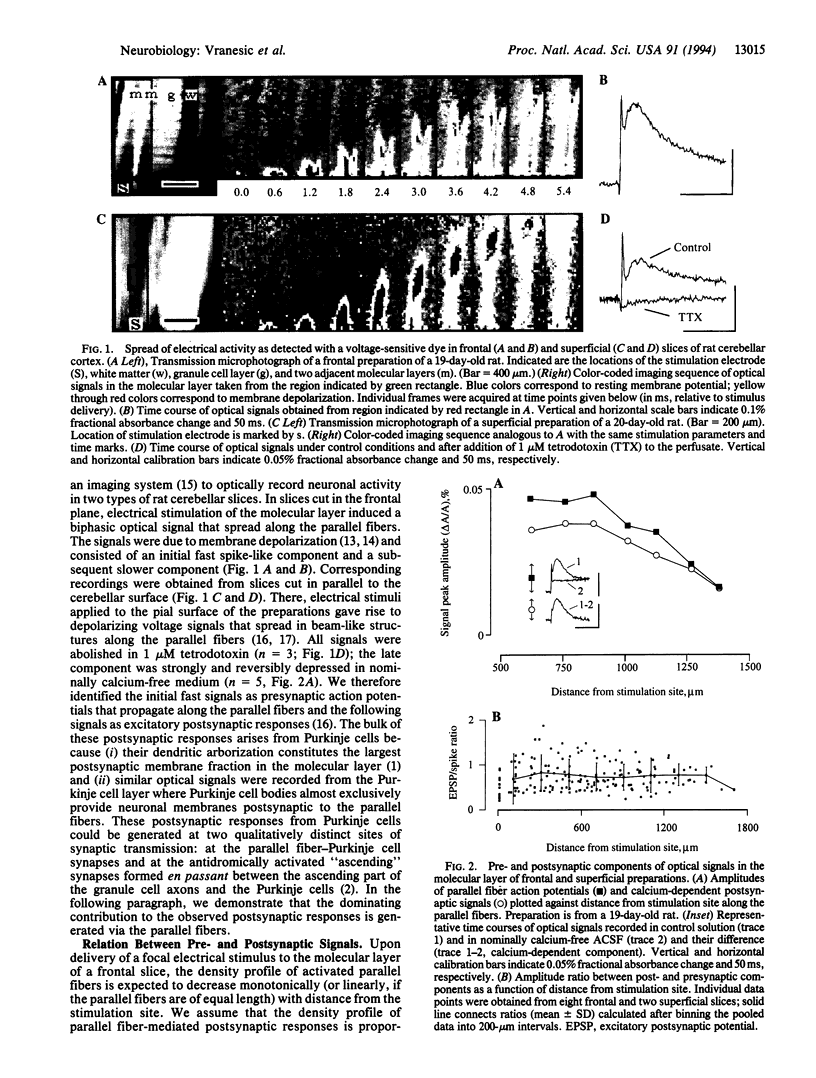
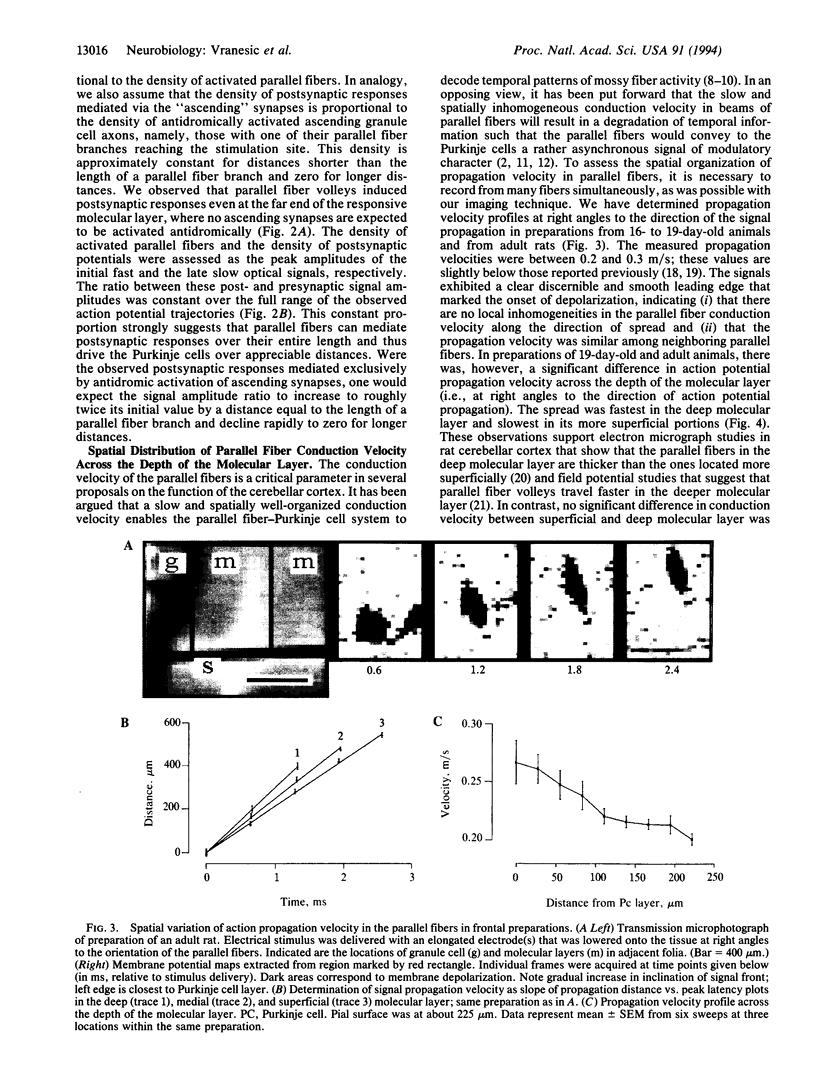
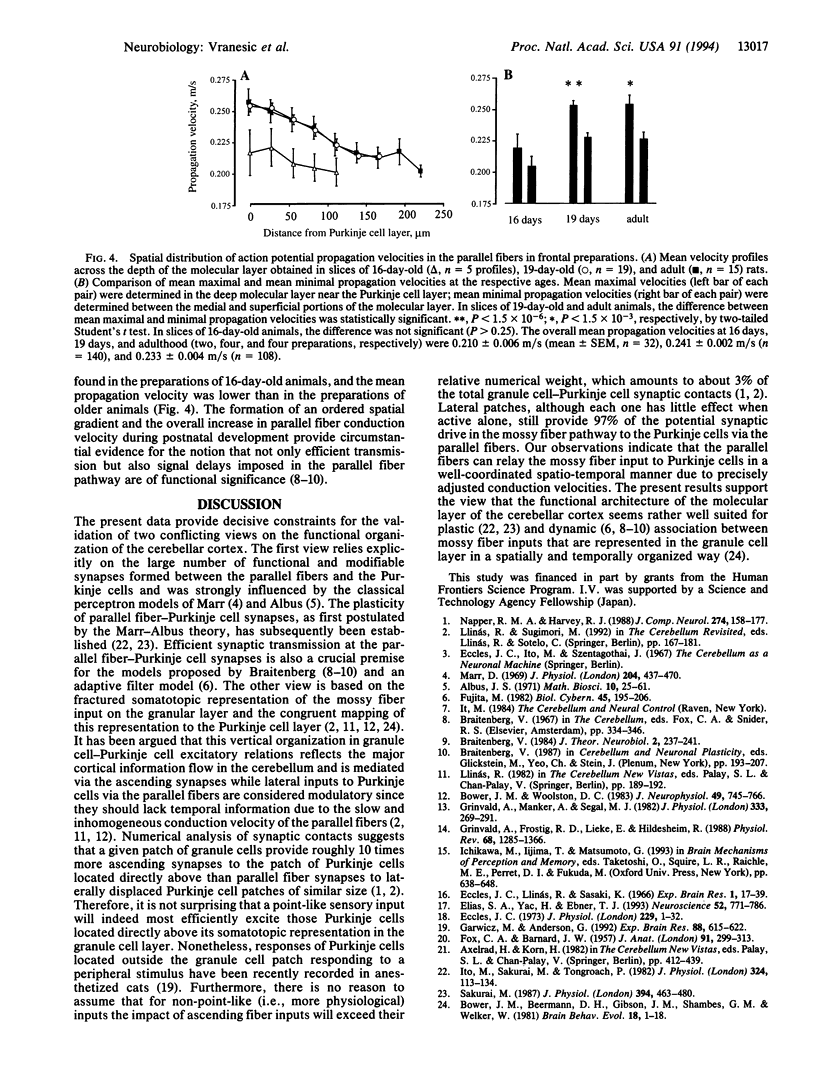
Abstract
We investigated the synaptic transmission in the parallel fiber-Purkinje cell system at high spatio-temporal resolution by using voltage-sensitive dyes and an imaging system. In rat cerebellar slices, cut in the frontal plane or in a plane of the cerebellar surface, local electrical stimulation induced volleys of action potentials in the parallel fibers; subsequent postsynaptic responses from Purkinje cells were observed along the volleys' entire trajectories. Furthermore, the formation of an ordered spatial gradient in parallel fiber conduction velocity across the depth of the molecular layer during postnatal development was observed. In preparations of adult, but not of immature rats, the conduction velocity of parallel fibers in the deep molecular layer was faster than in its more superficial regions. Our observations demonstrate that parallel fibers can mediate Purkinje cell excitation effectively and over considerable distances in a well-organized spatio-temporal manner, thus supporting the classical view of the physiological role assigned to the parallel fibers.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettler B., Boulter J., Hermans-Borgmeyer I., O'Shea-Greenfield A., Deneris E. S., Moll C., Borgmeyer U., Hollmann M., Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990 Nov;5(5):583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bettler B., Egebjerg J., Sharma G., Pecht G., Hermans-Borgmeyer I., Moll C., Stevens C. F., Heinemann S. Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron. 1992 Feb;8(2):257–265. doi: 10.1016/0896-6273(92)90292-l. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Bower J. M., Beermann D. H., Gibson J. M., Shambes G. M., Welker W. Principles of organization of a cerebro-cerebellar circuit. Micromapping the projections from cerebral (SI) to cerebellar (granule cell layer) tactile areas of rats. Brain Behav Evol. 1981;18(1-2):1–18. doi: 10.1159/000121772. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Is the cerebellar cortex a biological clock in the millisecond range? Prog Brain Res. 1967;25:334–346. doi: 10.1016/S0079-6123(08)60971-1. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Driscoll B. F., Deibler G. E., Law M. J., Crane A. M. Damage to neurons in culture following medium change: role of glutamine and extracellular generation of glutamate. J Neurochem. 1993 Nov;61(5):1795–1800. doi: 10.1111/j.1471-4159.1993.tb09818.x. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Heinemann S. F. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX C. A., BARNARD J. W. A quantitative study of the Purkinje cell dendritic branchlets and their relationship to afferent fibres. J Anat. 1957 Jul;91(3):299–313. [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Adaptive filter model of the cerebellum. Biol Cybern. 1982;45(3):195–206. doi: 10.1007/BF00336192. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Frostig R. D., Lieke E., Hildesheim R. Optical imaging of neuronal activity. Physiol Rev. 1988 Oct;68(4):1285–1366. doi: 10.1152/physrev.1988.68.4.1285. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Manker A., Segal M. Visualization of the spread of electrical activity in rat hippocampal slices by voltage-sensitive optical probes. J Physiol. 1982 Dec;333:269–291. doi: 10.1113/jphysiol.1982.sp014453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Herb A., Burnashev N., Werner P., Sakmann B., Wisden W., Seeburg P. H. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992 Apr;8(4):775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Honoré T., Drejer J. Chaotropic ions affect the conformation of quisqualate receptors in rat cortical membranes. J Neurochem. 1988 Aug;51(2):457–461. doi: 10.1111/j.1471-4159.1988.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Dingledine R., Heinemann S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991 Aug 30;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Ito M., Sakurai M., Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982 Mar;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Onishi H., Hattori S., Miyagi Y., Amaya Y., Mishina M., Okuda K. Functional expression of the alpha 1 subunit of the AMPA-selective glutamate receptor channel, using a baculovirus system. Biochem Biophys Res Commun. 1991 Dec 16;181(2):756–763. doi: 10.1016/0006-291x(91)91255-b. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kotewicz M. L., Sampson C. M., D'Alessio J. M., Gerard G. F. Isolation of cloned Moloney murine leukemia virus reverse transcriptase lacking ribonuclease H activity. Nucleic Acids Res. 1988 Jan 11;16(1):265–277. doi: 10.1093/nar/16.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M., Burnashev N., Sakmann B., Seeburg P. H. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993 Mar;10(3):491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Lomeli H., Wisden W., Köhler M., Keinänen K., Sommer B., Seeburg P. H. High-affinity kainate and domoate receptors in rat brain. FEBS Lett. 1992 Jul 28;307(2):139–143. doi: 10.1016/0014-5793(92)80753-4. [DOI] [PubMed] [Google Scholar]
- London E. D., Coyle J. T. Specific binding of [3H]kainic acid to receptor sites in rat brain. Mol Pharmacol. 1979 May;15(3):492–505. [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Napper R. M., Harvey R. J. Quantitative study of the Purkinje cell dendritic spines in the rat cerebellum. J Comp Neurol. 1988 Aug 8;274(2):158–167. doi: 10.1002/cne.902740203. [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Boel E., Johannessen M., Foster D. Quenching of the amidolytic activity of one-chain tissue-type plasminogen activator by mutation of lysine-416. Biochemistry. 1990 Apr 10;29(14):3451–3457. doi: 10.1021/bi00466a005. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993 Feb 18;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Sommer B., Burnashev N., Verdoorn T. A., Keinänen K., Sakmann B., Seeburg P. H. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992 Apr;11(4):1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- Werner P., Voigt M., Keinänen K., Wisden W., Seeburg P. H. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991 Jun 27;351(6329):742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]