Abstract
Copper has two key properties that are being exploited in consumer and medical device products in the last decade. On the one hand, copper has potent biocidal properties. On the other hand, copper is involved in numerous physiological and metabolic processes critical for the appropriate functioning of almost all tissues in the human body. In the skin, copper is involved in the synthesis and stabilization of extracellular matrix skin proteins and angiogenesis. This manuscript reviews clinical studies that show that the use of textile consumer and medical device products, embedded with microscopic copper oxide particles, improve the well-being of the skin. These include studies showing a) cure of athlete’s foot infections and improvement in skin elasticity, especially important for individuals suffering from diabetes; b) reduction of facial fine line and wrinkles; and c) enhancement of wound healing; by copper oxide embedded socks, pillowcases and wound dressings, respectively. The manuscript also reviews and discusses the mechanisms by which the presence of copper in these products improves skin well-being.
Keywords: Biocide, copper, extracellular matrix, skin, textiles, wound healing.
Copper has two key properties that endow it as an excellent active ingredient to be used in products, which come in contact with the skin, aiming to improve the skin’s well-being. Copper plays a key role in the synthesis and stabilization of skin proteins, and it also has potent biocidal properties. This manuscript discusses how these two important distinct properties are utilized in consumer and medically related products.
SKIN AGING
The skin is the largest organ of the body that protects our internal tissues from chemical, physical, and microbial damage. It also helps to prevent loss of water and other endogenous substances, participates in thermoregulation of the body and serves as an excretory organ [1, 2]. The skin is differentiated into the epidermis, dermis and subcutaneous layers. The external non-vascularized layer, the epidermis, consists of differentiating keratinocytes that overlay a basement membrane, melanocytes and langerhans cells, and serves as the main semipermeable protective barrier. The dermis, below the basement membrane, consists of fibroblasts, nerves, hair follicles, sebaceous glands, sweat glands, lymphatic and blood vessels. The dermal layer gives shape, firmness, sensation and nourishment to the skin. Finally, the subcutaneous layer consists mostly of adipose tissue, acting primarily as a heat insulator and a mechanical cushion [1, 2].
With age, the skin undergoes vast changes, becoming wrinkled and rigid, losing its firmness, elasticity, tone, texture, thickness, flexibility and moisture content [3]. Skin aging is attributed to several changes. These include alterations in the dermal extracellular matrix (ECM) made up mainly by collagen (which provides strength and structure) and elastin (which provides elasticity and resilience) fibres. Collagen and elastin are secreted mostly by fibroblasts [4, 5]. Transforming growth factor- β (TGF-β) is the primary stimulator of the collagen and elastin fiber formation and deposition [4, 6], and plays a significant role in scar formation [7]. Lysyl oxidase (LOX) is a key protein involved in the lysine-derived crosslinks of the ECM dermal proteins [8-10]. Heat shock protein-47 (HSp-47) is a collagen-specific chaperone needed for the formation of the collagen triple helical structure [11]. During aging, there is a reduction of elastin and collagen production by dermal fibroblasts, alterations in LOX, and breakdown of the collagen/elastin fibers forming the ECM by matrix metalloproteinases (MMp) [5, 6, 8-10, 12-16]. These events, accelerate by inflammation and oxidative stress in photoaged skin [3, 17, 18], are controlled chiefly via the mitogen activated protein kinase (MApK) and NF-κB/p65 signal transduction pathways [17, 19, 20].
COPPER AND ITS PHYSIOLOGICAL ROLE IN THE SKIN
Copper is one of those nine minerals that are recognized as essential nutrients for humans, as it plays a crucial role in different physiological normal processes in basically all human tissues [21], as well as in the skin [22]. The body of a 70-kg healthy individual has about 110 mg of copper, 50% of which is found in the bones and muscles, 15% in the skin, 15% in the bone marrow, 10% in the liver and 8% in the brain [23]. The uptake, distribution to different tissues, secretion of excess, and metabolism of copper, are very precise and efficient coordinated events [23, 24]. Copper is naturally found in many food sources such as meats, vegetables and grains, and the recommended daily intake of copper for adults is ~1 mg [22, 25].
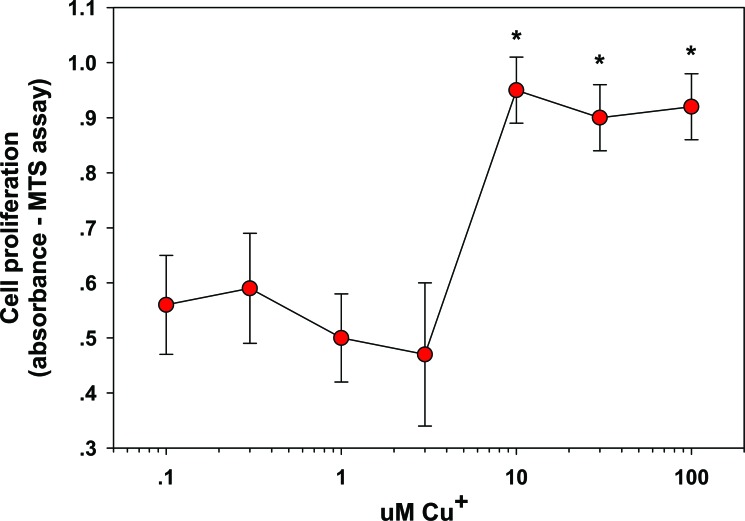
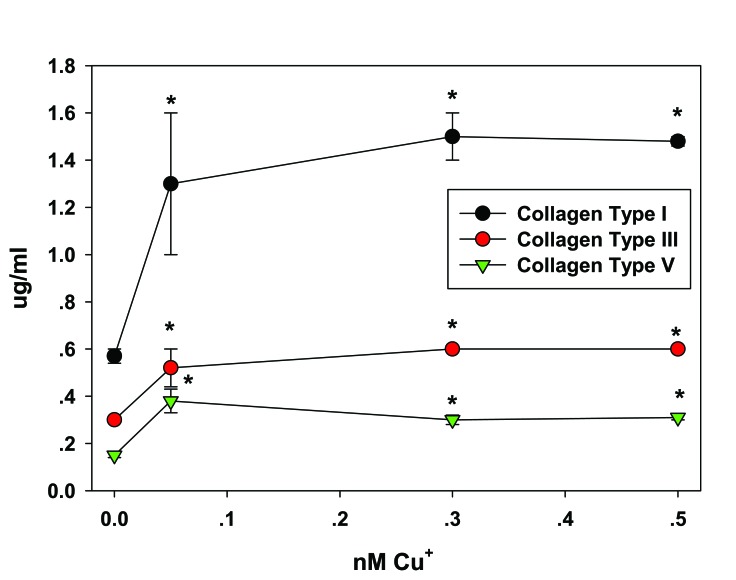
In the skin, copper a) stimulates dermal fibroblasts proliferation [26] (Fig. 1); b) upregulates collagen (types I, II, and V) and elastin fiber components (elastin, fibrillins) production by fibroblasts [27] (Fig. 2), seemingly through the induction of TGF-β [27]; c) stimulates HSp-47, essential to collagen fibril formation [27]; d) serves as a cofactor of LOX needed for efficient ECM protein cross-linking [10]; e) stabilizes the skin ECM once formed, as increased crosslinking of collagen and elastin matrices occurs in a copper dose dependant manner [28, 29]; f) serves as a cofactor of superoxide dismutase, an antioxidant enzyme present in the skin, important for protection against free radicals [30, 31]; g) inhibits cellular oxidative effects such as membrane damage and lipid peroxidation [27]; and h) serves as a cofactor of tyrosinase, a melanin biosynthesis essential enzyme responsible for skin and hair pigmentation [32, 33].
Fig. (1).
Stimulation of Fibroblast proliferation by Copper. Fibroblasts were exposed to 0–100 µM copper for 24 hr. The cells were examined for proliferation by MTS assay. Effects are represented as absorbance units; * = p < 0.05, relative to control (0 Cu2+). Error bars represent standard deviation, n = 4. Data taken from [26].
Fig. (2).
Upregulation of Fibrillar Collagens Secretion from Fibroblast by copper. Fibroblasts were dosed with 0, 0.05, 0.3, or 0.5 nM copper for 24 hr and examined for type I, type III and type V collagen protein levels. *p < 0.05 relative to control; Error bars represent standard deviation, n = 4. Data taken from [27].
In accordance with the above, Cu-GHK, a peptide found in human serum and cerebrospinal fluid that strongly binds copper, increases protein synthesis of collagen and elastin [34]. Cu-GHK also promotes proliferation and survival of epidermal basal stem cells, which are associated with increased expression of integrin [35]. Accordingly, this peptide has been found to improve wound healing. [34]. In addition, patients with an incapacity to metabolize copper (Menkes patients) have reduced LOX activity and collagen formation [36]. Copper is essential to wound healing, as it promotes angiogenesis and skin ECM formation and stabilization [37-41]. For a comprehensive review of the physiological processes in which copper is involved in the skin please see reference [17].
COPPER AS A BIOCIDE
Copper has also potent biocidal properties (reviewed in [42, 43]) and has been used as a biocide for centuries by many different civilizations [44, 45]. Both gram positive and gram negative bacteria, including antibiotic resistant bacteria as well as hard to kill bacterial spores, fungi and viruses, when exposed to high copper concentrations, are killed [42, 43]. In some cases, they are killed within minutes of exposure to copper or copper compounds (e.g. [46-48]). Accordingly, copper biocides have become indispensable, and many thousands of tons are used annually, all over the world, in agriculture [49], wood preservation [50], and in antifouling paints [51]. More recently, copper compounds are introduced into textiles and solid surfaces for odor and microbial control [52-55], including for reduction of microbial bioburden in medical institutions [43, 56-61].
Copper exerts its toxicity to microorganisms through several parallel mechanisms. These include direct contact killing and damage caused by exposure to released copper ions [42, 43, 62-64]. The damage is nonspecific and includes harm to the microorganisms’ envelope phospholipids, microbial envelope or intracellular proteins, and nucleic acids [48, 63-69]. Many bacteria and fungi, excluding viruses, deal with excess copper via intra- and extracellular sequestration through the cell envelopes and membrane efflux pumps. Furthermore, tolerance and adaptation occur by upregulating necessary genes in the presence of copper and by the precipitation of copper by secreted metabolites (reviewed in [42]). However, microorganisms cannot deal with copper overload. Therefore, when exposed to high concentrations of copper they are irreversibly damaged and killed.
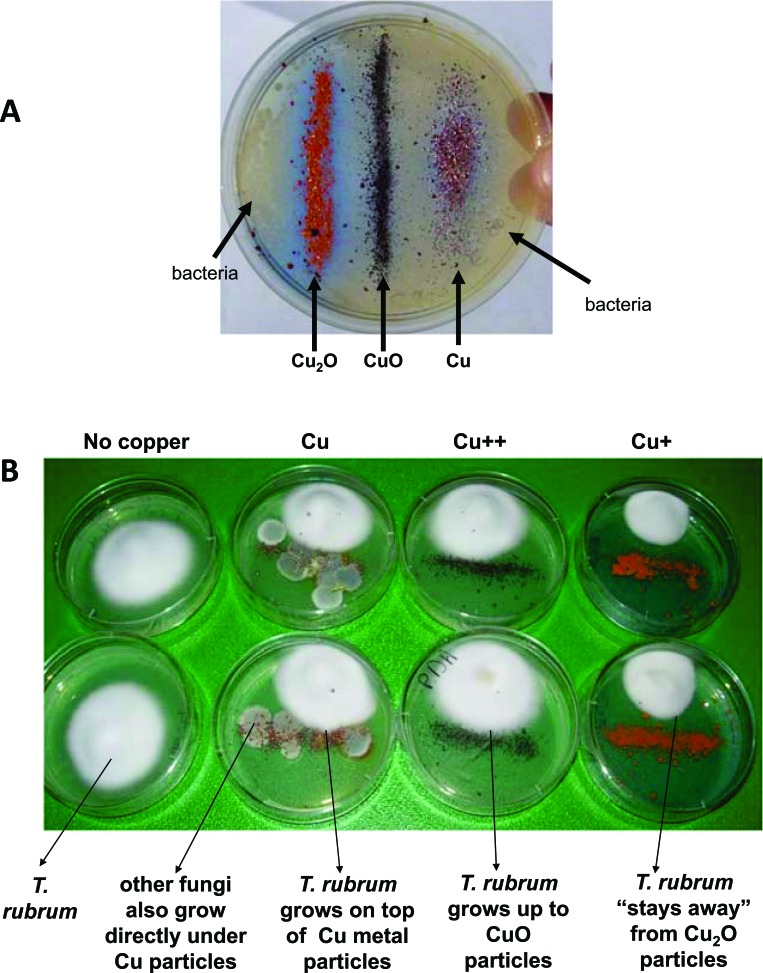
Copper can exist in several oxidation states, i.e. metallic copper (Cu0), monovalent copper (Cuprous, Cu+) or divalent copper (cupric, Cu++) ions. Cu+ ions may exhibit more cytotoxic effects with respect to bacteria in comparison to Cu++ ions [70, 71] (Fig. 3A). As shown in Figure 3B, cuprous ions exhibit greater cytotoxicity with respect to fungi. Importantly, redox cycling between Cu++ to Cu+ can catalyze the production of short lived hydroxyl radicals, which in turn may contribute to superior biocidal activity and thus the combined activity of both cuprous and cupric ions provide greater cytocidal activity than either oxidation state alone.
Fig. (3).
Antibacterial and antifungal efficacy of Cu, Cu++ and Cu+ ions. A) Staphylococcus aureus bacteria were homogenously plated in agar plates, followed by the addition of Cu, CuO and Cu2O particles (in strips). After 24 hours of culture at 37°C the bacteria formed colonies (beige areas). Notice no growth of bacteria especially around the strip of Cu2O particles; B) Cu, CuO and Cu2O particles (in strips) were added to agar plates. A Trichophyton rubrum colony was plated at the side of the plate. The pictures show the growth of the fungi after 4 days of culture at 37°C. plates without copper served as a negative control. Notice the inhibition of growth of the fungi especially when exposed to the cuprous particles. Experiment was conducted in duplicates.
In the presence of air, under ambient conditions, the external metallic copper layer oxidizes to Cu2O. Only at very high temperatures (>200°C), the Cu2O layer further oxidizes to CuO [72]. Significantly higher amounts of copper ions are released by metallic copper followed by Cu2O and CuO layers under different environmental conditions [73]. The Cu2O layer is as effective in contact killing as is metallic copper [73].
HISTORICAL USES OF COPPER FOR SKIN TREATMENT AND DISINFECTION
Interestingly, many different civilizations throughout human history, some in completely separate geographical locations, and mostly independent from one another, have discovered the capacity of copper to help improve/resolve skin and other tissue maladies (for a review see references [44] and [45]).
These include: the Sumerians (~4000–2300 B.C.), who used pulverized malachite (basic cupric carbonate) for generic medical purposes; the ancient Egyptian cultures (~3900–1550 B.C.), who also used pulverized malachite for the prevention and cure of eye infections, and later on (~1550 B.C. to 30 A.D.) additionally for healing postoperative wounds; the Babylonian-Assyrian culture (~1750–539 B.C.), who used different compounds containing copper, as well as copper bracelets, as generic therapeutic remedies; the ancient Indian culture (~2800–1000 B.C.), who used copper sulfide or copper sulfate for nonspecific medical purposes; the ancient Chinese culture (~3000 B.C. TO 1100 A.D.), who used copper (sulfate or sulfide) for topical treatment of skin and eye diseases and also for treatment of systemic infections by oral administration of copper; the Mayan, Aztec, and Inca cultures (~600 B.C. TO 1500 A.D.), who used gauzes soaked in a copper sulfate solution to ‘‘disinfect’’ surgical wounds afflicted during widely practiced drilling of a hole in the skull as a physical, mental, or spiritual treatment, with estimated survival rates above 50%; the ancient Greek culture (1300–323 B.C.), who used copper preparations for purifying drinking water and for the treatment of various cutaneous and eye diseases, pulmonary, vaginal and gastrointestinal disorders, and copper bracelets for arthritis; the early phoenicians (1550 BC to 300 B.C.) nailed copper strips to the bottom of their ships to inhibit fouling to increase speed and maneuverability; the ancient Roman culture (~600 B.C. TO 476 A.D.), who used various copper compounds for the treatment of eye and skin diseases, inflammation of the tonsils, hemorrhoids and generally wound treatment; and the Hindu store for centuries “holy water of the Ganges” in copper utensils to keep the water clean. Finally, copper sulphate is widely used by many inhabitants of the African continent for healing sores and skin diseases.
Today, copper and copper compounds are widely used in many medical related applications. Metallic copper is used already for many years in dental fillings [74] and in copper intrauterine devices for reversible contraception by millions worldwide [75, 76]. Copper compounds are widely used in anthroposophical medicine [77], via oral, subcutaneous injections, or topical applications, in order to stimulate the body to heal itself. Ointments containing copper, which liberate copper ions that are absorbed through the skin [77, 78], are used, for example, in the treatment of cramps, disturbances of renal function, peripheral, venous hypostatic circulatory disturbances, rheumatic disease and swelling associated with trauma [79]. There are also cosmetic facial creams that contain copper as their active ingredient (e.g. Neutrogena Visibly Firm® Face Lotion SpF 20).
SAFETY OF COPPER
As reviewed above, copper has been used throughout history by different civilizations, not only in utensils and adornments, but for many medicinal purposes. The topical or systemic forms of copper and copper compounds used included metallic copper, copper carbonate, copper silicate, copper oxide, copper sulfate, and copper chloride [44, 45], and in general were perceived as safe. As indicated above, copper is widely used today in dental amalgams and in IUDs, and their use is considered very safe [74-76, 80]. The use of copper, as the coating on the surface of hospital furniture, for the reduction of bioburden is gaining momentum following successful trials [59, 61]. Copper is a very weak sensitizer as compared with other metal compounds, and the risk of adverse reactions, due to dermal contact with copper, is exceptionally low [81, 82]. Application of ointment preparations, containing up to 20% metallic copper, were found not to cause any adverse reactions or toxicity [77].
INTRODUCTION OF COPPER OXIDE INTO POLYMERIC PRODUCTS
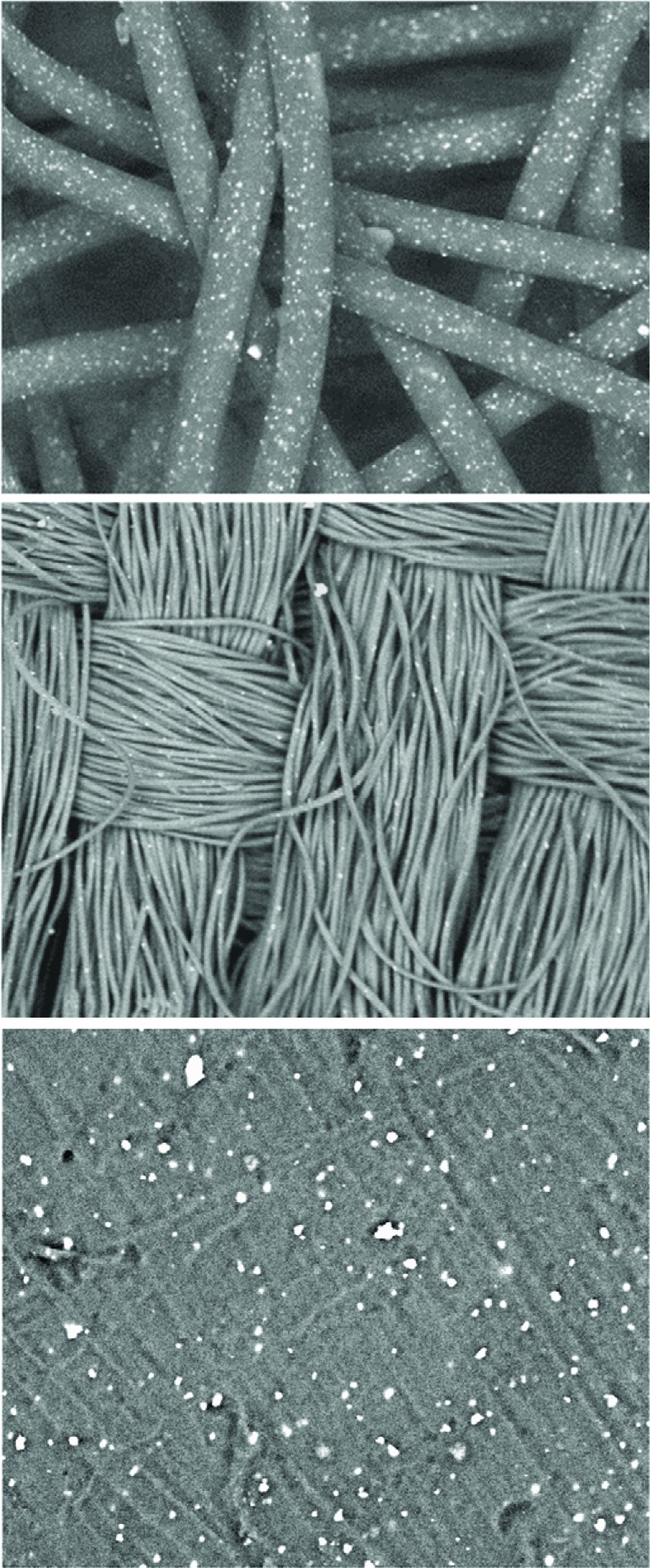
A novel durable platform technology has been industrialized, via which copper oxide particles are embedded (Fig. 4) in polymeric carriers (e.g. polyester, polypropylene and nylon). From these copper oxide embedded polymers, an endless variety of textile, extruded and cast products can be made, endowing these products with potent biocidal properties [52, 61, 83, 84]. The current consumer and health-related products made using this technology include: a) hospital linens, nurse clothing, patient robes and pajamas, with the aim of reducing bioburden and nosocomial infections [52, 55, 83, 85]; b) antiviral and antibacterial copper-impregnated personal protective equipment (ppE), such as protective respiratory masks [55]; c) socks for the prevention and treatment of fungal foot infections (athlete’s foot) [86] and for reducing the risk of skin pathologies, especially in diabetic patients [54]; d) pillowcases that reduce wrinkles [87, 88]; and e) wound dressings that reduce dressing and wound contamination, and enhance wound repair [38, 89].
Fig. (4).
Scanning electronic microscope pictures of non-woven, woven and plastic plate containing the copper oxide particles. The white dots are the copper oxide particles embedded in the polymeric matrices. For more detailed information please see references [52, 83].
The safety of using copper oxide containing products has been examined in several non-clinical studies and in more than 10 clinical trials. In all the studies, not even one adverse reaction was recorded. The products were found to be non-irritating, non-sensitizing, and safe to use, both when in contact with intact and broken skin [89-91].
Copper oxide particles are practicably non-soluble at aqueous solutions with a pH 5.5 and above. Textile products impregnated with copper oxide particles continue to be efficacious even after 50 repeated home or industrial washings [56, 86]. The copper oxide particles serve as a reservoir of copper ions. These ions are slowly liberated in the presence of humidity; for example as that present in the interior of the shoe or in the skin surface.
The following section summarizes the studies conducted with various textile products containing copper oxide that have been found to exert positive effects on the skin.
PILLOWCASES
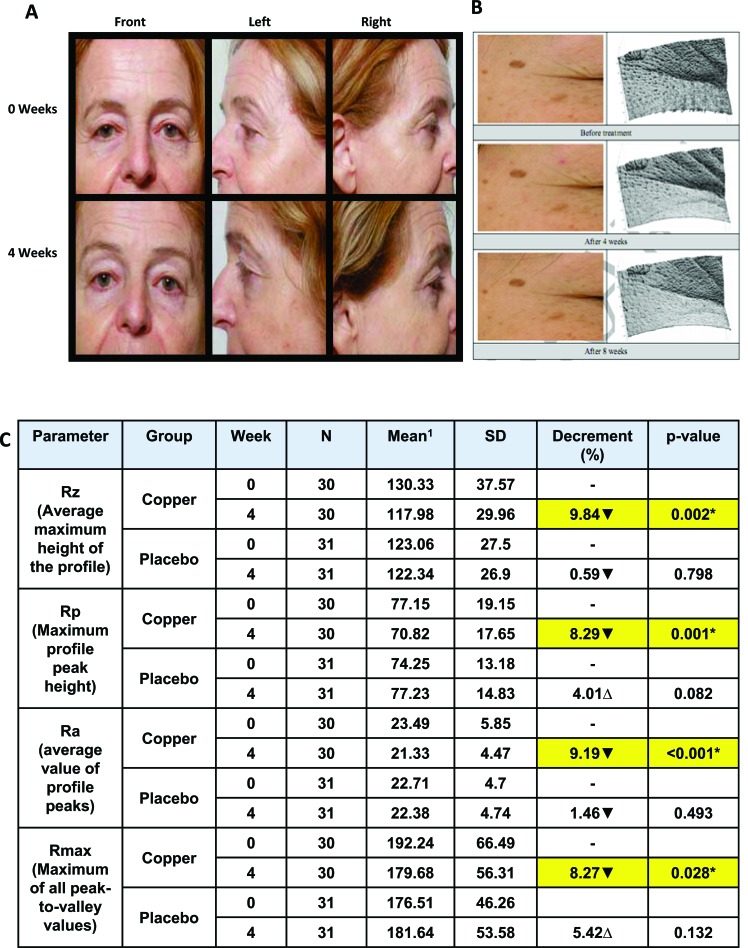
The capacity, of pillowcases containing copper oxide, in reducing wrinkles and improving the well-being of the skin was determined in several double blind placebo controlled clinicaltrials. These trials were conducted by independent researchers and specialized labs. In all the trials, half of the trial participants used pillowcases containing copper oxide while the other half used similar control pillowcases not containing copper oxide. The participants used the pillowcases during sleep for 4 or 8 weeks. In two studies, both the test and control groups used also a cleanser and moisturizing cream. The skin surface topography and condition was analyzed at the commencement of the trials and after 2, 4 and 8 weeks (where relevant) by skin imaging equipment (e.g. 3D Image Analysis GFM phaseshift Rapid in vivo Measurement of Skin (pRIMOS) system), photography, and by expert graders. In all studies, a statistically significant higher reduction of wrinkles and crow's feet/fine lines occurred in the group of individuals using the copper oxide containing pillowcases as compared to the control group [87, 88]. Some representative data and pictures are shown in (Fig. 5).
Fig. (5).
Representative pictures (A) and 3D images (B) data analysis showing reduction of fine lines in the group of individuals using the copper oxide containing pillowcases. pictures and data taken from references [87, 88]. 1Decrement of the mean value represents improvement of skin wrinkles (▼). *Statistically significant difference compare to before treatment (p<0.05).
SOCKS
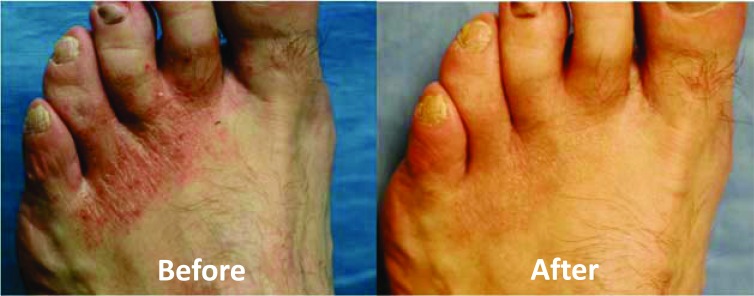
The first trial included 56 subjects suffering from chronic or acute severe tinea pedis (athlete’s foot infections). During the study, the participants used the socks for at least 8 hours a day. Within 8-12 days of use, there was a significant improvement or complete resolution of the podiatric conditions from which the patients suffered (erythema, fissuring, vesicular eruptions, scaling, burning, and/or itching) [86, 92] (Fig. 6). The results included improvement in the skin condition of 21 diabetic patients. Diabetes hinders the capacity to deal with skin damage and infections. Also, in areas of the foot where no fungal infection was present, the skin looked healthier (unpublished observations).
Fig. (6).
Representative pictures showing improvement in the foot skin well-being after using the copper oxide impregnated socks for 12 days. pictures taken from reference [54].
The second study included 53 soldiers undergoing basic training. This population is prone to fungal infections and related skin problems. The soldiers used the socks daily for 3 weeks during which a clear improvement in their skin condition was recorded [93]. Similarly, in an additional trial with ~ 200 soldiers that used the socks continuously for one week under harsh field conditions, the number of soldiers reporting to the infirmary due to foot problems was significantly lower as compared to soldiers using regular socks without copper (unpublished data).
Based on antifungal in vitro testing, conducted by independent laboratories, using the highest laboratory standard (GLp), Cupron Inc. obtained a unique registration with the USA Environmental protection Agency (EpA) to make public health claims, that their fibers/fabric kill 99.9% of the fungus that causes athlete’s foot, including following repeated washings (EpA registration numbers 84542-10 and 84542-11).
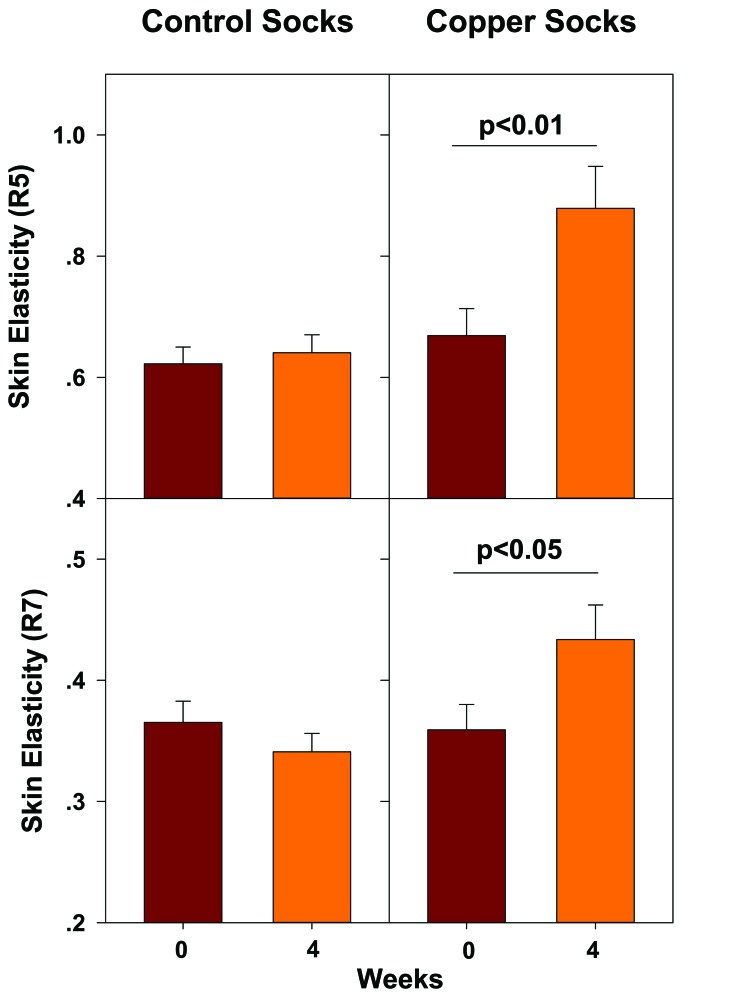
The positive effect of the socks on the skin, is not only due to the biocidal properties of the socks, but as determined in a double blind, placebo controlled study that was conducted with healthy volunteers by CuTest, a UK company that conducts dermatological clinical trials, the copper oxide containing socks improved significantly the elasticity of the skin [94] (Fig. 7).
Fig. (7).
Increased skin surface elasticity following the use of copper oxide containing socks. Using copper oxide containing socks for at least 10 h a day for 4 weeks resulted in 31.4% increased net skin elasticity (R5)(p = 0.004) and 20.7% increased mean biological elasticity (R7) (p=0.014). Data is taken from [94].
The capacity to heal minor wounds and cuts is significantly reduced in diabetic individuals, often resulting in hard to treat chronic ulcers. We hypothesized that part of their reduced capacity to heal wounds is related to the low in situ copper levels present, due to the reduced blood circulation that mainly occurs in the extremities [37]. We further hypothesized that using copper oxide containing socks, by diabetic individuals, may significantly reduce their risk of developing skin disorders in their feet [54]. We hypothesized that the copper impregnated socks continuously release copper ions that are absorbed through the skin. This results in the upregulation of the production of extracellular skin proteins and the stabilization of the ECM, which improve the skin’s well-being. Furthermore, the released copper ions reduce the risk of fungal and bacterial infection of minor wounds and cuts. In addition, especially in larger wounds, the copper ions stimulate angiogenesis and enhance wound healing. We thus suggested the use of copper impregnated socks as a preventive modality [54]. Indeed, such socks, designed specifically for diabetic patients, are sold in pharmacies (e.g. in Israel, by perrigo).
WOUND DRESSINGS
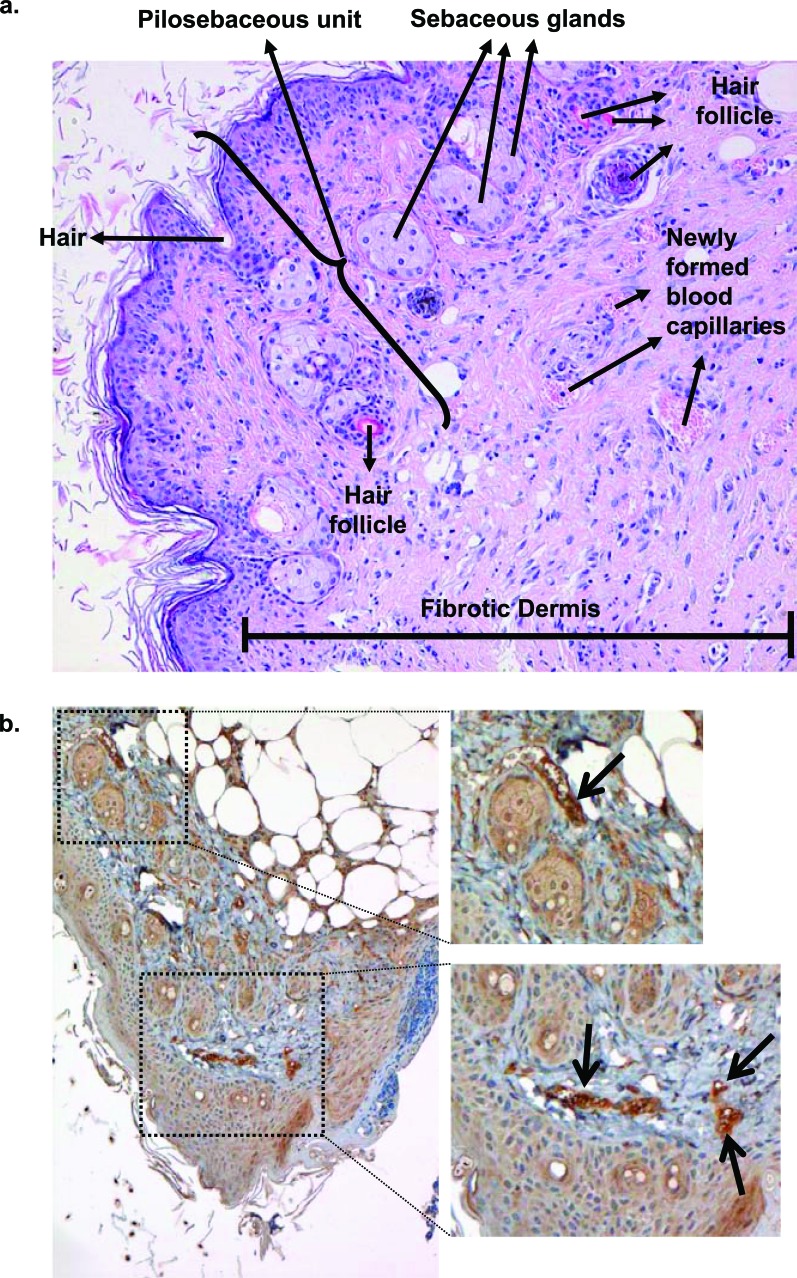
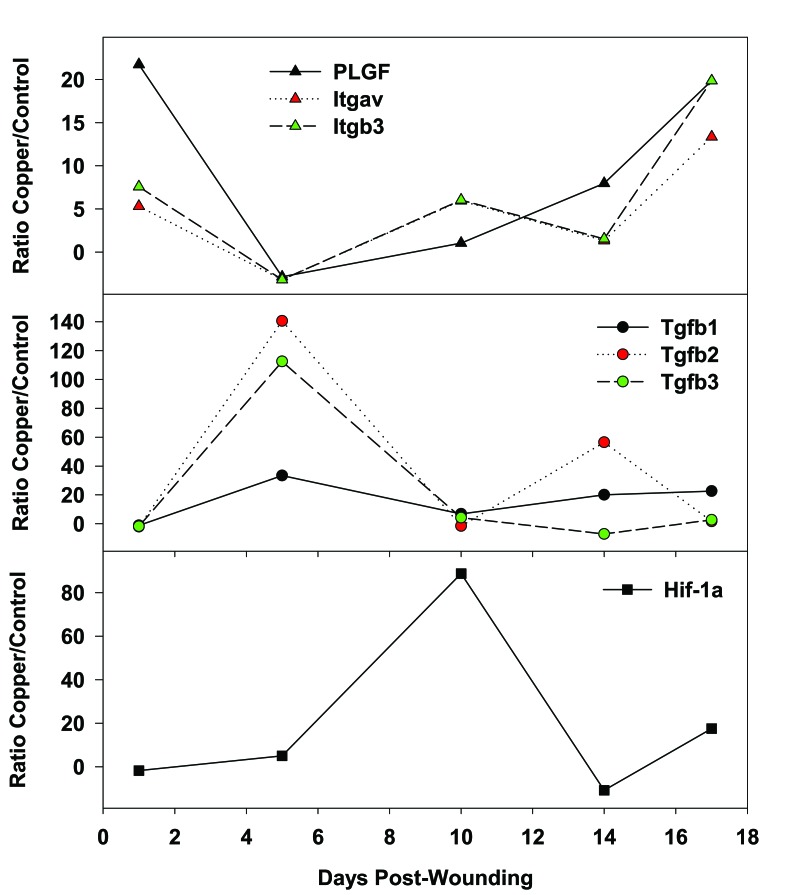
We tested how the continuous application of copper oxide impregnated wound dressing could help heal full thickness skin wounds inflicted in genetically engineered diabetic mice. We found that the wounds healed statistically significantly faster (p<0.01) than wounds treated with control wound dressing without copper or with wound dressing containing silver [38]. Histological analysis revealed normal epidermal and dermal regeneration, granulation tissue formation, formation of numerous new blood vessels, generation of new hair follicles and sebaceous glands, chronic inflammatory infiltrate, and fibroplasia, with no precancerous changes or atypia of any kind (Fig. 8A). mRNA expression analysis of biopsies taken from the wound beds at several days following wounding, revealed significant upregulation of key proteins involved in wound healing in the mice treated with the copper oxide wound dressings as compared to the control mice (Fig. 9). For example, one day after wounding there was ~6 fold increase in integrins (proteins that bind extracellular matrix proteins and transduce signals crucial for cell processes in wound healing [95]) and 22 fold increase in placental growth factor (pLGF; an angiogenic factor known to be markedly reduced in diabetic wounds). Five days after wounding there was a 33, 141 and 112 fold increase in TGF-β1, TGF-β2 and TGF-β3, proteins that stimulate collagen and elastin fiber formation and deposition [4, 6] and are involved in scar formation [7]. Hypoxia-inducible factor-1alpha (Hif-1α) was significantly upregulated by 5 and 88-fold at 5 and 10 days following wounding, respectively. Hif-1α, a regulatory protein involved in oxidative stress [96], activates many genes involved in wound angiogenesis and wound healing [97]. Redox between Cu2O and CuO bring about the production of H2O2 and hydroxyl radicals [42], leading to the upregulation of Hif-1α. Accordingly, in the copper oxide treated mice, we found in-situ increased expression of pro-angiogenic factors, such as pLGF, Hif-1α and vascular endothelial cell growth factor (VEGF, Fig. 8b) and consequently increased blood vessel formation (p<0.05), as compared to the control treated mice. For more detailed proposed molecular mechanisms of enhanced wound healing by the copper oxide containing wound dressings, please see reference [38].
Fig. (8).
Improved wound healing. Following wounding and treatment with copper oxide containing wound dressings there is a) normal skin regeneration (histological analysis after 17 days of wounding) and b) upregulation of angiogenic related factors, such as Vascular Endothelial Growth Factor (VEGF) – Immuno-histological analysis. Arrows show VEGF specific staining. For comparative data to control treated mice and other details, please see reference [38].
Fig. (9).
Increased expression of angiogenic factors and other proteins involved in wound healing. Significant and differential upregulation of mRNA expression of key proteins involved in the wound healing process in the group of mice treated with the copper oxide wound dressings as compared to mice treated with control wound dressing without copper. Itgav - Integrin alpha V; and Itgb3 - Integrin beta 3. Data taken from reference [38].
Importantly, the wound dressings did not induce any adverse reactions in intact skin as determined in rabbits exposed to the dressing for 72 hours, following the ISO-10993 international standard. This included no erythema, eschar or edema formation [89]. Similarly, normal clinical pathology, normal histological patterns and no adverse effects were noted in open wounds in a pig. There were no significant differences in erythema, edema, and crust formation between the wounds treated with control or copper oxide containing wound dressings at 3 and 7 days post-wounding [89].
CONCLUSION
Copper is an essential mineral that plays a key role in many physiological and metabolic processes, including angiogenesis, skin generation and expression and stabilization of extracellular skin proteins. Copper has also potent wide spectrum biocidal properties. The combination of these two distinct properties of copper makes copper a very attractive active material for the improvement of skin well-being. It is thus, not surprising that copper and copper compounds have been used by many different civilizations for more than 2 millennia to treat skin diseases, as well as other maladies. In the last decade, a platform technology has been developed that introduces non-soluble copper oxide into polymeric yarns from which many textile and other consumer products are widely produced. Among these products, pillowcases, socks and wound dressing have been found to exert positive effects both on intact and broken skin. pillowcases, containing copper oxide, have been demonstrated to reduce fine lines and wrinkles. Socks, containing copper oxide, have been found to increase skin elasticity and eliminate athlete’s foot infections when used regularly. Wound dressings, containing copper oxide, enhance wound healing. Thus, the introduction of copper oxide into ordinary products transforms them into extraordinary products.
CONFLICT OF INTEREST
The author is the Chief Medical Scientist of Cupron Inc., who uses copper oxide as its active ingredient.
ACKNOWLEDGEMENTS
I warmly thank Elizabeth Edelman Lipschutz for editing the manuscript.
REFERENCES
- 1.McLafferty E, Hendry C, Alistair F. The integumen-tary system anatomy, physiology and function of skin. Nurs. Stand. 2012;27:35–42. doi: 10.7748/ns2012.10.27.7.35.c9358. [DOI] [PubMed] [Google Scholar]
- 2.Kanitakis J. Anatomy, histology and immunohisto-chemistry of normal human skin. Eur. J Dermatol. 2002;12:390–9. [PubMed] [Google Scholar]
- 3.Rittie L, Fisher GJ. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. perspect. Med. 2015;5 doi: 10.1101/cshperspect.a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.philips N, Tuason M, Chang T, et al. Differential effects of ceramide on cell viability and extracellular matrix remodeling in keratinocytes and fibroblasts. Skin pharmacol. physiol. 2009;22:151–7. doi: 10.1159/000208168. [DOI] [PubMed] [Google Scholar]
- 5.philips N, Samuel M, Arena R, et al. Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J Cosmet. Sci. 2010;61:125–32. [PubMed] [Google Scholar]
- 6.philips N, Conte J, Chen YJ, et al. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factorbeta by polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. 2009;301:487–95. doi: 10.1007/s00403-009-0950-x. [DOI] [PubMed] [Google Scholar]
- 7.penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scar-ring a review. Int. J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 8.Szauter KM, Cao T, Boyd CD, Csiszar K. Lysyl oxi-dase in development, aging and pathologies of the skin. pathol. Biol (paris). 2005;53:448–56. doi: 10.1016/j.patbio.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Atsawasuwan p, Mochida Y, Katafuchi M, et al. Ly-syl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J Biol Chem. 2008;283:34229–40. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rucker RB, Kosonen T, Clegg MS, et al. lysyl oxidase, and extracellular matrix protein cross-linking. Am J Clin Nutr. 1998;67:996S–1002S. doi: 10.1093/ajcn/67.5.996S. [DOI] [PubMed] [Google Scholar]
- 11.Widmer C, Gebauer JM, Brunstein E, et al. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERpINH1 and its structure-specific client recognition. proc. Natl. Acad. Sci. USA. 2012;109:13243–7. doi: 10.1073/pnas.1208072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher GJ, Varani J, Voorhees JJ. Looking older fi-broblast collapse and therapeutic implications. Arch. Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher GJ, Quan T, purohit T, et al. Collagen frag-mentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. . Am. J pathol. 2009;174:101–14. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herouy Y. Matrix metalloproteinases in skin patholo-gy (Review). Int. J Mol Med. 2001;7:3–12. [PubMed] [Google Scholar]
- 15.philips N, Keller T, Hendrix C, et al. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. . Arch. Dermatol. Res. 2007;299:373–9. doi: 10.1007/s00403-007-0779-0. [DOI] [PubMed] [Google Scholar]
- 16.Watson RE, Griffiths CE, Craven NM, Shuttleworth CA, Kielty CM. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. J Invest Dermatol. 1999;112:782–7. doi: 10.1046/j.1523-1747.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- 17.Ekiguchi A, Imada M, philips N, Samuel p, Siomyk H, parakandi H, Gopal S, Shahin H. Improved cell metabolism and strength-ening of the extracellular matrix by nicotinamide, and copper for anti-skin aging. Skin Aging New Research ebook Nova Science. 2013:43–58. [Google Scholar]
- 18.Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res. Rev. 2015 doi: 10.1016/j.arr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Nichols JA, Katiyar SK. Skin photoprotection by nat-ural polyphenols anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaghan TM, Wilhelm Kp. A review of ageing and an examination of clinical methods in the assessment of ageing skin. part I Cellular and molecular perspec-tives of skin ageing. Int. J Cosmet. Sci. 2008;30:313–22. doi: 10.1111/j.1468-2494.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 21.Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am. J Clin. Nutr. 1998;67:952S–9S. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 22.polefka TG, Bianchini RJ, Shapiro S. Interaction of mineral salts with the skin a literature survey. Int. J Cosmet. Sci. 2012;34:416–23. doi: 10.1111/j.1468-2494.2012.00731.x. [DOI] [PubMed] [Google Scholar]
- 23.Linder MC, Wooten L, Cerveza p, et al. Copper transport. . Am. J. Clin. Nutr. 1998;67:965S–71S. doi: 10.1093/ajcn/67.5.965S. [DOI] [PubMed] [Google Scholar]
- 24.pena MM, Lee J, Thiele DJ. A delicate balance ho-meostatic control of copper uptake and distribution. J Nutr. 1999;129:1251–60. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- 25.Trumbo p, Yates AA, Schlicker S, poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am. Diet. Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 26.philips N, Hwang H, Chauhan S, Leonardi D, Gonza-lez S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010;51:224–9. doi: 10.3109/03008200903288431. [DOI] [PubMed] [Google Scholar]
- 27.philips N, Samuel p, parakandi H, et al. Beneficial regulation of fibrillar collagens, heat shock protein- 47, elastin fiber components, transforming growth factor-beta1, vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect. . Tissue Res. 2012;53:373–8. doi: 10.3109/03008207.2012.665970. [DOI] [PubMed] [Google Scholar]
- 28.Sajithlal GB, Chithra p, Chandrakasan G. An in vitro study on the role of metal catalyzed oxidation in gly-cation and crosslinking of collagen. Mol. Cell Bio-chem. 1999;194:257–63. doi: 10.1023/a:1006988719374. [DOI] [PubMed] [Google Scholar]
- 29.Kothapalli CR, Ramamurthi A. Copper nanoparticle cues for biomimetic cellular assembly of crosslinked elastin fibers. Acta Biomater. 2009;5:541–53. doi: 10.1016/j.actbio.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi T, Saito N, Takemori N, et al. Ultrastruc-tural localization of superoxide dismutase in human skin. Acta Derm. Venereol. 1993;73:41–5. doi: 10.2340/00015555734145. [DOI] [PubMed] [Google Scholar]
- 31.Sheng Y, Abreu IA, Cabelli DE, et al. Superoxide dismutases and superoxide reductases. Chem Rev. 2014;114:3854–918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivares C, Solano F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. pigment Cell Melanoma Res. 2009;22:750–60. doi: 10.1111/j.1755-148X.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- 33.Hearing VJ, Jimenez M. Mammalian tyrosinase--the critical regulatory control point in melanocyte pig-mentation. Int. J Biochem. 1987;19:1141–7. doi: 10.1016/0020-711x(87)90095-4. [DOI] [PubMed] [Google Scholar]
- 34.pickart L. The human tri-peptide GHK and tissue remodeling. J Biomater. Sci. polym. Ed. 2008;19:969–88. doi: 10.1163/156856208784909435. [DOI] [PubMed] [Google Scholar]
- 35.Kang YA, Choi HR, Na JI, et al. Copper-GHK in-creases integrin expression and p63 positivity by keratinocytes. . Arch. Dermatol. Res. 2009;301:301–6. doi: 10.1007/s00403-009-0942-x. [DOI] [PubMed] [Google Scholar]
- 36.peltonen L, Kuivaniemi H, palotie A, et al. Alterations in copper and collagen metabolism in the Menkes syndrome and a new subtype of the Ehlers-Danlos syndrome. . Biochemistry. 1983;22:6156–63. doi: 10.1021/bi00295a018. [DOI] [PubMed] [Google Scholar]
- 37.Borkow G, Gabbay J, Zatcoff RC. Could chronic wounds not heal due to too low local copper levelsκ. Med. Hypotheses. 2008;70:610–3. doi: 10.1016/j.mehy.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Borkow G, Gabbay J, Dardik R, et al. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. . Wound. Repair Regen. 2010;18:266–75. doi: 10.1111/j.1524-475X.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 39.Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem. 1998;69:326–35. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 40.Raju KS, Alessandri G, Ziche M, Gullino pM. Ceru-loplasmin, copper ions, and angiogenesis. J Natl. Cancer Inst. 1982;69:1183–8. [PubMed] [Google Scholar]
- 41.Sen CK, Khanna S, Venojarvi M, et al. Copper-induced vascular endothelial growth factor expression and wound healing. . Am J physiol Heart Circ. physiol. 2002;282:H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 42.Borkow G, Gabbay J. Copper as a biocidal tool. Curr. Med. Chem. 2005;12:2163–75. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- 43.Borkow G. Using copper to fight microorganisms. Curr Chem Biol. 2012;6:93–103. [Google Scholar]
- 44.Dollwet HHA, Sorenson JRJ. Historic uses of copper compounds in medicine. Trace Elements in Medicine. 2001;2:80–7. [Google Scholar]
- 45.Milanino R. Copper in medicine and personal care a historical overview. Copper and the skin. In: Hostynek JJ, Maibach HI, editors; pp. New York NY: Informa Healthcare USA; 2006. pp. 149–160. [Google Scholar]
- 46.Borkow G, Sidwell RW, Smee DF, et al. Neutralizing viruses in suspensions by copper oxide based filters. . Antimicrob. Agents Chemother. 2007;51:2605–7. doi: 10.1128/AAC.00125-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espirito SC, Taudte N, Nies DH, Grass G. Contribu-tion of copper ion resistance to survival of Escherich-ia coli on metallic copper surfaces. Appl. Environ Microbiol. 2008;74:977–86. doi: 10.1128/AEM.01938-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohsumi Y, Kitamoto K, Anraku Y. Changes induced in the permeability barrier of the yeast plasma mem-brane by cupric ion. J Bacteriol. 1988;170:2676–82. doi: 10.1128/jb.170.6.2676-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.La Torre A, Talocci S, Spera G, Valori R. Control of downy mildew on grapes in organic viticulture. Commun. Agric. Appl. Biol Sci. 2008;73:169–78. [PubMed] [Google Scholar]
- 50.Schultz Tp, Nicholas DD, preston AF. A brief review of the past, present and future of wood preservation. pest. Manag. Sci. 2007;63:784–8. doi: 10.1002/ps.1386. [DOI] [PubMed] [Google Scholar]
- 51.Cooney TE. Bactericidal activity of copper and noncopper paints. Infect. Control Hosp. Epidemiol. 1995;16:444–50. doi: 10.1086/648361. [DOI] [PubMed] [Google Scholar]
- 52.Borkow G, Gabbay J. putting copper into action copper-impregnated products with potent biocidal ac-tivities. FASEB J. 2004;18:1728–30. doi: 10.1096/fj.04-2029fje. [DOI] [PubMed] [Google Scholar]
- 53.Borkow G, Gabbay J. An ancient remedy returning to fight microbial, fungal and viral infections. Curr Chem Biol. 2009;3:272–8. [Google Scholar]
- 54.Borkow G, Zatcoff RC, Gabbay J. Reducing the risk of skin pathologies in diabetics by using copper im-pregnated socks. Med. Hypotheses. 2009;73:883–6. doi: 10.1016/j.mehy.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 55.Borkow G, Zhou SS, page T, Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. pLoS. One. 2010;5:e11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borkow G, Gabbay J. Endowing textiles with perma-nent potent biocidal properties by impregnating them with copper oxide. JTATM. 2006;5 [Google Scholar]
- 57.Borkow G, Monk AB. Fighting nosocomial infections with biocidal non-intrusive hard and soft surfaces. World J Clin Infect Dis. 2012;12:77–90. [Google Scholar]
- 58.Borkow G. Borkow G. pp. Switzerland:: Springer International publishing; 2014. Biocidal hard and soft surfaces containing copper oxide particles for the reduction of healthcare-acquired pathogens Use of Bio-cidal Surfaces for Reduction of Healthcare Acquired Infections. pp. 85–102. [Google Scholar]
- 59.Borkow G. Schmidt MG, Banks AL, Salgado CD. Switzerland:: Springer International publishing,; 2014. Role of the microbial burden in the acquisition and control of healthcare associated infections the utility of solid copper surfaces Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections. pp. 59–83. [Google Scholar]
- 60.Lazary A, Weinberg I, Vatine JJ, et al. Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with bi-ocidal copper oxide impregnated linens. . Int. J Infect Dis. 2014;24:23–9. doi: 10.1016/j.ijid.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 61.Monk AB, Kanmukhla V, Trinder K, Borkow G. po-tent bactericidal efficacy of copper oxide impregnated non-porous solid surfaces. BMC Microbiol. 2014;14:57. doi: 10.1186/1471-2180-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77:1541–7. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bleichert p, Espirito SC, Hanczaruk M, Meyer H, Grass G. Inactivation of bacterial and viral biothreat agents on metallic copper surfaces. Biometals. 2014;27:1179–89. doi: 10.1007/s10534-014-9781-0. [DOI] [PubMed] [Google Scholar]
- 64.Santo CE, Quaranta D, Grass G. Antimicrobial metal-lic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen. 2012;1:46–52. doi: 10.1002/mbo3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nan L, Liu Y, Lu M, Yang K. Study on antibacterial mechanism of copper-bearing austenitic antibacterial stainless steel by atomic force microscopy. J Mater. Sci. Mater. Med. 2008;19:3057–62. doi: 10.1007/s10856-008-3444-z. [DOI] [PubMed] [Google Scholar]
- 66.Rifkind JM, Shin YA, Hiem JM, Eichorn GL. Co-operative disordering of single stranded polynucleo-tides through copper crosslinking. Biopolymers. 2001;15:1879. doi: 10.1002/bip.1976.360151002. [DOI] [PubMed] [Google Scholar]
- 67.Sagripanti JL, Goering pL, Lamanna A. Interaction of copper with DNA and antagonism by other metals. Toxicol. Appl. pharmacol. 1991;110:477–85. doi: 10.1016/0041-008x(91)90048-j. [DOI] [PubMed] [Google Scholar]
- 68.Kim JH, Cho H, Ryu SE, Choi MU. Effects of metal ions on the activity of protein tyrosine phosphatase VHR highly potent and reversible oxidative inactiva-tion by Cu2+ ion. Arch. Biochem Biophys. 2000;382:72–80. doi: 10.1006/abbi.2000.1996. [DOI] [PubMed] [Google Scholar]
- 69.Karlstrom AR, Levine RL. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. proc. Natl. Acad. Sci. U. S. A. 1991;88:5552–6. doi: 10.1073/pnas.88.13.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abicht HK, Gonskikh Y, Gerber SD, Solioz M. Non-enzymic copper reduction by menaquinone enhances copper toxicity in Lactococcus lactis IL1403. Micro-biology. 2013;159:1190–7. doi: 10.1099/mic.0.066928-0. [DOI] [PubMed] [Google Scholar]
- 71.Beswick pH, Hall GH, Hook AJ, et al. Copper toxici-ty evidence for the conversion of cupric to cuprous copper in vivo under anaerobic conditions. . Chem Biol Interact. 1976;14:347–56. doi: 10.1016/0009-2797(76)90113-7. [DOI] [PubMed] [Google Scholar]
- 72.Cocke DL, Chuah GK, Kruse N, Block JH. Copper oxidation and urface copper oxide stability investigated by pulsed field desorption mass spectrometry. Appl. Surf. Sci. 1995;84:153–61. [Google Scholar]
- 73.Hans M, Erbe A, Mathews S, et al. Role of copper oxides in contact killing of bacteria. . Langmuir. 2013;29:16160–6. doi: 10.1021/la404091z. [DOI] [PubMed] [Google Scholar]
- 74.Ferracane JL. Lippincott Williams & Wilkins; 2001. Materials in Dentistry principles and Applications. [Google Scholar]
- 75.Bilian X. Intrauterine devices. Best. pract. Res. Clin. Obstet. Gynaecol. 2002;16:155–68. doi: 10.1053/beog.2002.0267. [DOI] [PubMed] [Google Scholar]
- 76.O'Brien pA, Kulier R, Helmerhorst FM, Usher-patel M, d'Arcangues C. Copper-containing, framed intrau-terine devices for contraception a systematic review of randomized controlled trials. Contraception. 2008;77:318–27. doi: 10.1016/j.contraception.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Gorter RW, Butorac M, Cobian Ep. Examination of the cutaneous absorption of copper after the use of copper-containing ointments. Am J Ther. 2004;11:453–8. doi: 10.1097/01.mjt.0000127148.83065.e5. [DOI] [PubMed] [Google Scholar]
- 78.Hostynek JJ, Dreher F, Maibach HI. Human stratum corneum penetration by copper in vivo study after occlusive and semi-occlusive application of the metal as powder. Food Chem Toxicol. 2006;44:1539–43. doi: 10.1016/j.fct.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Heilmittel W. Wala Therapeutic preparations (Hand-book). Weleda pharmacy Medi-cines Handbook for physicians. In: Heilmittel W, editor; pp. New York:: Weleda, Congers; 1994. p. 80. [Google Scholar]
- 80.Bastianelli C, Farris M, Benagiano G. Emergency contraception a review. Eur. J Contracept. Reprod. Health Care. 2008;13:9–16. doi: 10.1080/13625180701781096. [DOI] [PubMed] [Google Scholar]
- 81.Hostynek JJ, Maibach HI. Copper hypersensitivity dermatologic aspects--an overview. Rev. Environ. Health. 2003;18:153–83. doi: 10.1515/reveh.2003.18.3.153. [DOI] [PubMed] [Google Scholar]
- 82.Fage SW, Faurschou A, Thyssen Jp. Copper hyper-sensitivity. Contact Dermatitis. 2014;71:191–201. doi: 10.1111/cod.12273. [DOI] [PubMed] [Google Scholar]
- 83.Gabbay J, Mishal J, Magen E, et al. Copper oxide impregnated textiles with potent biocidal activities. . Journal of Industrial Textiles. 2006;35:323–35. [Google Scholar]
- 84.Borkow G, Lara HH, Covington CY, Nyamathi A, Gabbay J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Chemother. 2008;52:518–25. doi: 10.1128/AAC.00899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borkow G, Gabbay J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses. 2008;70:990–4. doi: 10.1016/j.mehy.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 86.Zatcoff RC, Smith MS, Borkow G. Treatment of tinea pedis with socks containing copper-oxide impregnated fibers. Foot (Edinb.). 2008;18:136–41. doi: 10.1016/j.foot.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Baek JH, Yoo MA, Koh JS, Borkow G. Reduction of facial wrinkles depth by sleeping on copper oxide-containing pillowcases a double blind, placebo con-trolled, parallel, randomized clinical study. J Cosmet. Dermatol. 2012;11:193–200. doi: 10.1111/j.1473-2165.2012.00624.x. [DOI] [PubMed] [Google Scholar]
- 88.Borkow G, Gabbay J, Lyakhovitsky A, Huszar M. Improvement of facial skin characteristics using cop-per oxide containing pillowcases a double-blind, pla-cebo-controlled, parallel, randomized study. Int J Cosmet. Sci. 2009;31:437–43. doi: 10.1111/j.1468-2494.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- 89.Borkow G, Okon-Levy N, Gabbay J. Copper oxide impregnated wound dressings biocidal and safety studies. Wounds. 2010;22:310–6. [PubMed] [Google Scholar]
- 90.Borkow G. Safety of using copper oxide in medical devices and consumer products. Curr Chem Biol. 2012;6:86–92. [Google Scholar]
- 91.Weinberg I, Lazary A, Jefidoff A, et al. Safety of using diapers containing copper oxide in chronic care elderly patients. . Open Biol J. 2013;6:54–9. [Google Scholar]
- 92.Gargiulo ME, Del Carmen-Elias A, Borkow G. Anal-ysis of the effect of wearing copper oxide impregnat-ed socks on tinea pedis based on "before and after" pictures - a statistical follow-up tool. Open Biol J. 2012;5:17–22. [Google Scholar]
- 93.Borkow G. protection of Soldiers' feet by copper ox-ide impregnated socks. Advances in Military Tech-nology. 2013;8:101–8. [Google Scholar]
- 94.Dykes p. Increase in skin surface elasticity in normal volunteer subjects following the use of copper oxide impregnated socks. Skin Res. Technol. 2014 doi: 10.1111/srt.12187. [DOI] [PubMed] [Google Scholar]
- 95.Agren MS, Werthen M. The extracellular matrix in wound healing a closer look at therapeutics for chronic wounds. Int. J Low Extrem. Wounds. 2007;6:82–97. doi: 10.1177/1534734607301394. [DOI] [PubMed] [Google Scholar]
- 96.McLaughlin K. HIF1{alpha} in hypoxia. Thorax. 2008;63:311. [Google Scholar]
- 97.Mace KA, Yu DH, paydar KZ, Boudreau N, Young DM. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound. Repair Regen. 2007;15:636–45. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]