with upregulation of ACAT2, but not Ceh/Hsl, while females were unaltered (Fig. 4, C and D). In contrast, while hepatic cholesterol accumulation in cholesterol-fed male DKO mice was not associated with up- and downregulation of ACAT2 and Ceh/Hsl, respectively, in females the decreased Ceh/Hsl, concomitant with unaltered ACAT2, appeared to contribute to increased liver cholesterol (Fig. 4, C and D).
In summary, the increased hepatic cholesterol accumulation in DKO mice was not associated with concomitant upregulation of Scarb1 or Ldlr. Accumulation of cholesteryl ester in control-fed male, but not female, DKO mice was associated in part with increased expression of ACAT2. The DKO-induced exacerbation of hepatic cholesteryl ester accumulation in high-cholesterol-fed mice was associated with decreased Ceh/Hsl rather than increased ACAT2.
Proteins involved in cytosolic transport/targeting of cholesterol.
The DKO resulted in complete ablation of both SCP-2 and SCP-x protein expression (Fig. 5, C and D). In control-fed DKO mice, this loss was compensated only in part by concomitant upregulation of the other major bile acid binding/transport protein L-FABP, whose expression was increased significantly in males and trended to increase slightly in females (Fig. 5E). The high-cholesterol diet alone increased expression of L-FABP and SCP-x in WT males but only SCP-2 and SCP-x in WT females (Fig. 5, C–E). DKO prevented the cholesterol diet-induced increase in hepatic L-FABP expression to decrease L-FABP level in males but did not alter L-FABP in females (Fig. 5E). While DKO increased or did not alter expression of cytosolic cholesterol transport proteins in control-fed male (but not female) mice, the net effect in DKO cholesterol-fed mice was to decrease the expression of proteins involved in cytosolic transport of cholesterol for oxidation and biliary elimination, thereby overall favoring retention of cholesterol in liver.
Fig. 5.
Analysis of key intracellular and membrane cholesterol transporters and examination of proteins involved in lipoprotein packaging by QrtPCR and Western blotting. QrtPCR was determined as in experimental materials to determine relative mRNA abundance of cholesterol efflux transporters Abcg5 (A) and Abcg8 (B). 18S rRNA was used to normalize mRNA expression levels. Western blots of liver homogenates isolated from WT and DKO mice were performed analyzed as in experimental materials to determine relative protein levels of SCP-2 (C), SCP-X (D), liver fatty acid binding protein (L-FABP; E), ATP-binding cassette transporter A1 (ABCA1; F), ApoA1 (G), ApoB (H), and microsomal triglyceride transfer protein (MTP; I). COX4 was used as a loading control to normalize protein expression. E–H, insets: representative Western blots were spliced from multiple blots showing relative protein expression in each mouse group, with the same matching representative COX4 captures from the corresponding blot shown in multiple figures. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 4–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
Hepatic proteins involved in secretion of cholesterol and other lipids into serum.
Assembly of neutral lipid-loaded nascent VLDL for secretion into serum requires the concerted action of L-FABP (and/or SCP-2) (12, 34, 48, 49, 66, 71, 95), MTP (82), and ApoB (82). Although hepatic levels of L-FABP and MTP were upregulated, ApoB was unaltered, in male control-fed DKO mice, while neither L-FABP, MTP, nor ApoB was increased in females (Fig. 5, E, H, and I). High-cholesterol diet alone induced expression of L-FABP (males only) but had no effect on ApoB and MTP (Fig. 5, E, H, and I). In contrast, DKO decreased L-FABP (males only), ApoB, and MTP in high-cholesterol-fed mice (Fig. 5, E, H, and I). Thus, while hepatic lipid accumulation in control-fed DKO mice was not due to loss of key proteins in VLDL assembly and secretion, it was associated at least in part with concomitant downregulation of these key proteins in cholesterol-fed DKO mice.
Nuclear receptors involved in hepatic de novo synthesis of fatty acids.
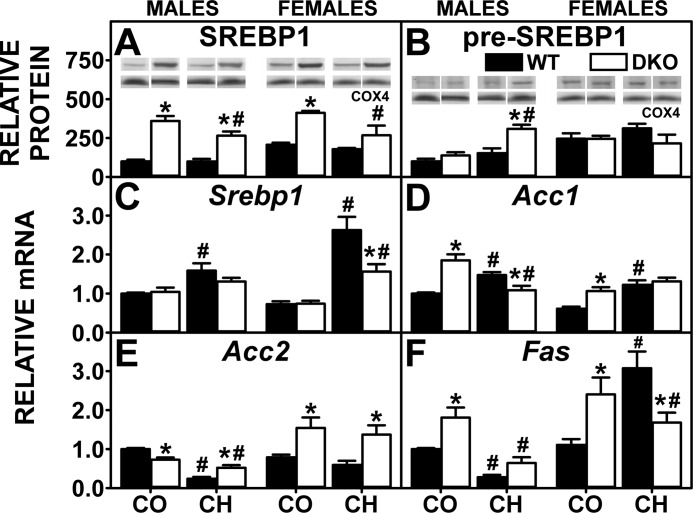
In liver, the primary SREBPs that regulate transcription of lipogenic genes (Acc, Fas) are SREBP1 and SREBP2 (93, 105). In addition, the Acc2 gene product produces malonyl CoA at the mitochondrial membrane where the malonyl CoA then inhibits CPT1, the rate-limiting step in fatty acid oxidation-thus increasing fatty acid availability for esterification. The high-cholesterol diet-induced hepatic accumulation of glycerides (triglyceride and phospholipid) was not associated with increased SREBP1 protein (Fig. 6A), and the proportion of SREBP1/pre-SREBP1 actually appeared to decrease (Fig. 6, A vs. B). However, the high-cholesterol diet did increase the hepatic level of SREBP2 and/or pre-SREBP2 (Fig. 7, B and C). Consequently, expression of lipogenic genes was increased in high-cholesterol-fed WT males (Acc1) and females (Acc1, Fas) while that of ACC2 was decreased or tended to decrease. DKO alone also induced hepatic glyceride accumulation that was associated with increased levels of SREBP1 protein (Fig. 6A) and SREBP2 protein (Fig. 7B). Consistent with increased SREBP1 and SREBP2 protein, DKO alone increased transcription of target genes Acc1 in both males and females (Fig. 6D) and Fas in females (Fig. 6F) concomitant with reduced transcription of Acc2 in males, but not females (Fig. 6E). The high-cholesterol diet did not exacerbate increases in mature SREBP1 and SREBP2 proteins in livers of DKO mice (Figs. 6A and 7B) and did not further increase the DKO-induced transcription of de novo fatty acid synthesis target genes (Fig. 6, D–F).
Fig. 6.
Effect of SCP-2/SCP-X gene ablation and cholesterol-rich diet on key regulators of fatty acid biosynthesis. Western blotting of liver homogenates isolated from WT and DKO mice were analyzed to determine relative protein levels of the 68-kDa sterol response element binding protein-1 (SREBP1; A) and 125-kDa pre-SREBP1 (B). COX4 was used as a loading control to normalize protein expression. QrtPCR was used to determine relative mRNA abundance of Srebp1 (C), Acc1 (D), Acc2 (E), and Fas (F). 18S rRNA was used to normalize mRNA expression levels. mRNA and protein expression levels were as described in experimental materials. A and B, insets: representative Western blots were spliced from multiple blots showing relative protein expression in each mouse group, with the same matching representative COX4 captures from the corresponding blot shown in multiple figures. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 4–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
Fig. 7.
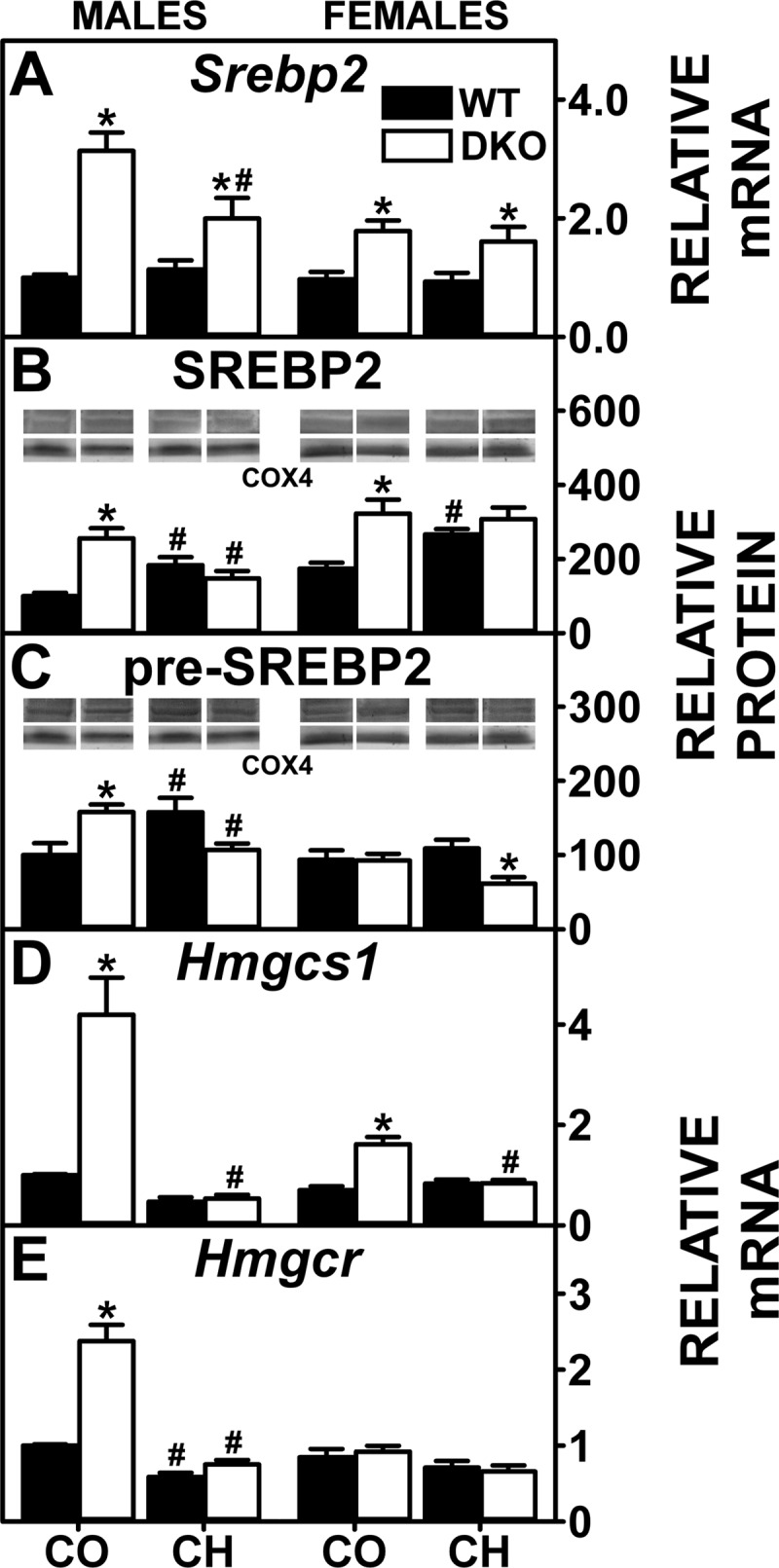
Effect of SCP-2/SCP-X gene ablation and cholesterol-rich diet on key regulators of cholesterol biosynthesis. QrtPCR was used to determine relative mRNA abundance of Srebp2 (A), Hmgcs1 (D), and Hmgcr (E). 18S rRNA was used to normalize mRNA expression levels. Western blots of liver homogenates isolated from WT and DKO mice were analyzed to determine relative protein levels of SREBP2 (68 kDa; B) and pre-SREBP2 (125 kDa; C). COX4 was used as a loading control to normalize protein expression. mRNA and protein expression levels were quantified as described in experimental materials. B and C, insets: Representative Western blots were spliced from multiple blots showing relative protein expression in each mouse group, with the same matching representative COX4 captures from the corresponding blot shown in multiple figures. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 4–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
In summary, the high-cholesterol and DKO associated hepatic accumulation of glycerides was attributable in part to increased expression of enzymes/proteins involved in de novo lipogenesis. The potential mechanistic basis for the increased SREBP1 and SREBP2 protein is further detailed in the discussion.
Nuclear receptors involved in hepatic lipoprotein secretion and de novo synthesis of cholesterol.
SREBP2, but much less so SREBP1, also regulates transcription of enzymes and transporters involved in hepatic accumulation of cholesterol (80, 82, 93, 105). The high-cholesterol diet-induced cholesterol accumulation was associated with increased levels of SREBP2 protein in WT males and females (Fig. 7B). Although this increase resulted in more transcription of Scarb1 (Fig. 4A), expression of other SREBP2 target genes, including Hmgcs1 (Fig. 7D), Hmgcr (Fig. 7E), and Ldlr (Fig. 4B), was not increased, suggesting antagonism by other pathways. The DKO alone increased hepatic levels of SREBP2 protein in both sexes (Fig. 7B) as well as pre-SREBP2 in males (Fig. 7C). The increased SREBP2 protein correlated with increased transcription of SREBP2 target genes in de novo cholesterol synthesis, i.e., Hmgcs1 (Fig. 7D) and Hmgcr (Fig. 7E), but not other SREBP2 target genes, i.e., Scarb1 (Fig. 4A) and Ldlr (Fig. 4B), which were unaltered. DKO did not further increase high-cholesterol diet-induced levels of SREBP2 proteins or mRNAs (Fig. 7, A–C). Likewise, DKO did not further alter transcription of other SREBP2 target genes (Figs. 4, A and B, and 7, D and E).
In summary, only in the DKO mice was the hepatic accumulation of cholesterol attributable in part to increased transcription of genes involved in de novo cholesterol synthesis. High-cholesterol diet alone or in combination with DKO did not induce de novo cholesterol synthesis genes. The potential mechanistic basis for the increased SREBP1 and SREBP2 protein is further detailed in the discussion.
Canalicular membrane proteins involved in biliary excretion of cholesterol.
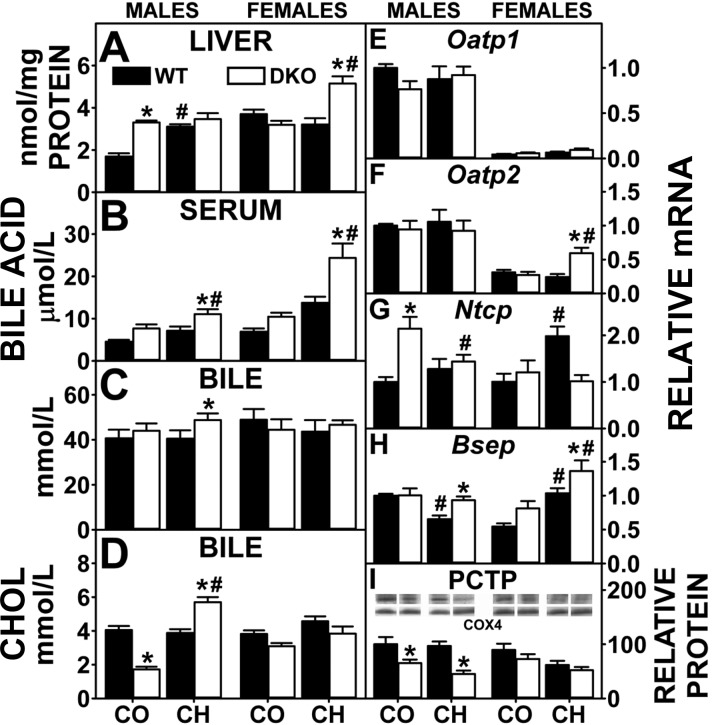
L-FABP, more than SCP-2, is involved in hepatic uptake and cytosolic transfer of HDL-derived cholesterol to bile canaliculus for biliary secretion (56). Biliary cholesterol was unaltered except in control (decreased)- and cholesterol-fed (increased) DKO males (Fig. 8D). The high-cholesterol diet alone increased transcription of canalicular membrane transporter Abcg5 in males, but not females (Fig. 5A), while increasing transcription of Abcg8 in both (Fig. 5B). The DKO alone increased transcription of Abcg5 in males (Fig. 5A), while transcription of Abcg8 was unaltered in either sex (Fig. 5B). DKO did not further exacerbate the high-cholesterol diet-induced transcription of Abcg5 in males but increased that in females (Fig. 5A) despite not increasing transcription of Abcg8 in either sex (Fig. 5B). In summary, the impact of the DKO on biliary cholesterol levels was associated only in part with altered expression of canalicular cholesterol transporters, since both members of the ABCG5/ABCG8 heterodimer pairs were not concomitantly upregulated.
Fig. 8.
Bile acid levels and hepatic expression of key proteins in bile acid reuptake and biliary excretion. Liver (A), serum (B), biliary (C) bile acids, and biliary cholesterol (D) levels were quantified was determined as in experimental materials. QrtPCR was performed as in experimental materials to determine relative mRNA abundance of Oatp1 (E), Oatp2 (F), Ntcp (G), and Bsep (H). 18S rRNA was used to normalize mRNA expression levels. Western blots of liver homogenates isolated from WT and DKO mice were obtained and analyzed as in experimental materials to determine relative protein level of phosphatidylcholine transfer protein (PCTP; I). COX4 was used as a loading control to normalize protein expression. I, inset: The Western blots for males and females were spliced each from 1 blot, respectively. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 4–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
Hepatic, serum, and biliary bile acid levels.
High-cholesterol diet alone increased hepatic, but not serum or biliary bile acid, levels in male but not female WT mice (Fig. 8, A–C). The DKO alone increased hepatic bile acid retention while not significantly altering that in serum or bile in male mice, but females were unaltered (Fig. 8A). High-cholesterol-fed DKO mice exhibited accumulation of bile acids in serum (males and females), liver (females), and bile (males) (Fig. 8, A–C). Since ∼90% of total bile acid is within the bile, the total bile acid pool (liver + serum + bile) was increased only in high-cholesterol-fed male DKO mice (3,763 ± 152 nmol) compared with cholesterol-fed male WT mice (3,088 ± 269 nmol) or control-fed male DKO mice (2,948 ± 221 nmol).
Expression of proteins involved in hepatic bile acid transport.
Hepatic expression of basolateral membrane (Oatp1, Oatp2, and Ntcp) bile acid transport for reuptake of bile acid from serum and canalicular membrane (Bsep) bile acid transporter for biliary excretion of bile acid were examined. The high-cholesterol diet alone and DKO alone in general had little overall effect on basolateral bile acid transport proteins (Fig. 8, E, F, and G) but did upregulate the canalicular Bsep, especially in high-cholesterol-fed DKO mice (Fig. 8H). In summary, the DKO-induced hepatic accumulation of bile acids was not due to concomitant massive upregulation of multiple basolateral bile acid transporters or downregulation of biliary bile acid transporter.
In contrast, because the DKO results in complete absence of both SCP-2 and SCP-x (Fig. 5, C and D), key proteins in cytosolic bile acid transport as well as bile acid synthesis, respectively, were affected. In control-fed DKO mice, this loss was compensated in part by concomitant upregulation of the other major bile acid binding/transport protein L-FABP, whose expression was increased significantly in males and trended to increase slightly in females (Fig. 5E). The cholesterol diet alone increased expression of L-FABP and SCP-x in WT males and increased that of SCP-2 and SCP-x (but not L-FABP) in WT females (Fig. 5, C–E). The DKO prevented the cholesterol diet-induced increase in hepatic L-FABP expression in males but did not alter L-FABP level in females (Fig. 5E).
Interestingly, the DKO significantly decreased hepatic expression of PCTP in DKO males and tended to decrease that in DKO females fed either diet (Fig. 8I). PCTP is the major cytosolic transport protein of phosphatidylcholine associated with phosphatidylcholine targeting to storage and/or VLDL secretion. While this change did not lead to decreased bile acid levels, it may have contributed in part to hepatic retention of phospholipids (Fig. 1C).
Expression of enzymes involved in hepatic bile acid and cholesterol synthesis.
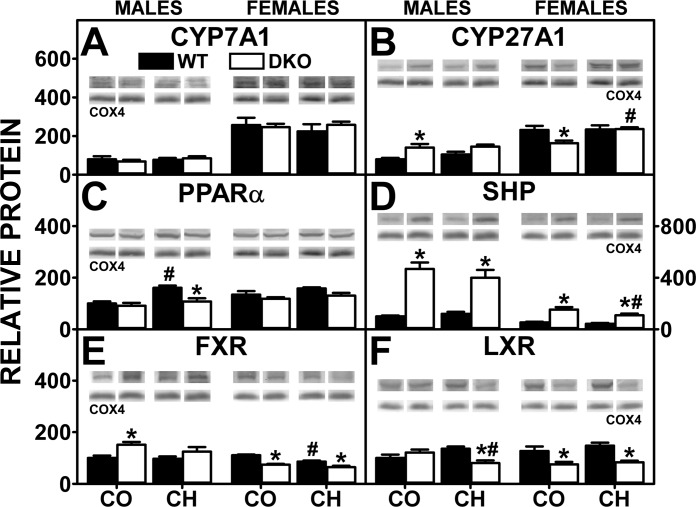
Mice and humans exhibit marked sex-dependent differential expression of the rate-limiting enzymes in the primary and secondary pathways for bile acid synthesis. Expression of CYP7A1 (rate-limiting enzyme in major/primary bile acid synthesis pathway) and CYP27A1 (rate-limiting enzyme in the alternate pathway of bile acid synthesis) was severalfold higher in female mice than male mice under all conditions examined (Fig. 9, A and B). Neither DKO alone, high-cholesterol diet alone, nor both together impacted expression of CYP7A1 in either sex (Fig. 9A). In contrast, DKO alone, but not cholesterol diet alone or both together, elicited concomitant upregulation the alternate pathway enzyme CYP27A1 in males, while decreasing that in females (Fig. 9B). Thus the higher total bile acid level (liver + serum + bile) of cholesterol-fed, male DKO mice was not associated with concomitant upregulation of bile acid synthetic enzymes or increased levels of nuclear receptors such as PPARα, LXR, and FXR (Fig. 9, C, E, and F) that induce CYP7A1 or CYP27A1 transcription (Fig. 9, A and B).
Fig. 9.
Effect of SCP-2/SCP-x gene ablation and cholesterol-rich diet on proteins and transcription factors involved in cholesterol synthesis and oxidation to bile acids. Western blotting of homogenates isolated from the livers of DKO and WT mice were analyzed to determine relative protein levels of cholesterol 7α-hydroxylase (CYP27A1; A), sterol 27-hydroxylase (CYP27A1; B), peroxisome proliferator-activated receptor-α (PPARα; C), small heterodimer partner (SHP; D), farnesoid x receptor (FXR; E), and liver x receptor (LXR; F). COX4 was used as a loading control to normalize protein expression. Expression levels were quantified as described in experimental materials. Insets: representative Western blots were spliced from multiple blots showing relative protein expression in each mouse group, with the same matching representative COX4 captures from the corresponding blot shown in multiple figures. Solid bars: WT mice; open bars: DKO mice. Values are means ± SE (n = 5–7). *P < 0.05 for DKO vs. WT. #P < 0.05 CH vs. CO.
DISCUSSION
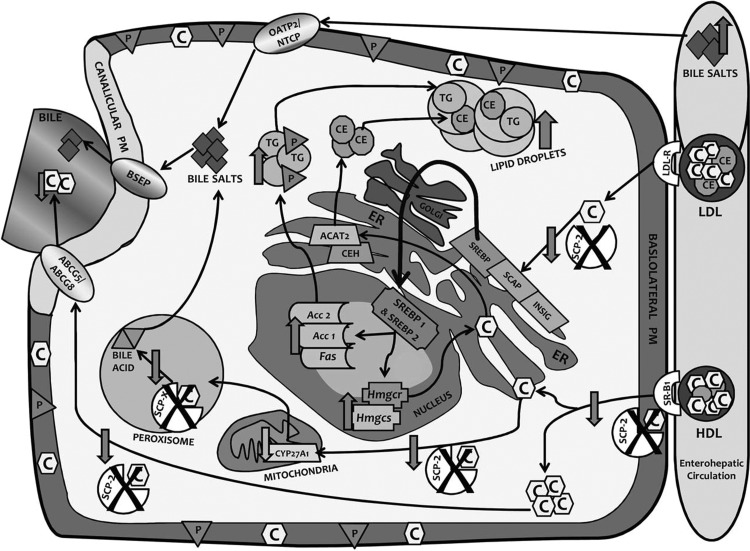
Since mammals have a limited ability to eliminate excess cholesterol, primarily via bile, cholesterol homeostasis is closely regulated. Little is known about how the very poorly soluble cholesterol is rapidly transported and targets within the hepatocyte, to the ER for esterification/storage and regulating SREBP release as well as to oxidative organelles and canalicular membrane for degradation/biliary excretion. Biliary excretion of HDL-derived cholesterol, for example, is extremely rapid with half-time of only 1–3 min (38, 78, 79, 114). Rather than spontaneous and vesicular cholesterol movement, increasing findings in vitro and with cultured cells indicate potential rapid transport via the chaperone intracellular cholesterol-binding proteins SCP-2, SCP-x, and L-FABP (14, 114). The physiological significance of SCP-2, SCP-x, or both (SCP-2/SCP-x) in hepatic cholesterol dynamics has been previously examined by adenovirus tissue-specific hepatic overexpression of SCP-2 (2, 5, 112) and, conversely, by whole body SCP-2 antisense treatment (76), whole body gene ablation of SCP-2/SCP-x (28, 51, 56, 91) or whole body SCP-x ablation alone (6). Likewise, the physiological impact of L-FABP on hepatic cholesterol dynamics has been addressed by adenovirus tissue-specific hepatic overexpression of L-FABP (73) and whole body L-FABP antisense treatment (72, 73), which produced a similar phenotype as whole body L-FABP gene ablation (56, 60, 62). Nevertheless, there have been no reports of tissue-specific knockdown of SCP-2, SCP-x, SCP-2/SCP-x, or L-FABP. Thus it is not possible to determine how whole body gene ablations affect experimental findings compared with what might be observed with tissue-specific knockdown strategies. For ease of comparisons with the literature, our studies were performed with whole body SCP-2/SCP-x gene ablation and high-dietary cholesterol, showing that hepatic lipid accumulation is regulated at least in part by SCP-2 chaperoning cholesterol transport to specific intracellular compartments:
First, SCP-2 facilitates cholesterol targeting to the ER, maintaining basal release of mature SREBP1 and SREBP2 in control-fed WT mice. Conversely, in the DKO mice, cholesterol transport to the ER is reduced, thereby increasing mature SREBP1 and SREBP2 release from the ER for trafficking to the nucleus, and induces nuclear transcription of multiple enzymes in de novo fatty acid and cholesterol synthesis (Fig. 10). Our studies showed that loss of SCP-2 increased release of mature SREBP1 and SREBP2 and induced transcription of their target genes in de novo synthesis of fatty acids and cholesterol. Conversely, adenovirus SCP-2 overexpression markedly diminished de novo cholesterol synthesis (113). Taken together, these findings suggest SCP-2 as a novel candidate for facilitating transport of cholesterol to the ER to regulate the release of SREBPs (Fig. 10). Consistent with this possibility, prior studies have shown that SCP-2 binds (15, 44, 61, 68, 85, 87, 96, 110) and preferentially enhances cholesterol transfer from plasma membranes to ER and from lysosomes/lysosomal membranes to plasma membranes or ER (3, 26, 27, 29, 30, 32, 33, 43, 65, 84, 98, 109). Although the intracellular protein L-FABP also binds cholesterol (45, 57), L-FABP more weakly transports cholesterol from the plasma membrane to the ER (26, 27). Furthermore, DKO upregulated L-FABP only in male but not female control-fed mice.
Fig. 10.
Schematic diagram of proposed roles of SCP-2/SCP-x and impact of SCP-2/SCP-x gene ablation on cholesterol transport and metabolism in C57BL/6Ncr mice. Pathway or product decreased due loss of SCP-2 or SCP-x (downward facing arrow), due to product increase (upward facing arrow), and loss of cholesterol transport protein activity in cellular pathway (cross). ABCG5, ATP-binding cassette transporter G5; ABCG8, ATP-binding cassette transporter G8; Acc 1, acetyl CoA carboxylase-1; Acc2, acetyl CoA carboxylase-2; BSEP, bile salt export pump; C, free cholesterol; CE, cholesteryl ester; CEH, cholesterol ester hydrolase; CYP27A1, cholesterol-27α-hydroxylase; ER, endoplasmic reticulum; Fas, fatty acid synthetase; Hm*gcs, hydroxymethylglutaryl CoA synthase; Hmgcr, hydroxymethylglutaryl CoA reductase; INSIG, insulin-induced gene protein; LDL-R, low density lipoprotein receptor; NTCP, Na+-taurocholate cotransporting polypeptide; OATP2, organic anion transporting polypeptide 2; P, phospholipid; SCAP, SREBP cleavage activating protein; SR-B1, scavenger receptor B1; TG, triacylglycerol.
Second, SCP-2 facilitates cholesterol targeting to the ER for esterification to cholesteryl ester (Fig. 10). SCP-2 overexpression by adenovirus induction increased hepatic cholesteryl ester production (113). However, the current study showed that SCP-2/SCP-x gene ablation also increased cholesteryl ester accumulation, attributed in part to concomitant upregulation of the other major cytosolic cholesterol binding protein L-FABP, at least in male DKO mice (45, 57). L-FABP also transports cholesterol from the plasma membrane to the ER (26, 27) for esterification (13, 47, 71). Total hepatic concentration of L-FABP is over eightfold higher than that of SCP-2 (86). The DKO also elicited concomitant upregulation of ACAT2 along with unaltered Ceh/Hsl in male mice. As suggested by little alteration in serum lipids, the DKO-induced hepatic cholesteryl ester accumulation was not compensated for by modest upregulation of key proteins in hepatic VLDL secretion (i.e., L-FABP, ApoB, and MTP) in control-fed male DKO mice. The DKO had little impact on L-FABP, ACAT2, or serum lipid levels in females.
Third, SCP-2 targets cholesterol to the ER for bile acid synthesis. SCP-2 stimulates hepatic cholesterol 7α-hydroxylase, the rate-limiting enzyme in hepatic bile acid synthesis (52, 92). Through an alternate transcription site, the SCP-2/SCP-x gene also encodes a larger protein, SCP-x (31, 90), which catalyzes the peroxisomal oxidation of cholesterol's branched side chain to form bile acids (31, 90). Adenovirus SCP-2 overexpression increased bile acid secretion and pool size (113). However, the DKO did not decrease total bile acid (liver + serum + bile), due in part to concomitant upregulation of the other major cytosolic bile acid binding protein L-FABP, at least in male DKO mice. L-FABP not only binds bile acids (16–18, 22, 35, 50, 103), it is the most prevalent hepatic FABP family member and bile acid binding protein (22, 64).
Fourth, a high-cholesterol diet alone induced hepatic glyceride and cholesterol accumulation in the C57BL/6N WT mice, consistent with earlier high-cholesterol diet studies in both male and female mice of this substrain (53, 58, 59) and with earlier studies in high-cholesterol-fed male C57BL/6J mice (21, 54). Although this high-cholesterol diet induced transcription of SREBP1 as shown herein and earlier (53), this induction was associated with a slightly decreased total mature SREBP1 in liver homogenate as shown in the male C57BL/6N substrain and a decreased nuclear level of mature SREBP1 as shown earlier in the male J substrain (21, 54). Likewise, although high-cholesterol diet did not alter transcription of SREBP2, the liver total mature SREBP2 was increased in the N substrain mice, regardless of sex, while that appearing in the nucleus of male J substrain mice was markedly decreased (21, 54). Consequently, transcription of most target genes of SREBP1 and SREBP2 was decreased more in male J substrain mice (21, 54) than in the male N substrain mice shown herein. Conversely, cholesterol depletion increased hepatic SREBP2 level in the hamster but surprisingly decreased SREBP1 levels of mRNA, precursor, and mature protein (93). These data indicate that cholesterol regulation of SREBP signaling is much more complex than dependence on dietary cholesterol alone, also being affected by sex, species, polyunsaturated fatty acids, oxysterols, and other factors (19, 21, 54, 93, 105, 111).
Additional contributions to the high-cholesterol diet-induced hepatic lipid accumulation include the severalfold higher cholesterol induction of hepatic L-FABP, SCP-2, and/or SCP-x (Fig. 5, E and D). Both SCP-2 (15, 44, 61, 68, 85, 87, 96, 110) and L-FABP (57) bind and preferentially enhance cholesterol transfer (SCP-2 more so than L-FABP) from plasma membranes as well as from lysosomes/lysosomal membranes to plasma membranes rather than to ER and mitochondria (3, 26, 27, 29, 30, 32, 33, 43, 65, 84, 98, 109). SCP-2 and L-FABP also increase basolateral plasma membrane cholesterol uptake/efflux (97) and target cholesterol to ER enzymes (ACAT2) for esterification to cholesteryl ester (11, 13, 47, 67, 95) and/or biliary excretion (24, 70, 83, 100). SCP-x is the only known enzyme for peroxisomal oxidation of cholesterol to form bile acids (90). Taken together, the high level of L-FABP as well as SCP-2 and SCP-x in the WT C57BL/6N mice may contribute to diverting excess cholesterol to other pathways besides targeting SREBP release from the ER. However, the loss of SCP-2/SCP-x (DKO) resulted in highest hepatic SREBP1 and SREBP2 levels, especially in high-cholesterol-fed DKO mice wherein SCP-2 is ablated and in females L-FABP is downregulated. We hypothesize that this DKO would decrease targeted delivery of cholesterol to the ER, thereby allowing increased release of mature SREBPs, as was observed (Figs. 6A and 7B). The current study examined total hepatic levels of mature SREBP1 protein and mature SREBP2 protein; however, the possibility that other factors may also contribute to nuclear distribution, retention, and efflux of these SREBPs must be considered.
Finally, it is important to note that the phenotype of control-fed DKO mice in this study differed from that reported in an earlier study with independently created male DKO mice (91). In the latter study, body weights were unaltered despite increased food intake, hepatic accumulation of cholesterol (especially cholesteryl ester) as well as glycerides (phospholipid and/or triglyceride) was not observed, and hepatic levels of phospholipid were unaltered, while triglycerides decreased (91). The earlier study differed in several key aspects since their DKO mice were as follows: 1) generated using a different ablation construct strategy (91); 2) backcrossed to a different background substrain (C57BL/6J rather than C57BL/6N); the J substrain differs significantly from the N in a number of genes and is much more susceptible to high-fat diet-induced obesity (reviewed in Ref. 4); and 3) the mice were fed a control diet containing high branched-chain lipid levels (i.e., 0.2 mg phytanic acid/g chow; 0.08 mg phytol/g chow) (91). In contrast, the diet in the present study was prepared phytoestrogen free and phytanic acid/phytol free, as confirmed by gas chromatography/mass spectroscopic analysis, which indicated no detectable phytanic acid, and phytol was present only at 0.0028 ± 0.0004 mg/g food (data not shown). The latter difference is especially important.
Phytanic acid is a ligand for both L-FABP and PPARα (25, 41, 63), and is one of the most potent naturally occurring inducers of PPARα (1, 36, 108). L-FABP is known to transport bound fatty acids into nuclei wherein it directly interacts with (40, 42, 94, 106, 107) and facilitates ligand activation of PPARα (40, 42, 46, 74, 75, 107). Ligand induction of PPARα elicits transcription of L-FABP as well as multiple enzymes in LCFA oxidation (46, 74, 75, 89, 107). Thus the >70-fold higher dietary level of phytanic acid in the control chow of the previously generated DKO mice would be expected to significantly induce PPARα transcription of L-FABP, as confirmed by fivefold concomitant upregulation of L-FABP (28, 91), nearly three- to fivefold greater than the present study. The much higher induction of L-FABP in turn would reinforce or potentiate phytanic acid uptake and transport into the nucleus to induce PPARα transcription of LCFA oxidative enzymes and reduce hepatic levels of fatty acids acylated to phospholipid and triglyceride (91). Since phytanic acid and pristanic acid are very potent PPARα agonists that induce transcription of fatty acid oxidative enzymes and L-FABP, increased fatty acid oxidation would compensate for any increase in food consumption and thereby maintain body weight (7, 9, 20, 37). Thus the significant dietary content of branched-chain lipids (phytanic acid and phytol) in the earlier study would account for the differences in observed phenotype.
In summary, the data suggest that the SCP-2/SCP-x double gene ablation affects cholesterol transport to particular intracellular compartments with 1) less cholesterol to ER for SREBP regulation; 2) more of what cholesterol that does arrive being used for cholesteryl ester synthesis and storage in lipid droplets; and 3) more cholesterol to peroxisomes and other organelles for bile salt synthesis and/or secretion. These data are important contributions to our understanding of regulation of sterol metabolism.
GRANTS
This work was supported in part by the US Public Health Service/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-41402 (to F. Schroeder and A. B. Kier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.K., D.L., G.G.M., A.L.M., K.K.L., J.T.M., and A.B.K. performed experiments; D.K., D.L., G.G.M., A.L.M., K.K.L., and J.T.M. analyzed data; D.K., D.L., G.G.M., A.L.M., K.K.L., J.T.M., F.S., and A.B.K. interpreted results of experiments; D.K., D.L., G.G.M., A.L.M., K.K.L., and J.T.M. prepared figures; D.K., F.S., and A.B.K. drafted manuscript; D.K., F.S., and A.B.K. edited and revised manuscript; D.K., D.L., G.G.M., A.L.M., K.K.L., J.T.M., F.S., and A.B.K. approved final version of manuscript; D.L., F.S., and A.B.K. conception and design of research.
REFERENCES
- 1.Adida A, Spener F. Intracellular lipid binding proteins and nuclear receptors inolved in branched-chain fatty acid signaling. Prost Leukot Essen Fatty Acids 67: 91–98, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Amigo L, Zanlungo S, Miquel JF, Glick JM, Hyogo H, Cohen DE, Rigotti A, Nervi F. Hepatic overexpression of sterol carrier protein-2 inhibits VLDL production and reciprocally enhances biliary lipid secretion. J Lipid Res 44: 399–407, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Atshaves BP, Gallegos A, McIntosh AL, Kier AB, Schroeder F. Sterol carrier protein-2 selectively alters lipid composition and cholesterol dynamics of caveolae/lipid raft vs non-raft domains in L-cell fibroblast plasma membranes. Biochemistry 42: 14583–14598, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and dietary obesity. J Nutr Biochem 21: 1015–1032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atshaves BP, McIntosh AL, Martin GG, Landrock D, Payne HR, Bhuvanendran S, Landrock K, Lyuksyutova OI, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J Lipid Res 50: 1429–1447, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atshaves BP, McIntosh AL, Landrock D, Payne HR, Mackie J, Maeda N, Ball JM, Schroeder F, Kier AB. Effect of SCP-x gene ablation on branched-chain fatty acid metabolism. Am J Physiol Gastrointest Liver Physiol 292: G939–G951, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem 279: 30954–30965, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Atshaves BP, McIntosh AL, Payne HR, Gallegos AM, Landrock K, Maeda N, Kier AB, Schroeder F. Sterol carrier protein-2/sterol carrier protein-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J Lipid Res 48: 2193–2211, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res 45: 812–830, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Atshaves BP, Petrescu A, Starodub O, Roths J, Kier AB, Schroeder F. Expression and intracellular processing of the 58 kDa sterol carrier protein 2/3-oxoacyl-CoA thiolase in transfected mouse l-cell fibroblasts. J Lipid Res 40: 610–622, 1999. [PubMed] [Google Scholar]
- 11.Atshaves BP, Starodub O, McIntosh AL, Roths JB, Kier AB, Schroeder F. Sterol carrier protein-2 alters HDL-mediated cholesterol efflux. J Biol Chem 275: 36852–36861, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Bordewick U, Heese M, Borchers T, Robenek H, Spener F. Compartmentation of hepatic fatty-acid-binding protein in liver cells and its effect on microsomal phosphatidic acid biosynthesis. Biol Chem Hoppe Seyler 370: 229–238, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J Lipid Res 44: 72–83, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Cohen DE. Hepatocellular transport and secretion of biliary lipids. Curr Opin Lipidol 10: 295–302, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Colles SM, Woodford JK, Moncecchi D, Myers-Payne SC, McLean LR, Billheimer JT, Schroeder F. Cholesterol interactions with recombinant human sterol carrier protein-2. Lipids 30: 795–804, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Di Pietro SM, Santome JA. Isolation, characterization, and binding properties of two rat liver fatty acid binding protein isoforms. Biochim Biophys Acta 1478: 186–200, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich A, Dieminger W, Fuchte K, Stoll GH, Schlitz E, Gerok W, Kurz G. Functional signficance of interaction of hepatic FABP with sulfated and nonsulfated taurine-conjugated bile salts in rat liver. J Lipid Res 36: 1745–1755, 1995. [PubMed] [Google Scholar]
- 18.Dietrich A, Dieminger W, Nelly SM, Gerok W, Kurz G. Synthesis and applicability of a photolabile 7,7-azi analogue of 3-sulfated taurine-conjugated bile acids. J Lipid Res 36: 1729–1744, 1995. [PubMed] [Google Scholar]
- 19.Eberle D, Hegarty BD, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochemie 86: 839–848, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein-2-/sterol carrier protein x-deficient mice. J Biol Chem 274: 2766–2772, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Engelking LJ, Liang G, Hammer RE, Takaishi K, Kuriyama H, Evers BM, Li WP, Horton JD, Goldstein JL, Brown MS. Schoenheimer effect explained–feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J Clin Invest 115: 2489–2498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favretto F, Assfalg M, Gallo M, Cicero DO, D'Onofrio M, Molinari H. Ligand binding promiscuity and human liver fatty acid binding protein: structural and dynamic insights from an interaction study with glycocholate and oleate. Chembiochemistry 14: 1807–1819, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Ferdinandusse S, Kostopoulos P, Denis S, Rusch R, Overmars H, Dillman U, Reith W, Haas D, Wanders RJ, Duran M, Marziniak M. Mutations in the gene encoding peroxisomal sterol carrier protein-x (SCP-x) cause leukencephalopathy with dystonia and motor neuropathy. Am J Hum Genet 78: 1046–1052, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis 208: 305–316, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Frolov A, Miller K, Billheimer JT, Cho TC, Schroeder F. Lipid specificity and location of the sterol carrier protein-2 fatty acid binding site: A fluorescence displacement and energy transfer study. Lipids 32: 1201–1209, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Frolov A, Woodford JK, Murphy EJ, Billheimer JT, Schroeder F. Spontaneous and protein-mediated sterol transfer between intracellular membranes. J Biol Chem 271: 16075–16083, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Frolov AA, Woodford JK, Murphy EJ, Billheimer JT, Schroeder F. Fibroblast membrane sterol kinetic domains: modulation by sterol carrier protein 2 and liver fatty acid binding protein. J Lipid Res 37: 1862–1874, 1996. [PubMed] [Google Scholar]
- 28.Fuchs M, Hafer A, Muench C, Kannenberg F, Teichmann S, Scheibner J, Stange EF, Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J Biol Chem 276: 48058–48065, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Gallegos AM, Atshaves BP, McIntosh AL, Storey SM, Ball JM, Kier AB, Schroeder F. Membrane domain distributions: analysis of fluorescent sterol exchange kinetics. Curr Analytic Chem 4: 1–7, 2008. [Google Scholar]
- 30.Gallegos AM, Atshaves BP, Storey S, McIntosh A, Petrescu AD, Schroeder F. Sterol carrier protein-2 expression alters plasma membrane lipid distribution and cholesterol dynamics. Biochemistry 40: 6493–6506, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh A, Martin G, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res 40: 498–563, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Gallegos AM, McIntosh AL, Atshaves BP, Schroeder F. Structure and cholesterol domain dynamics of an enriched caveolae/raft isolate. Biochem J 382: 451–461, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallegos AM, Storey SM, Kier AB, Schroeder F, Ball JM. Stucture and cholesterol dynamics of caveolae/raft and nonraft plasma membrane domains. Biochemistry 45: 12100–12116, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Gavey KL, Noland BJ, Scallen TJ. The participation of sterol carrier protein2 in the conversion of cholesterol to cholesterol ester by rat liver microsomes. J Biol Chem 256: 2993–2999, 1981. [PubMed] [Google Scholar]
- 35.Hagan RM, Worner-Gibbs J, Wilton DC. Tryptophan insertion mutagenesis of liver fatty acid binding protein. J Biol Chem 280: 1782–1789, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Hanhoff T, Benjamin S, Borchers T, Spener F. Branched-chain fatty acids as activators of peroxisome proliferators. Eur J Lip Sci Tech 107: 716–729, 2005. [Google Scholar]
- 37.Hanhoff T, Wolfrum C, Ellinghaus P, Seedorf U, Spener F. Pristanic acid is activator of PPARalpha. Eur J Lipid Sci 103: 75–80, 2001. [Google Scholar]
- 38.Hazard SE, Patel SB. Sterolins ABCG5 and ABCG8: regulators of whole body dietary sterols. Pflügers Arch 453: 745–752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109: 1125–1131, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hostetler HA, Balanarasimha M, Huang H, Kelzer MS, Kaliappan A, Kier AB, Schroeder F. Glucose regulates fatty acid binding protein interaction with lipids and PPARa. J Lipid Res 51: 3103–3116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hostetler HA, Kier AB, Schroeder F. Very-long-chain and branched-chain fatty acyl CoAs are high affinity ligands for the peroxisome proliferator-activated receptor alpha (PPARalpha). Biochemistry 45: 7669–7681, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hostetler HA, McIntosh AL, Atshaves BP, Storey SM, Payne HR, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) interacts with peroxisome proliferator activated receptor-a in cultured primary hepatocytes. J Lipid Res 50: 1663–1675, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang H, Gallegos A, Zhou M, Ball JM, Schroeder F. Role of sterol carrier protein-2 N-terminal membrane binding domain in sterol transfer. Biochemistry 41: 12149–12162, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Huang H, McIntosh AL, Martin GG, Landrock KK, Landrock D, Storey SM, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human L-FABP T94A variant enhances cholesterol uptake. Biochim Biophys Acta 1851: 946–955, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, McIntosh AL, Martin GG, Landrock KK, Landrock D, Storey SM, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human L-FABP T94A variant enhances cholesterol uptake. Biochim Biophys Acta 1851: 946–955, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, McIntosh AL, Martin GG, Petrescu AD, Landrock K, Landrock D, Kier AB, Schroeder F. Inhibitors of fatty acid synthesis induce PPARa-regulated fatty acid b-oxidative enzymes: synergistic roles of L-FABP and glucose. PPAR Res 2013: 1–22, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jefferson JR, Slotte JP, Nemecz G, Pastuszyn A, Scallen TJ, Schroeder F. Intracellular sterol distribution in transfected mouse L-cell fibroblasts expressing rat liver fatty acid binding protein. J Biol Chem 266: 5486–5496, 1991. [PubMed] [Google Scholar]
- 48.Jolly CA, Hubbell T, Behnke WD, Schroeder F. Fatty acid binding protein: stimulation of microsomal phosphatidic acid formation. Arch Biochem Biophys 341: 112–121, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Jolly CA, Murphy EJ, Schroeder F. Differential influence of rat liver fatty acid binding protein isoforms on phospholipid fatty acid composition: phosphatidic acid biosynthesis and phospholipid fatty acid remodeling. Biochim Biophys Acta 1390: 258–268, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Kaikaus RM, Bass NM, Ockner RK. Functions of fatty acid binding proteins. Experientia 46: 617–630, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Kannenberg F, Ellinghaus P, Assmann G, Seedorf U. Aberrant oxidation of the cholesterol side chain in bile acid synthesis of sterol carrier protein-2/sterol carrier protein-x knockout mice. J Biol Chem 274: 35455–35460, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Kawata S, Imai Y, Inada M, Inui M, Kakimoto H, Fukuda K, Maeda Y, Tarui S. Modulation of cholesterol 7-a hydroxylase activity by nsLTP in human liver–possibe altered regulation of its cytosolic level in patients with gallstones. Clin Chim Acta 197: 201–208, 1991. [DOI] [PubMed] [Google Scholar]
- 53.Kim HJ, Miyazaki M, Ntambi JM. Dietary cholesterol opposes PUFA-mediated repression of stearoyl-CoA desaturase-1 gene by SREBP-1 independent mechanism. J Lipid Res 43: 1750–1757, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Korn BS, Shimomura I, Bashmakov Y, Hammer RE, Horton JD, Goldstein JL. Blunted feedback suppression of SREBP processing by dietary cholesterol in transgenic mice expressing sterol-resistant SCAP (D443N). J Clin Invest 102: 2050–2060, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackie JT, Atshaves BP, Payne HR, McIntosh AL, Schroeder F, Kier AB. Phytol-induced hepatotoxicity in mice. Toxicol Pathol 37: 201–208, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin GG, Atshaves BP, Landrock KK, Landrock D, Storey SM, Howles PN, Kier AB, Schroeder F. Ablating L-FABP in SCP-2/SCP-x null mice impairs bile acid metabolism and biliary HDL-cholesterol secretion. Am J Physiol Gastrointest Liver Physiol 307: G1130–G1143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Pai PJ, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol Gastrointest Liver Physiol 297: G1053–G1065, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J 391: 549–560, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol Gastrointest Liver Physiol 290: G36–G48, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem 278: 21429–21438, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Martin GG, Hostetler HA, McIntosh AL, Tichy SE, Williams BJ, Russell DH, Berg JM, Spencer TA, Ball JA, Kier AB, Schroeder F. Structure and function of the sterol carrier protein-2 (SCP-2) N-terminal pre-sequence. Biochemistry 47: 5915–5934, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin GG, Huang H, Atshaves BP, Binas B, Schroeder F. Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochemistry 42: 11520–11532, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Martin GG, McIntosh AL, Huang H, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human liver fatty acid binding protein (L-FABP) T94A variant alters structure, stability, and interaction with fibrates. Biochemistry 52: 9347–9357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res 40: 1371–1383, 1999. [PubMed] [Google Scholar]
- 65.McIntosh AL, Huang H, Atshaves BP, Storey SM, Gallegos A, Spencer TA, Bittman R, Ohno-Iwashita Y, Kier AB, Schroeder F. Fluorescent sterols for the study of cholesterol trafficking in living cells. In: Probes and Tags to Study Biomolecular Function for Proteins, RNA, and Membranes, edited by Miller LW. Weinheim: Wiley VCH Verlag, 2008, p. 1–33. [Google Scholar]
- 66.Moncecchi DM, Nemecz G, Schroeder F, Scallen TJ. The participation of sterol carrier protein-2 (SCP-2) in cholesterol metabolism. In: Physiology and Biochemistry of Sterols, edited by Patterson GW, Nes WD. Champaign, IL: American Oil Chemists' Society Press, 1991, p. 1–27. [Google Scholar]
- 67.Murphy EJ, Schroeder F. Sterol carrier protein-2 mediated cholesterol esterification in transfected L-cell fibroblasts. Biochim Biophys Acta 1345: 283–292, 1997. [DOI] [PubMed] [Google Scholar]
- 68.Myers-Payne SC, Hubbell T, Wood WG, Schroeder F. Effects of in vitro and in vivo ethanol on liver cytosolic lipid binding proteins. Hepatology 66: 1648–1656, 1996. [DOI] [PubMed] [Google Scholar]
- 69.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for in vivo body composition in mice. Obes Res 8: 392–398, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. HDL as a biomarker, potential therapeutic target, and therapy. Diabetes 58: 2711–2717, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nemecz G, Schroeder F. Selective binding of cholesterol by recombinant fatty acid-binding proteins. J Biol Chem 266: 17180–17186, 1991. [PubMed] [Google Scholar]
- 72.Newberry EP, Kennedy SM, Xie Y, Luo J, Crooke RM, Graham MJ, Fu J, Piomelli D, Davidson NO. Decreased body weight and hepatic steatosis with altered fatty acid ethanolamide metabolism in aged L-FABP-/- mice. J Lipid Res 53: 744–754, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ong KT, Mashek MT, Davidson NO, Mashek DG. Hepatic ATGL mediates PPARa signaling and fatty acid channeling through an L-FABP independent mechanism. J Lipid Res 55: 808–815, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrescu AD, Huang H, Martin GG, McIntosh AL, Storey SM, Landrock D, Kier AB, Schroeder F. Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARa regulated b-oxidative enzymes. Am J Physiol Gastrointest Liver Physiol 304: G241–G256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrescu AD, McIntosh AL, Storey SM, Huang H, Martin GG, Landrock D, Kier AB, Schroeder F. High glucose potentiates liver fatty acid binding protein (L-FABP) mediated fibrate induction of PPARa in mouse hepatocytes. Biochim Biophys Acta 1831: 1412–1425, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puglielli L, Rigotti A, Amigo L, Nunez L, Greco AV, Santos MJ, Nervi F. Modulation on intrahepatic cholesterol trafficking: Evidence by in vivo antisense treatment for the involvement of sterol carrier protein-2 in newly synthesized cholesterol transfer into bile. Biochem J 317: 681–687, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 16: 459–481, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Robins SJ, Fasulo JM. High density lipoproteins, but not other lipoproteins, provide a vehicle for sterol transport to the bile. J Clin Invest 99: 380–384, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robins SJ, Fasulo JM. Delineation of a novel hepatic route for the selective transfer of unesterified sterols from high density lipoproteins to bile: studies using the perfused rat liver. Hepatology 29: 1541–1548, 1999. [DOI] [PubMed] [Google Scholar]
- 80.Rubin D, Schneider-Muntau A, Klapper M, Nitz I, Helwig U, Folsch UR, Schrezenmeir J, Doring F. Functional analysis of promoter variants in the MTTP gene. Hum Mutat 29: 123–129, 2008. [DOI] [PubMed] [Google Scholar]
- 81.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Sato R, Miyamoto W, Inoue J, Terada T, Imanaka T, Maeda M. SREBP negatively regulates MTTP gene transcription. J Biol Chem 274: 24714–24720, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Schaefer EJ, Santos RD, Asztalos BF. Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol 21: 289–297, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schoer J, Gallegos A, Starodub O, Petrescu A, Roths JB, Kier AB, Schroeder F. Lysosomal membrane cholesterol dynamics: role of sterol carrier protein-2 gene products. Biochemistry 39: 7662–7677, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Schroeder F, Butko P, Nemecz G, Scallen TJ. Interaction of fluorescent delta 5,7,9(11),22-ergostatetraen-3b-ol with sterol carrier protein-2. J Biol Chem 265: 151–157, 1990. [PubMed] [Google Scholar]
- 86.Schroeder F, Frolov A, Schoer J, Gallegos A, Atshaves BP, Stolowich NJ, Scott AI, Kier AB. Intracellular sterol binding proteins, cholesterol transport and membrane domains. In: Intracellular Cholesterol Trafficking, edited by Chang TY, Freeman DA. Boston, MA: Kluwer Academic, 1998, p. 213–234. [Google Scholar]
- 87.Schroeder F, Frolov A, Starodub O, Russell W, Atshaves BP, Petrescu AD, Huang H, Gallegos A, McIntosh A, Tahotna D, Russell D, Billheimer JT, Baum CL, Kier AB. Pro-sterol carrier protein-2: role of the N-terminal presequence in structure, function, and peroxisomal targeting. J Biol Chem 275: 25547–25555, 2000. [DOI] [PubMed] [Google Scholar]
- 88.Schroeder F, Huang H, McIntosh AL, Atshaves BP, Martin GG, Kier AB. Caveolin, sterol carrier protein-2, membrane cholesterol-rich microdomains and intracellular cholesterol trafficking. In: Cholesterol Binding and Cholesterol Transport Proteins, edited by Harris JR. New York: Springer, 2010, p. 279–318. [DOI] [PubMed] [Google Scholar]
- 89.Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock K, Landrock D, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 43: 1–17, 2008. [DOI] [PubMed] [Google Scholar]
- 90.Seedorf U, Brysch P, Engel T, Schrage K, Assmann G. Sterol carrier protein X is peroxisomal 3-oxoacyl coenzyme A thiolase with intrinsic sterol carrier and lipid transfer activity. J Biol Chem 269: 21277–21283, 1994. [PubMed] [Google Scholar]
- 91.Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KW, Wanders RJ, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev 12: 1189–1201, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seltman H, Diven W, Rizk M, Noland BJ, Chanderbhan R, Scallen TJ. Regulation of bile-acid syntheis. Role of sterol carrier protein 2 in biosynthesis of 7 alpha-hydroxy-cholesterol. Biochem J 230: 879–884, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shimano H. SREBPs: transcriptional regulators of lipid synthetic genes. Prog Lipid Res 40: 439–452, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Smathers RL, Galligan JJ, Shearn CT, Fritz KS, Mercer K, Ronis M, Orlicky DJ, Davidson NO, Petersen DR. Susceptibility of L-FABP-/- mice to oxidative stress in early-stage alcoholic liver. J Lipid Res 54: 1335–1345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Starodub O, Jolly CA, Atshaves BP, Roths JB, Murphy EJ, Kier AB, Schroeder F. Sterol carrier protein-2 immunolocalization in endoplasmic reticulum and stimulation of phospholipid formation. Am J Physiol Cell Physiol 279: C1259–C1269, 2000. [DOI] [PubMed] [Google Scholar]
- 96.Stolowich NJ, Frolov A, Petrescu AD, Scott AI, Billheimer JT, Schroeder F. Holo-sterol carrier protein-2: 13C-NMR investigation of cholesterol and fatty acid binding sites. J Biol Chem 274: 35425–35433, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Storey SM, Atshaves BP, McIntosh AL, Landrock KK, Martin GG, Huang H, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from primary cultured mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 299: G244–G254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Storey SM, Gallegos AM, Atshaves BP, McIntosh AL, Martin GG, Landrock K, Kier AB, Ball JA, Schroeder F. Selective cholesterol dynamics between lipoproteins and caveolae/lipid rafts. Biochemistry 46: 13891–13906, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 303: G837–G850, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Intracellular cholesterol binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 302: G824–G839, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thigpen JE, Setchell KD, Ahlmark KB, Kocklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci 49: 530–536, 1999. [PubMed] [Google Scholar]
- 102.Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Environ Health Perspect 107: A182–A183, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thumser AE, Wilton DC. The binding of cholesterol and bile salts to recombinant rat liver fatty acid-binding protein. Biochem J 320: 729–733, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83: 633–671, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Vallim T, Salter AM. Regulation of hepatic gene expression by saturated fatty acids. Prost Leukot Essen Fatty Acids 82: 211–218, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Velkov T. Interactions between human liver fatty acid binding protein and peroxisome proliferator activated receptor drugs. PPAR Res 2013: 1–14, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate PPARalpha and PPARgamma gene expresion via L-FABP: a signaling path to the nucleus. Proc Natl Acad Sci USA 98: 2323–2328, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F. Phytanic acid is ligand and transcriptional activator of murine liver fatty acid binding protein. J Lipid Res 40: 708–714, 1999. [PubMed] [Google Scholar]
- 109.Woodford JK, Colles SM, Myers-Payne S, Billheimer JT, Schroeder F. Sterol carrier protein-2 stimulates intermembrane sterol transfer by direct membrane interaction. Chem Phys Lipids 76: 73–84, 1995. [DOI] [PubMed] [Google Scholar]
- 110.Woodford JK, Hapala I, Jefferson JR, Knittel JJ, Kavecansky J, Powell D, Scallen TJ, Schroeder F. Mechanistic studies of sterol carrier protein-2 effects on L- cell fibroblast plasma membrane sterol domains. Biochim Biophys Acta 1189: 52–60, 1994. [DOI] [PubMed] [Google Scholar]
- 111.Ye J, DeBose-Boyd RA. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harbor Perspect Biol 3: a004754, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zanlungo S, Amigo L, Mendoza H, Glick J, Rodriguez A, Kozarsky K, Miquel JF, Rigotti A, Nervi F. Overexpression of sterol carrier protein-2 in mice leads to increased hepatic cholesterol content and enterohepatic circulation of bile acids. Gastroenterology 118: 135 A1165, 2000. [DOI] [PubMed] [Google Scholar]
- 113.Zanlungo S, Amigo L, Mendoza H, Miquel JF, Vio C, Glick JM, Rodriquez A, Kozarsky K, Quinones V, Rigotti A, Nervi F. Sterol carrier protein-2 gene transfer changes lipid metabolism and enterohepatic sterol circulation in mice. Gastroenterology 119: 1708–1719, 2000. [DOI] [PubMed] [Google Scholar]
- 114.Zanlungo S, Rigotti A, Nervi F. Hepatic cholesterol transport from plasma into bile: implications for gallstone disease. Curr Opin Lipidol 15: 279–286, 2004. [DOI] [PubMed] [Google Scholar]
- 115.Zhou M, Parr RD, Petrescu AD, Payne HR, Atshaves BP, Kier AB, Ball JA, Schroeder F. Sterol carrier protein-2 directly interacts with caveolin-1 in vitro and in vivo. Biochemistry 43: 7288–7306, 2004. [DOI] [PubMed] [Google Scholar]