Abstract
Physical therapists often treat older adults with marked deficits in physical function secondary to an acute hospitalization. These deficits are often collectively defined as hospital-associated deconditioning (HAD). However, there is a paucity of evidence that objectively demonstrates the efficacy of physical therapy for older adults with HAD. Older adults with HAD represent a highly variable and complex population and thus may be difficult to study and develop effective interventions for using our current rehabilitation strategies. This perspective article outlines an innovative framework to operationalize and treat older adults with HAD. This framework may help therapists apply emerging exercise strategies to this population and facilitate additional research to support the total value of physical therapy for older adults in postacute care settings—with value measured not only by improvements in physical performance but perhaps also by reduced rates of disability development, rehospitalization, and institutionalization.
Poor functional performance in older adults after an acute hospitalization is a growing concern. Medicare spending for postacute care after hospitalization has increased significantly over the last decade, representing 10% of the annual Medicare budget.1 Despite this spending, 68% of patients are discharged from postacute care settings below their prehospitalization level of function,2 which contributes to nearly 1 in 5 Medicare beneficiaries being rehospitalized within 30 days after an acute hospitalization.3
Physiologically, an acute hospitalization also causes significant stress on older adults. Within the hospital environment, older adults have prolonged periods of bed rest,4 relative inactivity,5 sleep disturbances,6 and nutritional deficits7,8—all of which contribute to critical failures in homeostatic mechanisms that make older adults vulnerable to adverse health events, such as rehospitalization. During an acute hospitalization, older adults spend approximately 83% of their hospital stay in bed and 12% of their time in a chair.4 Prolonged immobility in the hospital is associated with a number of impairments in older adults, including declines in muscle strength,9 muscle mass,10 cognitive function,11 muscle protein synthesis,12 and physical function.13 Hospitalization also is associated with a decline in activity of daily living (ADL) performance; strikingly, hospitalized older adults are 61 times more likely to develop disability in ADLs than those who are not hospitalized.14 This decline in function during acute hospitalization has been labeled as a partially avoidable physical dependence occurring over the course of care, or, more strikingly, as iatrogenic disability.15
Taken together, this multisystem decline in function has been described as part of a clinical sequela historically termed “medical deconditioning” or “hospital-associated deconditioning” (HAD)16 but more recently has evolved into a more formalized “post-hospital syndrome” (PHS).11 Although “post-hospital syndrome” is a valuable term of increasing importance, we will use the term “hospital-associated deconditioning” to describe our condition of interest in this article, as this term better describes deficits seen in physical therapist practice settings.16
Physical therapists frequently treat patients with HAD; often, these patients present with a myriad of metabolic, respiratory, or infectious causes for deconditioning that require medical supervision and rehabilitation to return to their prior level of function. However, the term “hospital-associated deconditioning,” when applied to this complex patient population, currently does little to guide physical therapist treatment—an assertion supported by the fact that patients with a primary rehabilitation diagnosis of deconditioning in rehabilitation hospitals have a poorer trajectory of functional recovery than patients with a serious, but clear, diagnostic label such as “hip fracture.”17 Older adults with HAD in postacute care settings have higher-than-average rates of readmission to an acute care hospital and lower rates of community discharge18—suggesting that older adults with HAD may be a unique population that requires additional research and clinical attention from the physical therapy community.
Deficits in the Current Management of Older Adults With HAD
After acute hospitalization, older adults with HAD often utilize postacute care settings for rehabilitation to return to their prehospitalization level of function.16,18 Considering the increasing utilization of rehabilitation after hospitalization, physical therapists have a responsibility to ensure they are responding to the changing landscape of value-driven health care by using intervention strategies that concurrently improve patient outcomes and contribute to reducing avoidable hospital readmissions. Given that responsibility, there are 2 main questions we have identified in current physical therapist treatment of older adults with HAD: (1) Are current physical therapist interventions delivered at the appropriate intensity for older adults with HAD? and (2) Do physical therapists need a more established role in models of transitional care for older adults with HAD?
Are Current Physical Therapy Interventions Delivered at the Appropriate Intensity for Older Adults With HAD?
The American Physical Therapy Association, as part of the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely campaign, has educated consumers to question the application of underdosed strength training programs for older adults—these programs are defined as including resistance exercise prescriptions that do not match intensity, duration, and frequency of training to functional goals.19 However, the few studies that have described usual physical therapist interventions for older adults with HAD described low-intensity and generalized treatments16,20 that may not adequately maximize physical function21—thus, leaving older adults with HAD vulnerable to rehospitalization,22 further disability development,2 and higher mortality rates.23 These adverse health outcomes are associated with the general loss of functional reserve commonly observed in older adults with HAD.11
In general terms, functional reserve reflects the capacity for older adults to handle additional stressors or illnesses without loss of independence.16 However, there is a paucity of literature supporting current rehabilitation approaches as intense enough to even return older adults with HAD to the threshold of independence, much less increase functional reserve. This is an important issue because older adults who are discharged with poor physical function have 3 times the odds of being rehospitalized within 30 days than older adults with medically complex conditions and high physical function.22
Despite the identification of physical function as a biomarker for adverse health outcomes, older adults with HAD are often treated by physical therapists in postacute settings with low-intensity exercises,16,20,24 chosen by therapists who may perceive this dosage as safer.25 However, low-intensity exercises may be physiologically inadequate to maximize functional performance21 and thus place an older adult at risk of developing further disability, being rehospitalized, or transitioning into a more frail state.
Similarly, physical therapy in this population has been described in a recent review as “almost exclusively based on historical tradition rather than rigorous scientific evaluation or evidence-based medicine,”16(p72) with lower extremity resistance training (RT), in particular, described as a supplement to basic transfer and gait training instead of a foundational treatment. This description does not reflect the importance of physical therapy for older adults with HAD but does suggest that there is a major opportunity to optimize interventions and improve functional outcomes for this population.
Do Physical Therapists Need a More Established Role in Models of Transitional Care for Older Adults With HAD?
In general, there is a dearth of physical therapist involvement in care transition models designed to reduce secondary complications in older adults after a hospitalization. Transitional care is especially important for older adults with HAD to help prevent secondary complications from the hospitalization, including avoidable readmission. However, the transitional care model for older adults with medically complex conditions established by the University of Pennsylvania—tested and refined with more than 20 years of supporting evidence26–28—does not specifically reference rehabilitation services in the model or within clinical applications.28 Most established transitional care models for older adults focus heavily on the involvement of advanced practice nurses to make home visits, engage older adults in their own medical care,29 promote independence with ADL,30 and coordinate other home health services.28 Evidence-based support for physical therapy in transitional care is lacking, despite physical activity levels31,32 and functional ability22 being robust independent predictors of rehospitalization risk in older adult populations.
HAD: Shifting the Rehabilitation Perspective
Returning (or reconditioning) patients with HAD to their prior level of function is a common therapy paradigm in postacute care, but this approach may miss physical biomarkers (eg, slow gait speed) that suggest a continued high degree of vulnerability33—biomarkers that are likely very responsive to appropriate exercise therapy. Even at their prior level of function, older adults with HAD may be functioning dangerously close to the threshold between independent functioning and dependency, meaning even a small decline in physical performance after hospital discharge may have catastrophic consequences.
Therefore, developing a novel lens through which to view older adults with HAD is the first step in changing the evaluation and treatment paradigms currently used to manage this population. Older adults are often hospitalized for different reasons and different lengths of time, but at discharge they often have a core cluster of symptoms in common.11 After an acute hospitalization, this population frequently experiences significant muscular weakness, decreased stamina, diminished appetite, fatigue, and decreased ability to carry out ADLs.11,16,18 For older adults with HAD, this loss of physical function at hospital discharge is accompanied by marked loss of functional reserve and physical performance, with concomitant elevation in the risk for adverse health events.16 Functional deficits and ADL disability commonly observed in older adults with HAD are strongly predictive of hospital readmission,22,32,34 institutionalization,35 and mortality.23,36,37
These deficits common to HAD strikingly resemble a similar decline in physical function common to the geriatric syndrome of frailty. Frailty has been defined in a myriad of ways,38–42 but most researchers agree that hallmark features of frailty are a generalized decline in physical reserves and a disruption in homeostasis that increases future risk for adverse health outcomes. One well-accepted and validated definition of frailty41 describes the condition as a physiologic syndrome characterized by having any 3 of the 5 diagnostic markers: muscle weakness, slow movement speed, self-reported exhaustion, low physical activity, and unintentional weight loss. When applied to large older adult cohorts, frailty confers independent risk of increased mortality,38,43–46 institutionalization,47 and disability38 similar to the adverse health risks attributed to an acute hospitalization.2,14,35,48 Thus, the acquisition of HAD is of particular concern to older adults who are already frail at the time of hospital admission. Older adults with frailty experience an accelerated loss of function during hospitalization compared with older adults without frailty15 and have higher rates of institutionalization47 and mortality41,49 after hospitalization.
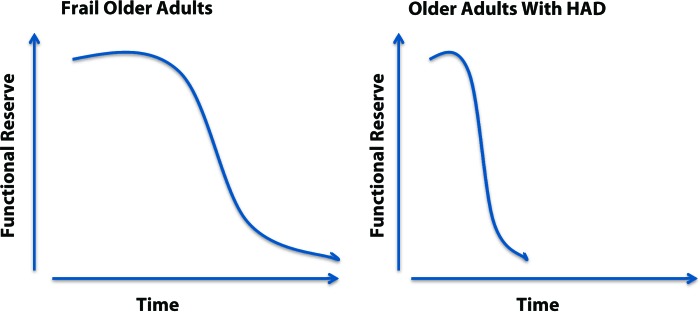
Frailty is related to, but distinctly separate from, comorbidity and disability,50 which makes it a unique clinical entity. The functional deficits present in older adults with frailty closely mirror the deficits seen in older adults with HAD,51 differing only in method of acquisition: frailty often develops insidiously over a period of months or years, whereas HAD can develop rapidly over a period of just a few days (Fig. 1). Thus, the clear similarities between HAD and frailty suggest that older adults with HAD represent a hospital-acquired phenotype of frailty.
Figure 1.
Differing trajectories leading to a loss of functional reserve in older adults. HAD=hospital-associated deconditioning.
Updating Practice Patterns for Older Adults With HAD
Applying exercise guidelines for older adults with frailty to older adults with HAD could be an immediately translatable clinical strategy with strong potential to improve functional outcomes and reduce adverse health events in this vulnerable population. However, shifting from historically performed conservative interventions toward a paradigm of treating underlying frailty symptoms in conjunction with disease-specific interventions requires a significant change in how physical therapists view older adults with HAD. Physical therapists have traditionally used general conditioning activities (GCAs) as a foundational treatment for older adults with HAD. These GCAs may include simple ambulation in the hallway of the facility without application of overload principles, group exercises without application of formal exercise training principles, or general nonspecific active range of motion exercises delivered at a subtherapeutic intensity.16 However, these activities do not adequately address impairments in physical function21 that represent biomarkers for adverse events (rehospitalization,22 institutionalization,35 and death37) in older adults with HAD and thus should be used sparingly in rehabilitation programs.
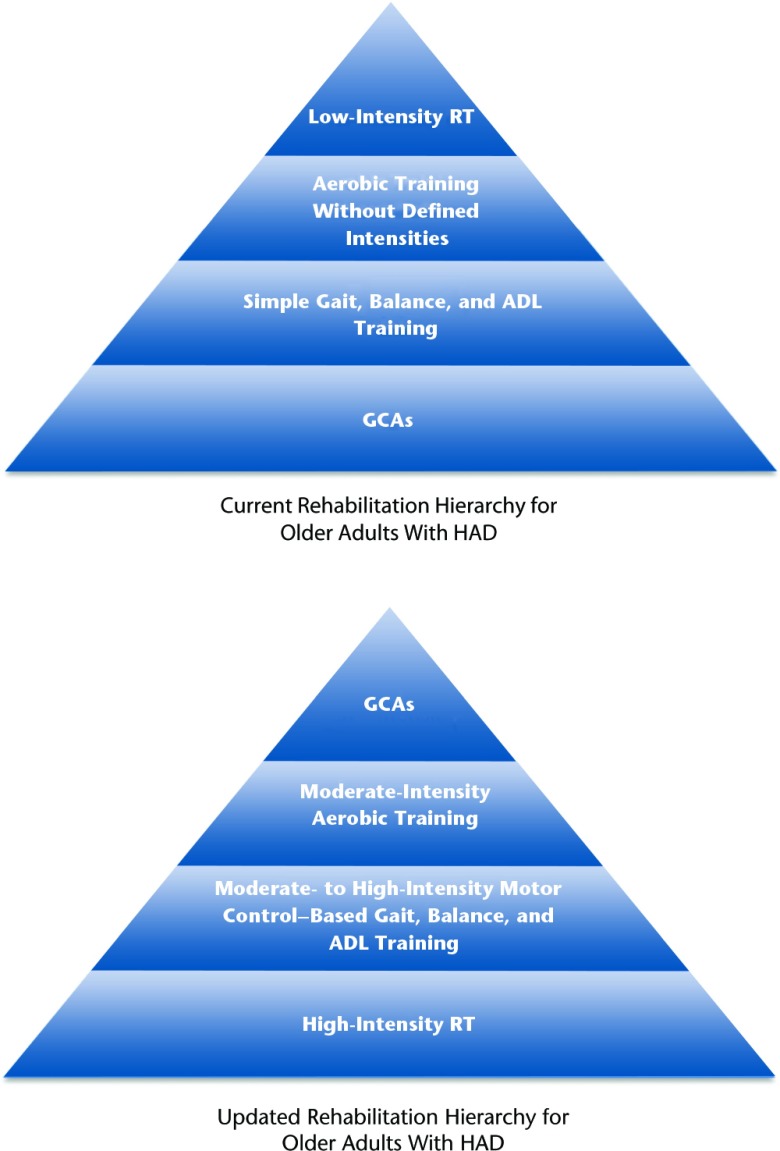
Outlined below is the general evidence-based exercise hierarchy that we believe should be applied to older adults with HAD. We have based the hierarchy on the available evidence for exercise in the frail older adult population, as well as current recommendations from the American College of Sports Medicine52 (ACSM) and the American Geriatrics Society (AGS).53 In general, we propose a treatment framework (Fig. 2) that increases emphasis on higher-intensity RT and de-emphasizes the commonly used GCAs. The evidence supporting our proposed treatment hierarchy is presented below.
Figure 2.
Current rehabilitation hierarchy for older adults with hospital-associated deconditioning (HAD) and hierarchy of an updated treatment approach for older adults with HAD. RT=resistance training, ADL=activities of daily living, GCAs=general conditioning activities.
Resistance Training
Despite the complexities of defining frailty and the multiple domains it influences, treatment of frailty has focused mainly on 2 factors: nutritional supplementation53,54 and exercise training55 with a heavy focus on RT.21,56 The biological evidence for RT in the older adult with frailty is strong; older adults have a diminished muscle protein synthetic response to protein intake and instead depend much more heavily on exercise to maintain a balance between muscle protein breakdown and synthesis.57 Resistance training acts to improve both the biochemical impairments in skeletal muscle58,59 and the low muscle mass60 that are thought to underlie muscle weakness seen in older adults with frailty. Optimal benefits of RT are observed when the programs are carried out at high intensities, defined as 70% to 80% of a patient's 1 repetition maximum (1RM). This intensity, when supervised, is appropriate for most older adults with frailty, including those with other comorbid conditions such as chronic obstructive pulmonary disease or osteoporosis.52
Clinical application of 70% to 80% of 1RM to older adults with frailty does not require maximal strength testing or any specialized equipment. A physical therapist can carry out a high-intensity strength program by simply providing a patient with enough resistance to cause muscle fatigue to the point of failure at 8 to 12 repetitions, with form deterioration over the last 2 repetitions.61,62 Strength training programs for older adults also can be monitored effectively and safely by using Borg Rating of Perceived Exertion, with a goal of 15 to 17 on the 6–20 scale.61,63 Although mild muscle soreness is commonly observed after appropriately dosed treatments, physical therapists should monitor older adults for excessive muscle soreness or fatigue following RT that impairs their ability to carry out basic ADL tasks and adjust dosages accordingly.64
Appropriately dosed high-intensity RT has been shown to improve muscle strength and power in even the frailest older adults and is often more feasible than aerobic training for this population.65 Resistance training specifically has been shown to improve ADL performance,56,66 lower body strength,60 and gait speed60,67 in older adults with frailty. Older adults with frailty have an established dose-dependent response to resistance RT,21,56 suggesting higher intensities are more beneficial and create gains in muscle strength that are more resistant to the effects of detraining.68 A number of studies within acute care,69 community,70 and home71 settings have shown that exercise interventions including RT21,54,72 for older adults with frailty carry no increased risk of serious adverse events72 and result in improved physical performance and reduced risk of health events73 that might necessitate a hospitalization. Despite this strong evidence, RT for older adults is often carried out with low intensities such as the use of 2-lb (0.91-kg) weights for functional quadriceps muscle strengthening or supine bed exercises for strengthening weight-bearing muscles.20,61 These intensities do not support an optimal return of function, nor do they contribute to increased functional reserve in older adults with HAD.
Motor Control Strategies for Gait, Balance, and ADL Improvements
Most evidence is generally supportive of motor control–based gait, balance, and ADL training for improving physical performance and frailty markers in older adults. We believe these interventions can be selected by clinicians based on patient-specific needs and note that further study may be needed in the postacute care setting to determine which interventions and intensities are most beneficial. Generally, task-specific ADL training74 has been shown to improve both muscle strength and motor learning in frail older adults and may be of particular importance to institutionalized or homebound patients. Balance training also is recommended by the ACSM for older adults with frailty and should be performed within a multicomponent program.72 Balance training has been shown to improve outcomes in older adults with frailty who are functionally dependent, including Berg Balance Scale scores75 and fall rates.73 Intensive tai chi training has similarly been shown to improve physical performance measures, including chair rises and gait speed.76 Motor control–based gait training with high-level, task-oriented activities promotes greater gains in gait speed77 and self-reported function78 than traditional endurance-based programs in older adults with gait impairments.
Aerobic Training
Older adults with frailty typically respond more robustly to RT than to aerobic training in the early stages of an exercise program, especially in the presence of significant sarcopenia.52 There is minimal evidence that aerobic training alone substantially improves functional performance or disability in older adults who are frail.66,72 However, aerobic exercise, when part of a multicomponent program including balance or RT, or both, does contribute to improved physical performance in older adults with frailty.56,79 Thus, formal aerobic training should be included only in combination with RT and balance training for older adults with HAD.
General Conditioning Activities
General conditioning activities are often considered a primary component of postacute physical therapy for older adults with HAD.16 These activities are often performed without application of the principles that define skilled exercise therapy—intensity, frequency, duration, or specificity. There is no evidence that GCAs impart substantial short-term or long-term functional benefits in older adults with frailty; thus, these activities that lack the formal components of exercise should be included sparingly in rehabilitation programs for older adults with HAD.
Future Directions for Treating Older Adults With HAD
Given the framework we proposed, there are 3 main future directions that we believe are important in establishing the role of physical therapy in treatment of older adults with HAD: (1) improved recognition of HAD as a unique syndrome, (2) application of higher-intensity treatment strategies when treating older adults with HAD, and (3) increased physical therapist involvement and leadership in developing or participating in interdisciplinary transitional care models for older adults with HAD.
1. Recognizing HAD as a Unique Syndrome
Older adults with HAD represent a unique cohort of patients for whom additional research and clinical attention are needed. This highly variable population has not been adequately defined or categorized, which has led to a disjointed collection of research that often does not center on physical therapist management strategies, despite the fact that older adults with HAD likely represents a large portion of a rehabilitation caseload in postacute care settings. A recent systematic review concluded that there are no well-designed trials that support rehabilitation treatment for older adults with HAD.80 Developing strategies to identify older adults with HAD at risk for poor outcomes could help guide future clinical and research efforts. These strategies also may help operationalize the hospital-associated frailty phenotype by delineating the characteristics of exercise “responders” and “nonresponders” in postacute care settings.
2. Application of Higher-Intensity Interventions to Older Adults With HAD
Low-intensity RT is commonly used within postacute care settings, despite evidence suggesting that older adults with multiple comorbidities not only tolerate62 but also respond robustly81 to supervised high-intensity resistance exercise. A recent research agenda53 published by the AGS suggests RT is a first-line intervention for managing frailty; similarly, the ACSM supports RT as a first-line management strategy for frailty65 with proper training and supervision. Physical therapists in postacute care settings have a tremendous opportunity to challenge and improve current practice patterns in postacute care—viewing HAD as a hospital-acquired phenotype of frailty provides a solid framework for this more progressive approach.
3. Involvement in Care Transition Models for Older Adults With HAD
Physical therapist involvement in care transition models is paramount as the current health care landscape transitions to more bundled models of care. Care transition models often target older adults with multiple comorbidities and at high risk for hospital readmissions. The goals of care transition programs are to improve continuity of care and reduce health care costs for these older adults. However, the existing guidelines make little mention of rehabilitation strategies for low mobility, even though physical activity levels are inversely correlated with risk of rehospitalization.31,32 Physical therapists need to increase their involvement within these care models and demonstrate the additive value that improved mobility and physical function might have on the outcomes of these programs. With an increasing focus on value in health care systems, physical therapists need to demonstrate to all stakeholders that their services offer a meaningful and cost-effective way to reduce rates of disability development and rehospitalization in older adults with HAD.
Conclusion
We have proposed a significant paradigm shift in the treatment of older adults with HAD. Our treatment pyramid, based on evidence for managing frailty, is a general hierarchy that leaves physical therapists open to develop specific protocols for individual patients, while providing a more evidenced-based approach for treating older adults with HAD. This population has tremendous potential to benefit from increased rehabilitation attention. Therefore, we hope this model will facilitate a shift in how physical therapists define successful postacute rehabilitation of older adults with HAD to the following: not only achieving meaningful improvement in physical function but also contributing to concurrent declines in rehospitalizations, future disability development, and mortality.
Footnotes
All authors provided concept/idea/project design and writing. Dr Mangione and Dr Stevens-Lapsley provided consultation (including review of manuscript before submission).
This work was funded, in part, by a Florence P. Kendall Scholarship from the Foundation for Physical Therapy (Dr Falvey), a Fellowship for Geriatric Research from the Academy of Geriatric Physical Therapy (Dr Falvey), the Private Foundation of Abington Memorial Hospital–Innovators' Circle Award (Dr Mangione), an American Physical Therapy Association Home Health Section Research Grant (Dr Stevens-Lapsley), and the National Institutes of Health (NIH K23 AG029978).
References
- 1. US Department of Health and Human Services. CMS Financial Report–Fiscal Year 2007. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CFOReport/downloads/2007_CMS_Financial_Report.pdf Accessed July 24, 2014.
- 2. Gill TM, Gahbauer EA, Han L, Allore HG. Functional trajectories in older persons admitted to a nursing home with disability after an acute hospitalization. J Am Geriatr Soc. 2009;57:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 4. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665. [DOI] [PubMed] [Google Scholar]
- 5. Zisberg A, Shadmi E, Sinoff G, et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273. [DOI] [PubMed] [Google Scholar]
- 6. Missildine K, Bergstrom N, Meininger J, et al. Sleep in hospitalized elders: a pilot study. Geriatr Nurs. 2010;31:263–271. [DOI] [PubMed] [Google Scholar]
- 7. Hiesmayr M, Schindler K, Pernicka E, et al. ; NutritionDay Audit Team. Decreased food intake is a risk factor for mortality in hospitalised patients: the NutritionDay survey 2006. Clin Nutr. 2009;28:484–491. [DOI] [PubMed] [Google Scholar]
- 8. Drevet S, Bioteau C, Mazière S, et al. Prevalence of protein-energy malnutrition in hospital patients over 75 years of age admitted for hip fracture. Orthop Traumatol Surg Res. 2014;100:669–674. [DOI] [PubMed] [Google Scholar]
- 9. Bodilsen AC, Pedersen MM, Petersen J, et al. Acute hospitalization of the older patient: changes in muscle strength and functional performance during hospitalization and 30 days after discharge. Am J Phys Med Rehabil. 2013;92:789–796. [DOI] [PubMed] [Google Scholar]
- 10. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drummond MJ, Dickinson JM, Fry CS, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–E1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gill TM, Allore H, Guo Z. The deleterious effects of bed rest among community-living older persons. J Gerontol Series A Biol Sci Med Sci. 2004;59:755–761. [DOI] [PubMed] [Google Scholar]
- 14. Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. [DOI] [PubMed] [Google Scholar]
- 15. Lafont C, Gérard S, Voisin T, et al. Reducing “iatrogenic disability” in the hospitalized frail elderly. J Nutr Health Aging. 2011;15:645–660. [DOI] [PubMed] [Google Scholar]
- 16. Kortebein P. Rehabilitation for hospital-associated deconditioning. Am J Phys Med Rehabil. 2009;88:66–77. [DOI] [PubMed] [Google Scholar]
- 17. Kortebein P, Granger CV, Sullivan DH. A comparative evaluation of inpatient rehabilitation for older adults with debility, hip fracture, and myopathy. Arch Phys Med Rehabil. 2009;90:934–938. [DOI] [PubMed] [Google Scholar]
- 18. Kortebein P, Bopp MM, Granger CV, Sullivan DH. Outcomes of inpatient rehabilitation for older adults with debility. Am J Phys Med Rehabil. 2008;87:118–125. [DOI] [PubMed] [Google Scholar]
- 19. White NT, Delitto A, Manal TJ, Miller S. The American Physical Therapy Association's Top Five Choosing Wisely Recommendations. Phys Ther. 2015;95:9–24. [DOI] [PubMed] [Google Scholar]
- 20. Mangione KK, Lopopolo RB, Neff NP, et al. Interventions used by physical therapists in home care for people after hip fracture. Phys Ther. 2008;88:199–210. [DOI] [PubMed] [Google Scholar]
- 21. Seynnes O, Fiatarone Singh MA, Hue O, et al. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J Gerontol A Biol Sci Med Sci. 2004;59:503–509. [DOI] [PubMed] [Google Scholar]
- 22. Hoyer EH, Needham DM, Atanelov L, et al. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baztan JJ, Galvez CP, Socorro A. Recovery of functional impairment after acute illness and mortality: one-year follow-up study. Gerontology. 2009;55:269–274. [DOI] [PubMed] [Google Scholar]
- 24. Lenze EJ, Host HH, Hildebrand MW, et al. Enhanced medical rehabilitation increases therapy intensity and engagement and improves functional outcomes in postacute rehabilitation of older adults: a randomized-controlled trial. J Am Med Dir Assoc. 2012;13:708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor NF, Dodd KJ, Damiano DL. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Phys Ther. 2005;85:1208–1223. [PubMed] [Google Scholar]
- 26. Naylor M, Brooten D, Jones R, et al. Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial. Ann Intern Med. 1994;120:999–1006. [DOI] [PubMed] [Google Scholar]
- 27. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–620. [DOI] [PubMed] [Google Scholar]
- 28. Naylor MD, Bowles KH, McCauley KM, et al. High-value transitional care: translation of research into practice. J Eval Clin Pract. 2013;19:727–733. [DOI] [PubMed] [Google Scholar]
- 29. Naylor MD, Hirschman KB, O'Connor M, et al. Engaging older adults in their transitional care: what more needs to be done? J Comp Eff Res. 2013;2:457–468. [DOI] [PubMed] [Google Scholar]
- 30. Coleman EA, Parry C, Chalmers S, Min S. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. [DOI] [PubMed] [Google Scholar]
- 31. Fisher SR, Kuo YF, Sharma G, et al. Mobility after hospital discharge as a marker for 30-day readmission. J Gerontol A Biol Sci Med Sci. 2013;68:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen HQ, Chu L, Amy Liu I-L, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:695–705. [DOI] [PubMed] [Google Scholar]
- 33. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32:46–49. [PubMed] [Google Scholar]
- 34. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Portegijs E, Buurman BM, Essink-Bot ML, et al. Failure to regain function at 3 months after acute hospital admission predicts institutionalization within 12 months in older patients. J Am Med Dir Assoc. 2012;13:569.e561–e567. [DOI] [PubMed] [Google Scholar]
- 36. Sleiman I, Rozzini R, Barbisoni P, et al. Functional trajectories during hospitalization: a prognostic sign for elderly patients. J Gerontol A Biol Sci Med Sci. 2009;64:659–663. [DOI] [PubMed] [Google Scholar]
- 37. Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049–1054. [DOI] [PubMed] [Google Scholar]
- 38. Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. [DOI] [PubMed] [Google Scholar]
- 39. Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown I, Renwick R, Raphael D. Frailty: constructing a common meaning, definition, and conceptual framework. Int J Rehabil Res. 1995;18:93–102. [PubMed] [Google Scholar]
- 41. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 42. Rockwood K, Abeysundera MJ, Mitnitski A. How should we grade frailty in nursing home patients? J Am Med Dir Assoc. 2007;8:595–603. [DOI] [PubMed] [Google Scholar]
- 43. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. [DOI] [PubMed] [Google Scholar]
- 44. Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. [DOI] [PubMed] [Google Scholar]
- 45. Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. [DOI] [PubMed] [Google Scholar]
- 46. Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213:37–42; discussion 42–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lahousse L, Maes B, Ziere G, et al. Adverse outcomes of frailty in the elderly: the Rotterdam Study. Eur J Epidemiol. 2014;29:419–427. [DOI] [PubMed] [Google Scholar]
- 50. Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 51. Covinsky KE, Pierluissi E, Johnston C. Hospitalization-associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306:1782–1793. [DOI] [PubMed] [Google Scholar]
- 52. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 53. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. [DOI] [PubMed] [Google Scholar]
- 54. Rosendahl E, Lindelof N, Littbrand H, et al. High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Aust J Physiother. 2006;52:105–113. [DOI] [PubMed] [Google Scholar]
- 55. Landi F, Abbatecola AM, Provinciali M, et al. Moving against frailty: does physical activity matter? Biogerontology. 2010;11:537–545. [DOI] [PubMed] [Google Scholar]
- 56. Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–1928. [DOI] [PubMed] [Google Scholar]
- 57. Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009;106:2040–2048. [DOI] [PubMed] [Google Scholar]
- 58. Yarasheski KE, Pak-Loduca J, Hasten DL, et al. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am J Physiol. 1999;277(1 pt 1):E118–E125. [DOI] [PubMed] [Google Scholar]
- 59. Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–482. [DOI] [PubMed] [Google Scholar]
- 60. Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. [DOI] [PubMed] [Google Scholar]
- 61. Avers D, Brown M. White paper: strength training for the older adult. J Geriatr Phys Ther. 2009;32:148–152, 158. [PubMed] [Google Scholar]
- 62. Mangione KK, Craik RL, Tomlinson SS, Palombaro KM. Can elderly patients who have had a hip fracture perform moderate- to high-intensity exercise at home? Phys Ther. 2005;85:727–739. [PubMed] [Google Scholar]
- 63. Eston R, Evans HJ. The validity of submaximal ratings of perceived exertion to predict one repetition maximum. J Sports Sci Med. 2009;8:567–573. [PMC free article] [PubMed] [Google Scholar]
- 64. Krishnathasan D, Vandervoort AA. Eccentric strength training prescription for older adults. Top Geriatr Rehabil. 2000;15:29–40. [Google Scholar]
- 65. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand: exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. [DOI] [PubMed] [Google Scholar]
- 66. Daniels R, van Rossum E, de Witte L, et al. Interventions to prevent disability in frail community-dwelling elderly: a systematic review. BMC Health Serv Res. 2008;8:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Freiberger E, Haberle L, Spirduso WW, Zijlstra GA. Long-term effects of three multicomponent exercise interventions on physical performance and fall-related psychological outcomes in community-dwelling older adults: a randomized controlled trial. J Am Geriatr Soc. 2012;60:437–446. [DOI] [PubMed] [Google Scholar]
- 68. Fatouros IG, Kambas A, Katrabasas I, et al. Strength training and detraining effects on muscular strength, anaerobic power, and mobility of inactive older men are intensity dependent. Br J Sports Med. 2005;39:776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mallery LH, MacDonald EA, Hubley-Kozey CL, et al. The feasibility of performing resistance exercise with acutely ill hospitalized older adults. BMC Geriatr. 2003;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Timonen L, Rantanen T, Ryynanen OP, et al. A randomized controlled trial of rehabilitation after hospitalization in frail older women: effects on strength, balance and mobility. Scand J Med Sci Sports. 2002;12:186–192. [DOI] [PubMed] [Google Scholar]
- 71. Gill TM, Baker DI, Gottschalk M, et al. A prehabilitation program for the prevention of functional decline: effect on higher-level physical function. Arch Phys Med Rehabil. 2004;85:1043–1049. [DOI] [PubMed] [Google Scholar]
- 72. Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sihvonen SE, Sipila S, Taskinen S, Era PA. Fall incidence in frail older women after individualized visual feedback-based balance training. Gerontology. 2004;50:411–416. [DOI] [PubMed] [Google Scholar]
- 74. Alexander NB, Galecki AT, Grenier ML, et al. Task-specific resistance training to improve the ability of activities of daily living-impaired older adults to rise from a bed and from a chair. J Am Geriatr Soc. 2001;49:1418–1427. [DOI] [PubMed] [Google Scholar]
- 75. Sihvonen SE, Sipila S, Era PA. Changes in postural balance in frail elderly women during a 4-week visual feedback training: a randomized controlled trial. Gerontology. 2004;50:87–95. [DOI] [PubMed] [Google Scholar]
- 76. Wolf SL, O'Grady M, Easley KA, et al. The influence of intense Tai Chi training on physical performance and hemodynamic outcomes in transitionally frail, older adults. J Gerontol A Biol Sci Med Sci. 2006;61:184–189. [DOI] [PubMed] [Google Scholar]
- 77. Brach JS, Van Swearingen JM, Perera S, et al. Motor learning versus standard walking exercise in older adults with subclinical gait dysfunction: a randomized clinical trial. J Am Geriatr Soc. 2013;61:1879–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van Swearingen JM, Perera S, Brach JS, et al. Impact of exercise to improve gait efficiency on activity and participation in older adults with mobility limitations: a randomized controlled trial. Phys Ther. 2011;91:1740–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sauvage LR, Jr, Myklebust BM, Crow-Pan J, et al. A clinical trial of strengthening and aerobic exercise to improve gait and balance in elderly male nursing home residents. Am J Phys Med Rehabil.. 1992;71:333–342. [DOI] [PubMed] [Google Scholar]
- 80. Timmer AJ, Unsworth CA, Taylor NF. Rehabilitation interventions with deconditioned older adults following an acute hospital admission: a systematic review. Clin Rehabil. 2014;28:1078–1086. [DOI] [PubMed] [Google Scholar]
- 81. Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3:CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]