Abstract
Several influences modulate biochemical responses to a weight-adjusted levothyroxine (l-T4) replacement dose. We conducted a secondary analysis of the relationship of l-T4 dose to TSH and free T3 (FT3), using a prospective observational study examining the interacting equilibria between thyroid parameters. We studied 353 patients on steady-state l-T4 replacement for autoimmune thyroiditis or after surgery for malignant or benign thyroid disease. Peripheral deiodinase activity was calculated as a measure of T4–T3 conversion efficiency. In euthyroid subjects, the median l-T4 dose was 1.3 μg/kg per day (interquartile range (IQR) 0.94,1.60). The dose was independently associated with gender, age, aetiology and deiodinase activity (all P<0.001). Comparable FT3 levels required higher l-T4 doses in the carcinoma group (n=143), even after adjusting for different TSH levels. Euthyroid athyreotic thyroid carcinoma patients (n=50) received 1.57 μg/kg per day l-T4 (IQR 1.40, 1.69), compared to 1.19 μg/kg per day (0.85,1.47) in autoimmune thyroiditis (P<0.01, n=76) and 1.08 μg/kg per day (0.82, 1.44) in patients operated on for benign disease (P< 0.01, n=80). Stratifying patients by deiodinase activity categories of <23, 23–29 and >29 nmol/s revealed an increasing FT3–FT4 dissociation; the poorest converters showed the lowest FT3 levels in spite of the highest dose and circulating FT4 (P<0.001). An l-T4-related FT3–TSH disjoint was also apparent; some patients with fully suppressed TSH failed to raise FT3 above the median level. These findings imply that thyroid hormone conversion efficiency is an important modulator of the biochemical response to l-T4; FT3 measurement may be an additional treatment target; and l-T4 dose escalation may have limited success to raise FT3 appropriately in some cases.
Keywords: thyroid hormone replacement, l-T4 therapy, levothyroxine, TSH, triiodothyronine, deiodinase, conversion
Introduction
Thyroid disorders are among the most prevalent diseases in the western world, affecting as many as one out of seven adults (1). They are frequently associated with overt thyroid dysfunction, particularly various degrees of hypothyroidism that require thyroid hormone replacement (2, 3). This is mainly done by administration of synthetic levothyroxine (l-T4), which is a well-established, convenient, safe and inexpensive treatment modality (4, 5). However, this does not accurately reflect the natural direct secretion pattern of both thyroid hormones triiodothyronine (T3) and thyroxine (T4) by the thyroid gland (6, 7). Unlike other drugs, dosing of l-T4 is not fixed but has to be titrated according to individual needs. Dose adequacy is mainly defined by reference to suitable biochemical standards, particularly thyrotropin (TSH) (8). This parameter has evolved into the main treatment target to be monitored and kept within an assumed euthyroid range (9). A number of studies have attempted to predict T4 requirement, and various regimes for a starting dose have been proposed based on an average of 1.6 μg/kg body weight (BW) or by more refined weight- or BMI-related algorithms (10, 11, 12, 13, 14, 15, 16).
Although TSH measurement has dominated procedural management of thyroid replacement by its apparent ease and good standardisation, a disturbingly high proportion of patients remains unsatisfied with the treatment they receive (17, 18). This has prompted some authors including our group to question the validity of relying on the TSH level as the sole measure of dose adequacy in l-T4-treated patients (19, 20, 21). We have shown that the homeostatic equilibria between TSH and peripheral thyroid hormones are modulated by various influences such as age, body mass and the treatment modality itself (22). As a controlling element, the effective TSH level derived in a healthy normal population cannot necessarily be inferred to be equally optimal for a given patient on l-T4 medication, because the constitutive equilibria between TSH and thyroid hormones, especially FT3, differ in health and disease (22).
In the present analysis, we examined the relationship of the l-T4 dose with clinical categories and biochemical outcomes such as TSH, FT4 and FT3 levels. We sought to define the interaction between TSH and the FT3 target and also to analyse the influences of modulators such as gender, age, disease category or the efficiency of T3 conversion from T4.
Subjects and methods
Study design and objective
An open prospective observational study (ClinicalTrials.gov NCT01969552) was conducted at the Department of Nuclear Medicine at Klinikum Luedenscheid, Germany, between July 2013 and February 2014 and approved by the Ethics Committee of the University of Muenster, Germany. Participants gave written informed consent.
The present secondary analysis is restricted to the subgroup of patients on steady l-T4 treatment, examining dose requirements of l-T4 including conditioning modulators, thyroid hormone conversion efficiency and relationships with biochemical outcomes such as TSH, FT4 and FT3 levels. The primary study outcome, namely the analysis of the interacting equilibria and interrelations between thyroid parameters under various conditioning influences such as gender, age and body mass and l-T4 treatment has already been reported (22).
Patients
The original study involved 1912 adult patients who were consecutively seen, were free of severe comorbidity and provided written informed consent. For this subgroup analysis, 353 patients on thyroid hormone replacement meeting the following criteria were included: being seen as outpatients, presenting in a controlled functional state (FT4 ≥10 pmol/l and TSH ≤4 mU/l) and having reached a steady state on a constant l-T4 medication. Although infrequently seen in an ambulatory setting, patients with severe non-thyroidal illness or potentially interfering comorbidities were ineligible to participate in the study. This exclusion extended to other conditions and the use of comedications that may interfere with the resorption or measurement of thyroid hormones or with pituitary TSH. Patients with T3/T4 combination therapy (n=9), anti-thyroid drug use (n=99), hypothalamic/pituitary diseases (n=5) or pregnancy (n=3) were excluded before analysis.
Diagnostic procedures included a detailed history, physical examination, standardised questionnaire documenting gender, age, height, weight, smoking habits (75% answered), prior surgery or radioiodine treatment, thyroid medication (brand, dosage, duration, time of intake), other drugs, laboratory tests (FT3, FT4, TSH and, if autoimmune thyroiditis was suspected or to be excluded, thyroid peroxidase antibodies (TPO-Ab) or TSH-receptor antibodies (TSH-R Ab)) and thyroid imaging.
Laboratory methods
TSH, FT3 and FT4 were measured with an automated direct chemoluminescence method (Advia Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany). TSH is traceable to the 3rd International Standard for TSH (WHO, IRP 81/565). A TSH range from 0.006 to 160 mU/l was linear, and coefficient of variations of inter-assay imprecision ranged from 0.9% to 2.4%. Reference intervals were laboratory established and pre-evaluated for the local population, using 10–23 pmol/l for FT4, 3.1–6.8 pmol/l for FT3 and 0.4–4.0 mU/l for TSH (23).
TPO-Abs were determined by a competitive chemoluminescence method (ADVIA Centaur XP, Siemens Healthcare Diagnostics, reference range <60 U/ml) and TSH-R Abs by competitive ELISA (Euroimmun AG, Lübeck, Germany, reference range <2 U/l).
FT3–FT4 ratio and calculated deiodinase activity
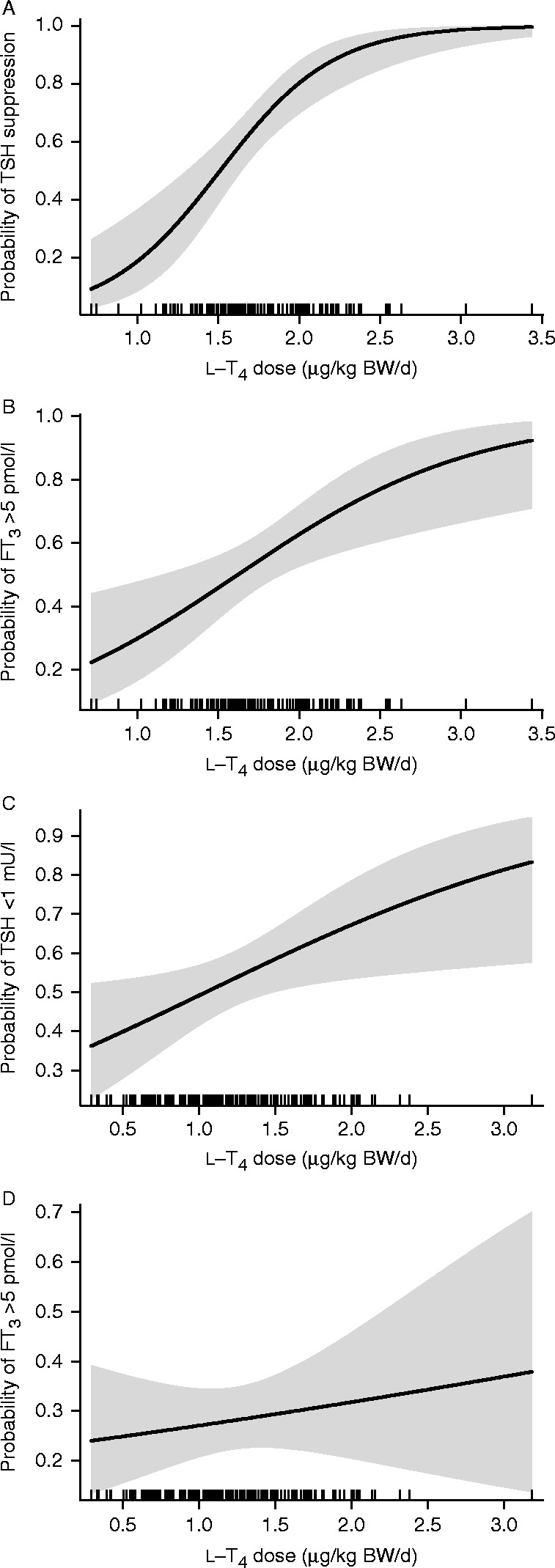
As measures of conversion efficiency, we calculated the FT3–FT4 ratio by simple division of both parameters in pmol/l and the sum activity of peripheral deiodinases (SPINA-GD, termed ‘deiodinase activity,’ thereafter, nmol/s) from equilibrium levels of FT4, FT3 and estimated constant parameters for plasma protein binding, distribution and elimination with
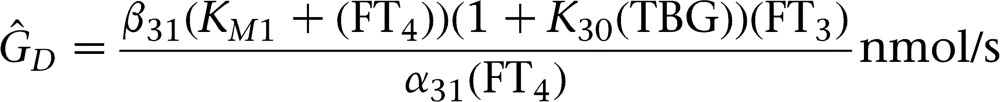 |
as previously described (20, 21, 24).
Although the two measures are closely related in the linear part of the substrate relationship defined by Michaelis-Menten kinetics, only the more complex formula (GD) accounts for the saturation kinetics of the enzyme.
In addition to using estimated deiodinase activity as a continuous variable, we divided deiodinase activity in three distinct categories defining poor (<23 nmol/s), intermediate (23–29 nmol/s) or good (>29 nmol/s) converters. The cut-offs were pre-specified based on observations in l-T4-treated patients vs healthy untreated subjects and in low (<5 ml) vs higher thyroid volumes (22). They approximate turning points in the relationship between deiodinase activity and FT3, defining a central region with a derivative of about 0 and low or high regions with steeper slopes.
Thyroid ultrasound and scintigraphy
Thyroid volume was sonographically (10 MHz transducer) determined according to the ellipsoid formula. Reference values were <18 ml for females and <25 ml for males. A volume <1 ml was considered athyreotic. Larger nodules were further examined by scintigraphy.
Statistical methods
Descriptive data are reported as median plus interquartile range (IQR). We used Wilcoxon's rank sum or a χ2 test in case of categorical variables for comparison of baseline characteristics. Correlations are based on Pearson's product-moment when suitable or Kendall's τ. Multiple variables and conditional influences were analysed by a generalised linear model (GLM) and approximated by a linear regression function over restricted intervals. β coefficients were derived from a linear model. TSH was used after logarithmic transformation. We tested for collinearity in the models using the variance inflation factor. A GLM with a binomial function (logistic regression) was used to assess success rates of the l-T4 dose for reaching a TSH or FT3 target and to create dose-related probability plots. Relative proportions were statistically compared by receiver operating characteristic curves and Delong's test. P values <0.05 were considered significant for all tests. Statistical analyses were performed using Deducer (version 0.7-7) and the R statistical package (Mac version 3.1.2) (25, 26).
Results
The present analysis comprises 353 patients in a stable controlled non-hypothyroid state on thyroid hormone replacement with l-T4. Patient characteristics are shown in Table 1. Of the total study group, 304 patients were euthyroid according to FT4, 342 according to FT3 and 216 according to TSH, based on their respective reference intervals with all displaying clinically satisfactory levels of medication.
Table 1.
Characteristics of study group (n=353). For referencing purpose, parameters in 146 disease-free individuals from the same study were as follows: median age 38 (26, 49) years, TSH 1.62 (1.12, 2.25) mU/l, FT3 5.0 (4.8, 5.2) pmol/l, FT4 14.0 (13.0,15.1) pmol/l, calculated deiodinase activity 32.8 (30.0, 36.2) nmol/s, thyroid volume 10 (8,13) ml (22).
| Parameter | Median (IQR) or percentage |
|---|---|
| Gender (female, male) | 280 (79%), 73 (21%) |
| Age (years) | 56 (46, 66) |
| In women vs men | 53 (45, 66) vs 59 (53, 64), P=0.03 |
| Disease aetiology (%) | Autoimmune thyroiditis 27% |
| Benign thyroid disease after surgery 32% | |
| Thyroid carcinomaa 41% | |
| Surgery, radioiodine treatment (%) | 73%, 42% |
| BMI (kg/m2) | 27.5 (24.1, 30.8) |
| Dose (μg/day) | 100 (75, 150) |
| Weight-adjusted daily dose (μg/kg per day) | 1.47 (1.09, 1.72) |
| TSH (mU/l) | 0.64 (0.12, 1.47) |
| FT3 (pmol/l) | 4.80 (4.40, 5.30) |
| FT4 (pmol/l) | 18.6 (16.2, 21.1) |
| TPO-Ab (U/l) | 450 (48, 1300), positive 65%, n=97 |
| FT3–FT4 ratio | 0.26 (0.24, 0.29) |
| Deiodinase activity (nmol/s) | 24.3 (21.8, 27.1) |
| Thyroid volume (ml) – total group | 2 (0, 7) |
| Autoimmune thyroiditis | 7 (4,11) |
| Benign thyroid disease post surgery | 6 (2,10) |
| Thyroid carcinomab | 0 (0, 0) |
82% of the thyroid carcinoma patients had a higher TNM stage than 1.
96% had no detectable residual thyroid volume by ultrasound after total thyroidectomy and radioiodine treatment.
Dose requirements associated with biochemical euthyroidism (n=208) defined by the reference ranges of all three parameters varied widely from 25 to 275 μg/day l-T4 (mean 98, median 100 (IQR 75, 125)) or 0.3 to 2.2 μg/kg BW per day (mean 1.2, median 1.3 (IQR 0.94, 1.60)). In univariate linear models, the l-T4 dose in the treated euthyroid panel was significantly associated with gender, age, body mass index, aetiology of disease, T3–T4 ratio and calculated deiodinase activity (all P<0.001) but not with TSH (P=0.94). The influences remained independently predictive in a multivariable model (Table 2).
Table 2.
β coefficients in a linear model of covariates predicting dose of l-T4 in the euthyroid panel. The multivariable model was simultaneously fitted with the parameters listed, all of which were significant predictors of the l-T4 dose in univariate models. All variance inflations factors were <1.2.
| Variable | β coefficient (95% CI) |
|---|---|
| Gender male vs female | 0.22 (0.11, 0.33), P<0.001 |
| Disease aetiology | |
| Autoimmune vs malignant disease | −0.33 (−0.47, −0.19), P<0.001 |
| Benign goitre vs malignant disease | −0.34 (−0.48, −0.20), P<0.001 |
| Age | −0.26 (−0.37, −0.15), P<0.001 |
| BMI | 0.33 (0.22, 0.44), P<0.001 |
| Deiodinase activity | −0.27 (−0.39, −0.15), P<0.001 |
TSH levels in the euthyroid range were unrelated to any of the above influences except disease category (P=0.003), as might be expected considering the lower TSH target in malignant disease. Deiodinase activity was positively associated with thyroid volume (τ=0.23, P<0.001, n=208) but inversely correlated with weight-adjusted l-T4 dose (r=−0.37, P<0.001, n=208).
In a biochemically defined euthyroid state excluding subclinically hyperthyroid subjects, athyreotic thyroid carcinoma patients received significantly higher doses of l-T4 (1.57 μg/kg BW per day (IQR 1.40, 1.69), n=50)) than patients with autoimmune thyroiditis (1.19 μg/kg BW per day (IQR 0.85, 1.47), n=76, P<0.001)) or benign thyroid disease post surgery (1.08 μg/kg BW per day (IQR 0.82, 1.44), n=80, P<0.001)). Furthermore, after adjusting for differing levels of TSH suppression in a linear model the weight-adjusted l-T4 dose was higher in athyreotic carcinoma patients compared to autoimmune thyroiditis or benign disease (P<0.001, Fig. 1A). Similarly, the dose required to achieve the same FT3 concentration was higher in the carcinoma group (P<0.001, Fig. 1B).
Figure 1.
TSH (A) or FT3 (B) vs weight-adjusted l-T4 dose in three groups of patients on thyroid hormone replacement, with autoimmune thyroiditis (n=96), after surgery for benign goitre (n=111) or thyroid carcinoma (n=143). Between group differences in both panels were significant (P<0.01) and remained so after adjusting for volume (not shown, P<0.01), as evidenced by linear models with the diagnostic group as a covariate. See text for further details. AIT, autoimmune thyroiditis; Goitre, goitre post surgery for benign nodular thyroid disease.
Median thyroid volume was 0 ml (IQR 0, 0 ml) in carcinomas, 7 ml (IQR 4, 11 ml) in autoimmune thyroiditis and 6 ml (IQR 3, 8 ml) in benign goitre post surgery. The weight-adjusted l-T4 dose was inversely correlated with the thyroid volume in the three diagnostic groups (r=−0.22, P=0.002, n=208).
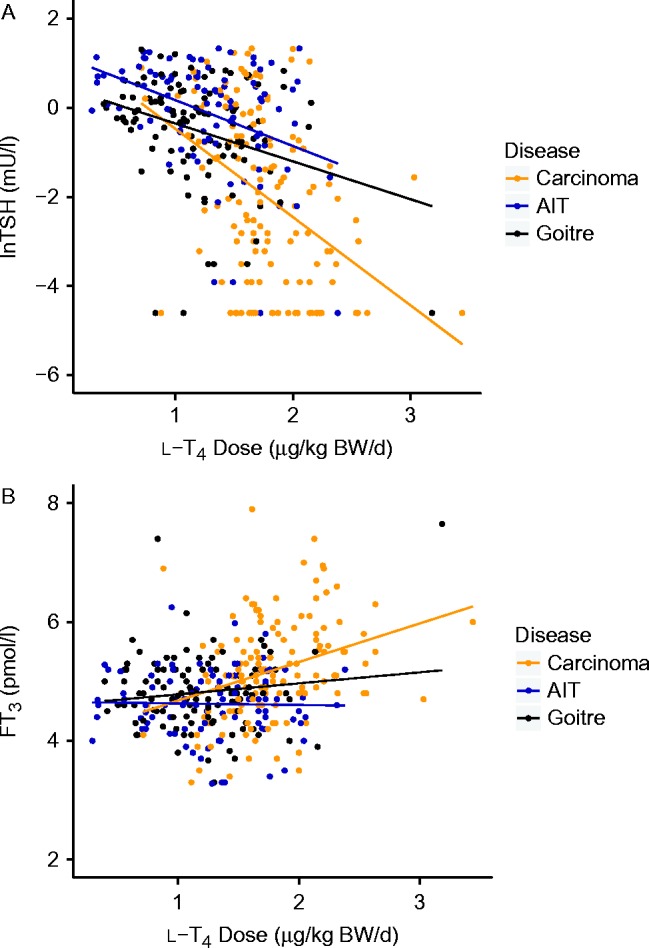
Three distinct categories of conversion efficiency were defined (see Subjects and methods) as follows: poor converters <23 nmol/s, intermediate converters 23–29 nmol/s and good converters >29 nmol/s deiodinase activity. The poor converters reached significantly (P<0.001) higher FT4 concentrations in the circulation than intermediate or good converters but, at the same time, showed significantly (P<0.001) lower absolute FT3 levels compared to the other two groups (Fig. 2). While the FT3–FT4 dissociation was apparent in all three disease entities, it was most pronounced in the carcinoma group (n=143, Fig. 2). The latter group showed the highest proportion of poor converters (Fig. 2). The converter groups were similar (P>0.1) in their age, BMI, weight-adjusted l-T4 dose and TSH levels except for men being overrepresented in the good converter group (P<0.01). Converter categories of the carcinoma group were comparable (P=0.42) in their thyroid residual volumes, which were below 1 ml in 96% of all cases. In contrast, in the combined group of benign diseases the converter status was significantly associated with thyroid volume (4 (2, 8) vs 7 (4, 11) vs 8 (5, 12) ml, P=0.009). Thyroid volumes differed between the carcinoma group and the benign diseases (P<0.001) but not between autoimmune thyroiditis and goitre post surgery (P=0.25, Table 1).
Figure 2.
FT3 (A), FT4 (B) and TSH (C) levels in l-T4-treated patients stratified by disease and conversion efficiency. The disease entities were closely associated with categories of the thyroid volume (see Table 1 and text). The red box refers to poor converters (calculated deiodinase activity <23 nmol/s), green to intermediate converters (deiodinase activity 23–29 nmol/s) and blue to good converters (deiodinase activity >29 nmol/s). Remarkably, absolute FT3 concentrations were lowest in the poor converter group in all disease categories, while FT4 levels were highest in the poor converters. Wilcoxon test, revealed significant differences compared to each first group; *P<0.05, **P<0.001. AIT, autoimmune thyroiditis; goitre, goitre post surgery for benign nodular thyroid disease.
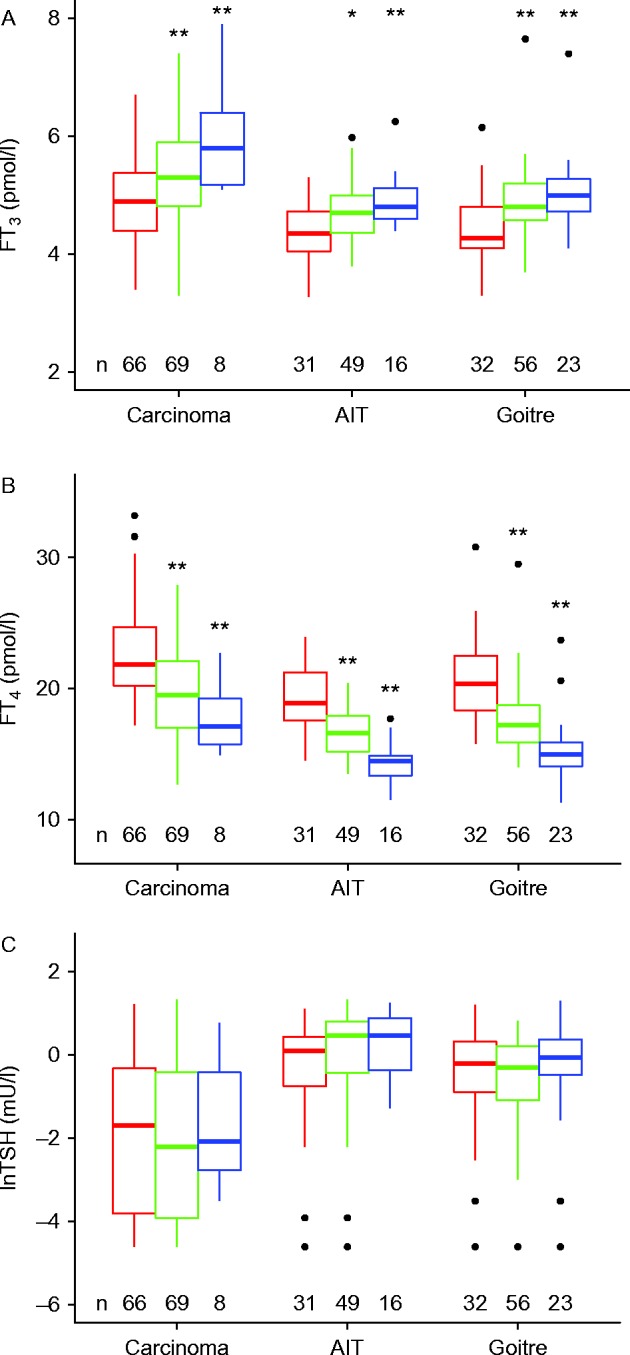
A given weight-adjusted dose suppressed the TSH below the lower reference limit (<0.4 mU/l) in a higher proportion of carcinoma patients, than it raised their FT3 level above the median level typical of the euthyroid controls (>5 pmol/l) (Fig. 3A and B). Conversely, much lower doses reached a target of a fully suppressed TSH compared to the FT3 median (Fig. 3A and B). The same tendency is true for a more modest target below 1 mU/l for TSH in autoimmune thyroiditis or benign disease post surgery, although variation was higher in this panel (Fig. 3C and D). Overall, a significantly higher proportion of patients achieved TSH suppression compared to FT3 above median by Delong's test employing receiver operating characteristic curves (P<0.001).
Figure 3.
Probability plot of weight adjusted l-T4 dose to (A) suppress TSH below its lower reference limit (0.4 mU/l) or (B) raise FT3 above the median of euthyroid controls (>5 pmol/l) in the carcinoma patients (n=143), and (C) suppress TSH <1 mU/l or (D) elevate FT3 above 5 pmol/l in benign disease (patients with autoimmune thyroiditis, n=75 and nodular thyroid disease post surgery, n=111). Probability plots were created by logistic regression. The shaded areas indicate the confidence interval surrounding the fitted curve. The TSH targets were more frequently reached at a lower dose than the FT3 target (see Results).
Discussion
In this cohort, dose requirements for l-T4-treated patients varied in a large euthyroid panel and were associated with many influences including gender, age, disease category and thyroid hormone conversion efficiency. However, not all of the treatment conditions necessarily aim at a biochemically euthyroid thyroid state as a comprehensive therapeutical goal, as defined by maintaining the respective reference ranges of all three parameters: TSH, FT4 and FT3. Particularly, in the treatment of thyroid carcinomas, for many patients in our sample the target was a lower or suppressed TSH below the reference range, which, as a consequence, raised FT4 levels above the upper reference range in a proportion of these patients. At both comparable levels of TSH suppression or similar FT3 concentrations, athyreotic thyroid carcinoma patients were taking a higher weight-adjusted dose of l-T4. Three remarkable and linked observations from this study were a dissociation between FT3 and FT4, an apparent disjoint between TSH and FT3 and an inverse association between l-T4 dose and conversion efficiency.
The present study was a cross-sectional secondary analysis not involving a randomised design. As previously reported in a separate communication (22), the primary aim of this prospective observational study was to analyse further the interacting equilibria. While introducing some uncontrolled variations, this allowed for the study of a broader natural spectrum of responses, as observed in consecutive patients. FT3 or FT4 measurements were not compromised in any way by problematic conditions such as the non-thyroid illness syndrome, as the study was conducted in a cohort of otherwise ‘healthy’ out-patients without relevant comorbidity. There was no evidence for a potential bias stemming from a variable time interval between l-T4 intake and blood sampling, which might result in an expected slight temporary elevation of circulating FT4 concentrations, as previously discussed (22). There were neither linear (P=0.27) nor non-linear (P=0.28) relationships with deiodinase activity.
In l-T4 treatment, equilibria typical of the healthy state were found not to be invariant, but profoundly altered (22). Here we disclose further consequences that are associated with alterations in the regulatory patterns in patients under l-T4 therapy. In particular, one aspect relates to the l-T4 dose and conversion efficiency. We estimated T4–T3 conversion by calculating the sum activity of peripheral deiodinases (see Subjects and methods). The measure is similar to the FT3–FT4 ratio, albeit more precise wherein it accounts for non-linear enzyme saturation kinetics. However, it does not further differentiate global activity by type of deiodinase. Thus, the source of T3 or contribution of various tissues to the T3 plasma pool cannot be discerned. We found that a poor converter status was associated with a higher l-T4 dose and higher serum FT4 levels but still lower absolute FT3 concentrations, compared to the more efficient converters. This paradoxically relates the higher T4 supply to a worsened rather than improved absolute FT3 level. This is not to say that an increasing dose will not raise on average the FT3 but that the dose response varies widely among individuals, and conversion inefficiency in some patients may outweigh the dose effect in terms of achievable absolute FT3 concentrations. How can this be explained? A high l-T4 dose may not invariably remedy T3 deficiency owing to T4-induced conversion inefficiency but could actually hinder its attainment through the inhibitory actions of the substrate itself and/or reverse T3 (rT3) on deiodinase type 2 activity (27). A study by Cettour-Rose et al. (28) confirmed that rT3, when infused into rats, inhibited deiodinase type 2 activity in the pituitary, cerebral cortex and brown adipose tissue, but interestingly, this did not have much impact on circulating T4, T3 and TSH concentrations in the animals. However, in this model the rT3 effect was studied under rather artificial conditions in the absence of an abundant T4 supply with elevated FT4 levels that characterizes the treatment situation. In contrast, another recent experimental study has shown that escalating only the l-T4 dose fails to normalize serum T3 in the rat, and as a result, irrespective of local variations by type of deiodinase, all organs examined such as the brain, liver and skeletal muscle were hypothyroid at the tissue level in the presence of a normal serum TSH (29). This study suggests ubiquitination may be the limiting factor for T4 alone to restore true tissue euthyroidism in the rodent (29). The lack of TSH stimulation and absence or functional deficiency of the thyroid gland may also impair T4–T3 conversion (30). Another important consideration is that, just as FT4 and FT3 dissociate under l-T4 therapy, so do TSH and FT3. While a high proportion of patients was able to achieve a target of a suppressed TSH below the lower reference limits or a TSH value <1 mU/l in autoimmune thyroiditis, their FT3 levels at the same time frequently remained below the median FT3 level found in normal subjects. The situation differs from conditions in which l-T4 absorption may be impaired and, as a consequence, elevated TSH levels persist (31, 32, 33). Thus, not even an l-T4 dose in which TSH is fully suppressed and FT4 by far exceeds its upper reference limit can guarantee above average FT3 levels in these patients, indicating an FT3–TSH disjoint. As a consequence, although dose escalation may help some patients who maintained a sufficiently efficient thyroid hormone conversion to raise their FT3 for euthyroidism and well-being, the strategy may not be invariably successful in all patients. In two studies, ∼15% of athyreotic patients could not even raise their FT3 above the lower reference limit on l-T4 (19, 20). Another controlled follow-up study after hemithyroidectomy for benign euthyroid goitre suggests that this deficiency may have unwanted clinical consequences. In this study, weight gain after 2 years in association with a lowered thyroid function within the laboratory reference range was interpreted as a clinical manifestation of a permanently decreased metabolic rate (34).
l-T4 dose requirements have been well studied and various regimes based on weight, BMI or more refined algorithms have been proposed to put patients on a presumed adequate dose from the very beginning (10, 11, 12, 13, 14, 15, 16, 35, 36, 37, 38, 39). As useful as these algorithms may be for average predictions and initial guidance in the general population, they do not take into account individual variations in the response to l-T4, such as conversion efficiency. Dosing strategies solely based on a TSH definition of euthyroidism neglect the important role of FT3, which has recently emerged as an equally significant parameter in defining thyroid physiology (20, 22, 29, 30, 40, 41). Central and peripheral regulatory mechanisms do not constitute divided levels of control, as has previously been assumed. Rather they are integrated via feed-forward control of deiodinase activity by TSH and operate jointly to maintain T3 homeostasis as an overarching goal (30).
While acknowledging the role of genetically determined differences in deiodinase activity affecting conversion rates, the poor converter status described here appears to emerge mainly as a consequence of the T4 monotherapy itself, induced by the mechanisms discussed above (42, 43, 44, 45). Compared to untreated subjects, deiodinase activity and conversion efficiency tend to be diminished in l-T4 treatment (20, 22). However, individual pre-treatment measurements were not available for comparison. We found conversion inefficiency to be significantly correlated with low residual thyroid volume and most prevalent in athyreotic patients. However, differences in deiodinase activities were also apparent in the absence of a functioning thyroid gland within the group of thyroidectomised carcinoma patients. Overall, patients differ widely in the degree of the conversion impairment they suffer. This, in turn, may influence their dose requirements of l-T4 and, at a comparable weight-adjusted l-T4 dose, their levels of TSH suppression and circulating FT3 concentrations.
We speculate that l-T4-induced conversion inefficiency could prevent some vulnerable subjects from reaching true tissue normality on T4 monotherapy alone. Those were not analysed separately in the numerous earlier T3/T4 trials and could be possible candidates for a combined T3/T4 treatment option, as recognized by some authors and the guidelines of the European Thyroid Association (46, 47). As a limitation, this study addresses biochemical treatment responses but did not evaluate patient-reported outcomes or biomarkers of thyroid hormone action.
Whether conversion efficiency and the resulting differences in relationships between TSH, FT4 and FT3 are clinically useful markers of dosing inadequacy requires further well-designed prospective studies. Patient satisfaction, complaints and symptoms play an essential part in the clinical assessment. However, owing to considerable inter-individual variation, these measures apparently lack statistical power in a trial setting and have not been clearly linked to prognosis. For example, even a change in thyroid function as profound as the transition from the hypothyroid to the euthyroid state may be associated with only modest improvements in thyroid-related quality-of-life measures in patients with autoimmune thyroiditis (48). As a result, a trial size of several thousand subjects may be required to produce a credible result with adequate discriminatory power. Additionally, the exact outcome would depend on the overall makeup of the panel as regards the mixture of T4–T3 conversion capabilities. Possible long-term consequences of the observed biochemical alterations such as the altered FT3–FT4 ratio are also presently unknown.
The findings of the present study have several clinical implications. First, they recognize thyroid hormone conversion efficiency, as defined by the calculated global deiodinase activity or more simply the T3–T4 ratio, is an important determinant of l-T4 dose requirements and the biochemical response to treatment. Second, in view of a T4-related FT3–TSH disjoint, FT3 measurement should be adopted as an additional treatment target. Third, in cases where an FT3–FT4 dissociation becomes increasingly apparent following dose escalation of l-T4, an alternate treatment modality, possibly T3/T4 combination therapy, should be considered, but further randomized controlled trials are required to assess the benefit versus risk in this particular group.
Acknowledgements
The authors are grateful to Hans-Günther Wahl, Institute of Laboratory Medicine, Klinikum Lüdenscheid, for measuring thyroid hormones.
Declaration of interest
J W Dietrich is co-owner of the intellectual property rights for the patent ‘System and Method for Deriving Parameters for Homeostatic Feedback Control of an Individual’ (Singapore Institute for Clinical Sciences, Biomedical Sciences Institutes, Application Number 201208940e20120895). All other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Bjoro T, Holmen J, Kruger O, Midthjell K, Hunstad K, Schreiner T, Sandnes L, Brochmann H. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT) European Journal of Endocrinology. 2000;143:639–647. doi: 10.1530/eje.0.1430639. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clinical Endocrinology. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 3.Roberts CGP, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]
- 4.Mandel SJ, Brent GA, Larsen PR. Levothyroxine therapy in patients with thyroid disease. Annals of Internal Medicine. 1993;119:492–502. doi: 10.7326/0003-4819-119-6-199309150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocrine Reviews. 2014;35:433–512. doi: 10.1210/er.2013-1083. [DOI] [PubMed] [Google Scholar]
- 6.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine Reviews. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 7.Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. American Journal of Physiology. Endocrinology and Metabolism. 1990;258:E715–E726. doi: 10.1152/ajpendo.1990.258.4.E715. [DOI] [PubMed] [Google Scholar]
- 8.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baloch ZW, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry J-F, LiVosli VA, Niccoli-Sire P, John R, uf J, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 10.Roos A, Linn-Rasker SP, van Domburg RT, Tijssen JP, Berghout A. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Archives of Internal Medicine. 2005;165:1714–1720. doi: 10.1001/archinte.165.15.1714. [DOI] [PubMed] [Google Scholar]
- 11.Santini F, Pinchera A, Marsili A, Ceccarini G, Castagna MG, Valeriano R, Giannetti M, Taddei D, Centoni R, Scartabelli G, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. Journal of Clinical Endocrinology and Metabolism. 2005;90:124–127. doi: 10.1210/jc.2004-1306. [DOI] [PubMed] [Google Scholar]
- 12.Sukumar R, Agarwal A, Gupta S, Mishra A, Agarwal G, Verma AK, Mishra SK. Prediction of lT4 replacement dose to achieve euthyroidism in subjects undergoing total thyroidectomy for benign thyroid disorders. World Journal of Surgery. 2010;34:527–531. doi: 10.1007/s00268-009-0345-3. [DOI] [PubMed] [Google Scholar]
- 13.Mistry D, Atkin S, Atkinson H, Gunasekaran S, Sylvester D, Rigby AS, England RJ. Predicting thyroxine requirements following total thyroidectomy. Clinical Endocrinology. 2011;74:384–387. doi: 10.1111/j.1365-2265.2010.03940.x. [DOI] [PubMed] [Google Scholar]
- 14.Jin J, Allemang MT, McHenry CR. Levothyroxine replacement dosage determination after thyroidectomy. American Journal of Surgery. 2013;205:360–363. doi: 10.1016/j.amjsurg.2012.10.015. discussion 363–364. [DOI] [PubMed] [Google Scholar]
- 15.Ojomo KA, Schneider DF, Reiher AE, Lai N, Schaefer S, Chen H, Sippel RS. Using body mass index to predict optimal thyroid dosing after thyroidectomy. Journal of the American College of Surgeons. 2013;216:454–460. doi: 10.1016/j.jamcollsurg.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Donna V, Santoro MG, de Waure C, Ricciato MP, Paragliola RM, Pontecorvi A, Corsello SM. A new strategy to estimate levothyroxine requirement after total thyroidectomy for benign thyroid disease. Thyroid. 2014;24:1759–1764. doi: 10.1089/thy.2014.0111. [DOI] [PubMed] [Google Scholar]
- 17.Wiersinga WM. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nature Reviews. Endocrinology. 2014;10:164–174. doi: 10.1038/nrendo.2013.258. [DOI] [PubMed] [Google Scholar]
- 18.Pepper GM, Casanova-Romero PY. Conversion to Armour thyroid from levothyroxine improved patient satisfaction in the treatment of hypothyroidism. Journal of Endocrinology, Diabetes & Obesity. 2014;2:1055–1060. [Google Scholar]
- 19.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS ONE. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment? European Journal of Endocrinology. 2013;168:271–280. doi: 10.1530/EJE-12-0819. [DOI] [PubMed] [Google Scholar]
- 21.Midgley JEM, Hoermann R, Larisch R, Dietrich JW. Physiological states and functional relation between thyrotropin and free thyroxine in thyroid health and disease: in vivo and in silico data suggest a hierarchical model. Journal of Clinical Pathology. 2013;66:335–342. doi: 10.1136/jclinpath-2012-201213. [DOI] [PubMed] [Google Scholar]
- 22.Hoermann R, Midgley JEM, Giacobino A, Eckl WA, Wahl HG, Dietrich JW, Larisch R. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clinical Endocrinology. 2014;81:907–915. doi: 10.1111/cen.12527. [DOI] [PubMed] [Google Scholar]
- 23.Larisch R, Giacobino A, Eckl W, Wahl HG, Midgley JEM, Hoermann R. Reference range for thyrotropin. Post hoc assessment. Nuklearmedizin. 2015;54:112–117. doi: 10.3413/Nukmed-0671-14-06. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich JW, Landgrafe G, Fotiadou EH. TSH and thyrotropic agonists: key actors in thyroid homeostasis. Journal of Thyroid Research. 2012;2012:1–29. doi: 10.1155/2012/351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fellows I. Deducer: a data analysis GUI for R. Journal of Statistical Software. 2012;49:1–15. [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing 2014. Vienna, Austria: R Foundation for Statistical Computing. (available at: http://www.R-project.org/)
- 27.Silva EJ, Gordon MB, Crantz FR, Leonard JL, Larsen PR. Qualitative and quantitative differences in the pathways of extrathyroidal triiodothyronine generation between euthyroid and hypothyroid rats. Journal of Clinical Investigation. 1984;73:898–907. doi: 10.1172/JCI111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cettour-Rose P, Visser TJ, Burger AG, Rohner-Jeanrenaud F. Inhibition of pituitary type 2 deiodinase by reverse triiodothyronine does not alter thyroxine-induced inhibition of thyrotropin secretion in hypothyroid rats. European Journal of Endocrinology. 2005;153:429–434. doi: 10.1530/eje.1.01984. [DOI] [PubMed] [Google Scholar]
- 29.Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla SM, Wittmann G, Lechan RM, Gereben B, Bianco AC. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. Journal of Clinical Investigation. 2015;125:769–781. doi: 10.1172/JCI77588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoermann R, Midgley JEM, Larisch R, Dietrich J. Integration of peripheral and glandular regulation of triiodothyronine production by thyrotropin in untreated and thyroxine-treated subjects. Hormone and Metabolic Research. 2015;47:674–680. doi: 10.1055/s-0034-1398616. [DOI] [PubMed] [Google Scholar]
- 31.Checchi S, Montanaro A, Pasqui L, Ciuoli C, De Palo V, Chiappetta MC, Pacini F. l-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. Journal of Clinical Endocrinology and Metabolism. 2008;93:465–469. doi: 10.1210/jc.2007-1544. [DOI] [PubMed] [Google Scholar]
- 32.Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Practice & Research. Clinical Endocrinology & Metabolism. 2009;23:781–792. doi: 10.1016/j.beem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Robertson HMA, Narayanaswamy AKP, Pereira O, Copland SA, Herriot R, McKinlay AW, Bevan JS, Abraham P. Factors contributing to high levothyroxine doses in primary hypothyroidism: an interventional audit of a large community database. Thyroid. 2014;24:1765–1771. doi: 10.1089/thy.2013.0661. [DOI] [PubMed] [Google Scholar]
- 34.Toft Kristensen T, Larsen J, Pedersen PL, Feldthusen A-D, Ellervik C, Jelstrup S, Kvetny J. Weight gain and serum TSH increase within the reference range after hemithyroidectomy indicate lowered thyroid function. Journal of Thyroid Research. 2014;2014:892573. doi: 10.1155/2014/892573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. New England Journal of Medicine. 1987;316:764–770. doi: 10.1056/NEJM198703263161302. [DOI] [PubMed] [Google Scholar]
- 36.Banovac K, Carrington SA, Levis S, Fill MD, Bilsker MS. Determination of replacement and suppressive doses of thyroxine. Journal of International Medical Research. 1990;18:210–218. doi: 10.1177/030006059001800305. [DOI] [PubMed] [Google Scholar]
- 37.Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocrine Practice. 1999;5:233–238. doi: 10.4158/EP.5.5.233. [DOI] [PubMed] [Google Scholar]
- 38.Baehr KM, Lyden E, Treude K, Erickson J, Goldner W. Levothyroxine dose following thyroidectomy is affected by more than just body weight. Laryngoscope. 2012;122:834–838. doi: 10.1002/lary.23186. [DOI] [PubMed] [Google Scholar]
- 39.de Lima JG, de Mesquita DJTM, da Costa Fernandes F, de Souza ABC, Santos Juniordos AC, Reboucas B, de Lima NN, Sousa AGP, Nobrega LHC. Comparison among the daily levothyroxine doses according to the etiology of hypothyroidism. Journal of Endocrinology and Metabolism. 2013;3:1–6. doi: 10.4021/jem165w. [DOI] [Google Scholar]
- 40.Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clinical Endocrinology. 2014;81:633–641. doi: 10.1111/cen.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonseca TL, Correa-Medina M, Campos MPO, Wittmann G, Werneck-de-Castro JP, Arrojo e Drigo R, Mora-Garzon M, Ueta CB, Caicedo A, Fekete C, et al. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. Journal of Clinical Investigation. 2013;123:1492–1500. doi: 10.1172/JCI61231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoftijzer HC, Heemstra KA, Visser TJ, le Cessie S, Peeters RP, Corssmit EPM, Smit JWA. The type 2 deiodinase ORFa-Gly3Asp polymorphism (rs12885300) influences the set point of the hypothalamus–pituitary–thyroid axis in patients treated for differentiated thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism. 2011;96:E1527–E1533. doi: 10.1210/jc.2011-0235. [DOI] [PubMed] [Google Scholar]
- 43.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. Journal of Clinical Endocrinology and Metabolism. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 44.Taylor PN, Panicker V, Sayers A, Shields B, Iqbal A, Bremner AP, Beilby JP, Leedman PJ, Hattersley AT, Vaidya B, et al. A meta-analysis of the associations between common variation in the PDE8B gene and thyroid hormone parameters, including assessment of longitudinal stability of associations over time and effect of thyroid hormone replacement. European Journal of Endocrinology. 2011;164:773–780. doi: 10.1530/EJE-10-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAninch EA, Jo S, Preite NZ, Farkas E, Mohácsik P, Fekete C, Egri P, Gereben B, Li Y, eng Y, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. Journal of Clinical Endocrinology and Metabolism. 2015;100:920–933. doi: 10.1210/jc.2014-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiersinga WM. Do we need still more trials on T4 and T3 combination therapy in hypothyroidism? European Journal of Endocrinology. 2009;161:955–959. doi: 10.1530/EJE-09-0879. [DOI] [PubMed] [Google Scholar]
- 47.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MPJ. ETA guidelines: the use of l-T4+l-T3 in the treatment of hypothyroidism. European Thyroid Journal. 2012;1:55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watt T, Cramon P, Hegedüs L, Bjorner JB, Bonnema SJ, Rasmussen ÅK, Feldt-Rasmussen U, Groenvold M. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. Journal of Clinical Endocrinology and Metabolism. 2014;99:3708–3717. doi: 10.1210/jc.2014-1322. [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a