Abstract
Babesiosis is a tick-borne hemoprotozoan disease of domestic and wild animals. The disease is caused by various species of Babesia and some species of Babesia have also zoonotic significance. The parasite in vertebrate hosts’ remains in erythrocytes and the morphology of Babesia spp. is not uniform in all vertebrate hosts. With the advancement of science, particularly the use of molecular techniques made it easy to study the evolution of parasites and thereby reclassifying Babesia spp. as per their phylogeny and to establish the relation of one isolate of Babesia spp. with isolates throughout the world. An attempt also made in this communication to enlighten the readers regarding relationship of one isolate of Babesia spp. of a particular area to another isolate of Babesia spp. of that area or other parts of the world and phylogenetic classification of Babesia spp. was also discussed. It has been concluded that as the study on Babesia is complex in nature so monitoring of the infection with the use of modern techniques is very much needed to control the infection. Second, more research work on phylogenetic relationship of Babesia spp. isolated from different hosts is needed, particularly in India to know the evolution of Babesia spp. of a particular area, as it has great importance to study the trans boundary diseases of animals.
KEY WORDS: Babesia, morphology, phylogeny
INTRODUCTION
In the year 1888, Babes investigated outbreaks of diseases with symptoms of hemoglobinuria in cattle in Romania and was the first to discover piroplasm in the blood of cattle.[1] Initially, he thought it to be a bacterium that had named as Hematococeus bovis and later it was changed to Babesia bovis. After 5 years, Smith and Kilborne demonstrated the causative organism of “Texas Fever” (babesiosis) as Pyrosoma bigeminum (Babesia bigemina).[2] After that the first demonstration of transovarian transmission of Babesia through its tick vector – a finding of historic significance was done.[3] Babesiosis was discovered gradually in various parts of the world with its zoonotic potential particularly in the tropical and subtropical countries along with its economic impact.[4,5] The first confirmed case of human fatal babesiosis caused by Babesia divergens was recorded in 1956.[6] Since then, zoonotic significance of babesiosis that too as a potentially life threatening zoonotic infection in human came out.[7] There are several species of Babesia which causes human infections throughout the world[8] but major one is Babesia microti particularly in North America.[9] Babesiosis is transmitted during blood feeding by infected ticks. The disease is considered as the most economically important tick-borne disease in tropical and subtropical areas.[10] If we take the cattle babesiosis in respect of economic impact then we could see losses occurs due to mortality, decreased milk or meat production, abortions, reduction of draft power, cost under the head of control measures, including increased cost of management to maintain ill animals. An annual loss of 16.9, 5.1, 5.4, 6.8, 21.6, 19.4, 57.2, 3.1, and 0.6 million US dollars in Australia, Kenya, Zimbabwe, Tanzania, South Africa, China, India, Indonesia, and Philippines, respectively, have been estimated due to babesiosis and anaplasmosis.[2] From India it is reported that due to clinical babesiosis in a crossbred cattle decreased milk production could be noticed for 30 days and during this period total loss of 51.6 L of milk has been estimated.[11]
MORPHOLOGY
On the basis of morphology, babesias are divided into two groups – small babesias (1.0–2.5 μm long) which included B. bovis, Babesia gibsoni, B. microti, Babesia rodhaini, etc., and large babesias (2.5–5.0 μm long) which included B. bigemina, Babesia caballi, Babesia Canis, etc., The orientation of the parasite in the red blood cells (RBCs) depends on its size because large pyriform parasites meet at their pointed ends at an acute angle to each other and small forms make an obtuse angle to each other.[12] More than 100 species of Babesia have been identified that are infecting many mammalian and some avian species.[7] The following are the morphology of different Babesia spp. of animals according to their hosts.
CATTLE AND BUFFALOES
B. bigemina - large form (4.5 μm × 2.0 μm) of Babesia. The parasites are characteristically pear shaped. Round (2–3 μm in diameter) oval or irregularly shaped form may also be found.
B. bovis - small form (2.0 μm × 1.5 μm) of Babesia. Slightly larger than B. divergens, vacuolated signet ring forms are particularly common.[13]
B. divergens - small form (1.5 μm × 0.4 μm) of Babesia. Generally remained as paired form, superficially lie on the RBC, stout and pyriform or circular forms may be found.
B. major - large form (3.2 μm × 1.5 μm) of Babesia. Pyriform bodies, the angle between the organism is <90°. Round forms with a diameter of about 1.8 μm are also available.
CANINE
Canine babesiosis is caused by two species of Babesia viz; B. canis and B. gibsoni, which are morphologically differentiated on the basis of their size.
B. canis - large form (4–5 μm long) of Babesia. Pyriform in shape, pointed one end, and round other. In a single RBC, multiple infection that is, more than one organism up to 16 may be found [Figure 1].
Figure 1.
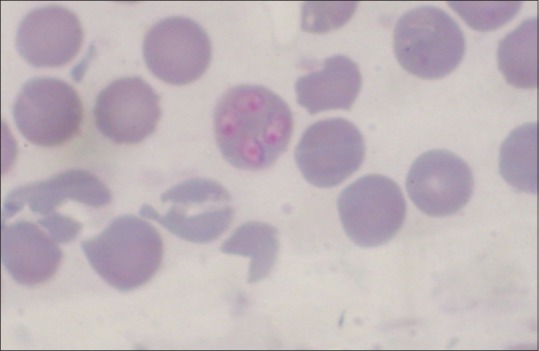
Babesia canis within red blood cell in Giemsa stained blood smear of a dog
B. gibsoni - small form (1.5–2.5 μm) of Babesia. Lack usual pyriform shapes, trophozoites are annular or oval; signet ring forms may occur [Figure 2].
Figure 2.
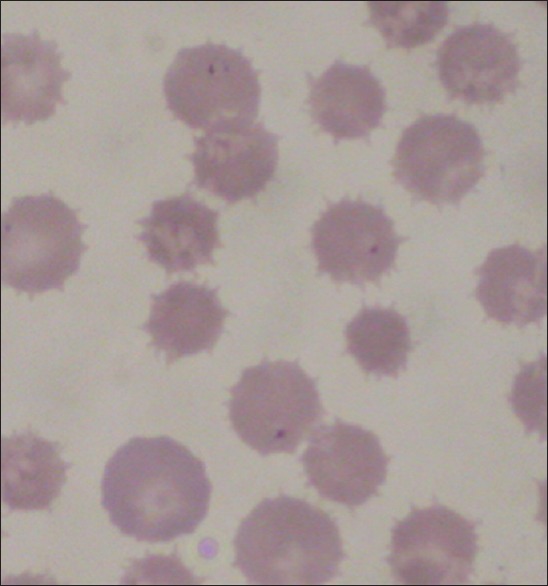
Babesia canis within red blood cell in Giemsa stained blood smear of a dog
OVINE AND CAPRINE
B. motasi - large form (2.5–4.5 μm) of Babesia, organisms are pyriform.
B. ovis - small form (1.0–2.5 μm in length) of Babesia, maximum are round, remain at the margin of the RBC.
Babesia foliate - small form of Babesia. Leaf-shaped, located more centrally in RBC.
Babesia taylori - small form (1.5–2.0 μm long) of Babesia, ovoid to round in shape, multiple infections up to 16 parasites in a RBC may be seen.
EQUINES
B. caballi - large form of Babesia. Commonly occur as pair. Pyriform and measures 2.5–4 μm long. Round or oval forms with 1.5–3.0 μm in diameter may be found.
Babesia equi - small form (2.0 μm) of Babesia. The piroplasms characteristically form a Maltese cross of four organisms.
B. equi is also named as Theileria equi due to its preerythrocytic development as recorded in other Theileria species.[14] The differences and similarities between these organism have been worked out using molecular techniques and 18S rDNA sequence analysis reclassified recently B. equi as T. equi.[15] Among two species of infecting equines, B. equi (T. equi) considered as more pathogenic and widespread than B. caballi and there is no confirmed report regarding the occurrence of B. caballi from India.[16] On the contrary, B. caballi is more prevalent in Southern African countries than T. equi.
SWINE
Babesia trautmanni - large form of Babesia, 2.5–4.5 μm × 2 μm in diameter. Frequently occurs in pairs, but oval, amoeboid, and ring forms may also be found. Multiple infection in single RBC (up to 4 parasites) may be found.
FELINE
Babesia cati - large form of Babesia and Babesia felis - small form of Babesia. Babesia sp. in a cat from Kerala with a mean size of 2.4 μm × 1.3 μm has been reported.[17] This Babesia was found to present in RBC as single, in pairs, or multiple up to eight trophozoites within RBC. The large form of Babesia, B. cati from Indian wild cat was reported in Madras.[18]
EPIDEMIOLOGY
The epidemiology of babesiosis in general depended on several parameters such as availability of host, presence of ticks that act as vector of transmission of infections, presence of parasites within vectors, as well as hosts and environmental condition. These parameters are responsible for spread of infections. Absence of any one parameter will discontinue the spread of infections. As regarding epidemiology of babesiosis, a state of “Endemic Stability” where the relationship between host, parasite, vector, and environment remained in such a way that clinical disease occur rarely or not at all,[19] can also be taken into consideration. The parasite Babesia itself is the weakest point of this system of spread of infection as it needs both vectors and host for its survival,[20] and thus dependent on them. The second weakest point is the vector which depends on host and finally the host which support these two parameters to spread the infection but is not dependent on these two parameters.
HOST
One important fact is that Babesia spp. are not strictly host specific that might be a reason of ever increasing list of new species. Babesia infection in host depending on age of the host and inverse age resistance like young animals are less and older animals are more susceptible to infection. Breeds are also important for getting infection where Bos indicus are more resistant than Bos taurus. Besides, low grade immunity could be observed in previously infected animals. Several reports on occurrence of babesiosis in cattle in India are available.[21,22,23,24,25,26,27,28] From North-Eastern region of India, B. bigemina infections in 3.6% cattle using polymerase chain reaction (PCR) has been reported.[29] Lower prevalence of B. bigemina has been reported from different parts of India both by conventional (Giemsa staining) and molecular methods. By conventional method, 0.48–2.7% prevalence rate has been reported from Punjab,[30,31] Kerala,[32] and Karnataka.[33] By using PCR, prevalence of 0.66% and 2.43% has been reported from Kerala[32] and Punjab,[30] respectively. In India, in case of cattle, B. bigemina infection predominates over B. bovis infection and very few reports of B. bovis are available.[33] From Pakistan 9% cattle were found as positive for Babesia spp. infections out of which 6% were found infected with B. bigemina and 3% were found infected with B. bovis after examination of stained blood smear. In the same study, PCR detected 20% positive animals with a distribution of 13% with B. bigemina and 7% with B. bovis.[34]
In India, the situation of canine babesiosis is not clear[35] except few reports.[36,37,38,39,40,41] The prevalence of canine babesiosis in Assam has been reported as 21.7% without mentioning the species.[38] The incidence reported from Northern part of India varied between 0.66% and 8.9%[37,42] while from Southern India 11.6% prevalence of hemoprotozoa and among hemoprotozoa B. canis and B. gibsoni were recorded as 3.9% and 84.9%, respectively.[39] A total of 44 (39.63%) dogs were diagnosed as positive for Babesia infections after microscopic examination from North-Eastern region of India.[43] Among these, B. canis infection was diagnosed in 5 dogs (4.50%) and B. gibsoni infection in 39 (35.13%) dogs microscopically in Giemsa stained blood smears. Molecular diagnosis using PCR detected 63 (56.75%) dogs positive for Babesia infection. Single infection with B. canis was found in 9 (8.10%) dogs while B. gibsoni alone was detected in 3 (2.70%) dogs. Mixed infections by both these species were detected in 51 (45.94%) dogs. Overall, PCR detected 54 (48.64%) dogs as B. gibsoni and 60 (54.05%) dogs as B. canis positive. Equine babesiosis due to T. equi has been reported from India.[44,45,46,47]
PARASITE
The parasite Babesia itself is essential for causing the disease babesiosis as well as the transmitting agent. For infection to the vectors, parasitemia in the host and for infection to host, parasitemia in vectors is essential. But, there is no direct relation between the level of parasitemia in the host and the tick infection rate.[48] The study on interaction between Babesia microplus ticks and Babesia spp. infections revealed no detrimental effect of the parasite on naturally infected B. microplus and pathogenic effect of Babesia on ticks need not to be considered.[20]
VECTOR
All species of Babesia are naturally transmitted from animal to animal through the bites of ticks and within ticks’ transovarian transmission (transmission of infection through eggs from mother ticks), and stage-to-stage transmission (transmission of infection from egg to larvae to nymph to adult) occurs. Ticks are widely distributed throughout the world particularly in tropical and subtropical countries, and 80% of the world cattle are affected with ticks and ticks borne diseases.[49]
The ticks of genera Boophilus, Rhipicephalus, Hemaphysalis, Hyalomma, and Ixodes acts as vector for transmission of B. bigemina and ticks of genera Boophilus, Rhipicephalus, and Ixodes are responsible for transmission of B. bovis. The presence of B. microplus ticks in B. bigemina infected animals reported earlier.[50] In Pakistan, in cattle, the prevalence of Hyalomma tick was highest followed by Boophilus, Hemaphysalis, and Rhipicephalus.[34]
Babesia spp. in dogs are transmitted by ticks Rhipicephalus sanguineus, Hemaphysalis longicornis, Hemaphysalis leachi, and Dermacentor marginatus.[51] Other modes of transmission are through blood transfusion[52] and through the transplacental transmission as reported.[53]
T. equi infection is transmitted among equines through Ixodid ticks of the genera Hyalomma, Rhipicephalus, and Dermacentor. In donkeys and horses of India, Hyalomma species have been found as a potential vector for transmission of the infections.[14] Reservoir of B. caballi infection is ticked rather than horses because transovarian transmission occurs in ticks with B. caballi (except Rh. evertsi evertsi), and infections persist in ticks throughout several generations. But, the reservoir of B. equi infection are horses rather than ticks as only stage-to-stage transmission occurs in ticks with B. equi infections.[54] Besides transmission of infection through ticks, transplacental transmission of T. equi from carrier mother to foals has been reported from Punjab.[16] Intra-uterine transmission of equine babesiosis has also been reported.[55]
With the advancement of molecular diagnosis using PCR, now-a-days several authors detected the presence of Babesia organism in ticks. In a study, PCR-based detection of bovine Babesia spp. in ticks R. sanguineus and R. bursa has been done successfully.[10] B. bigemina DNA in ticks by DNA hybridization using a nonradioactive probe has been detected[56] and it is opined that this newly developed DNA probe could be used in epidemiological study of the disease. The frequency of B. bovis and B. bigemina infection in B. microplus engorged female ticks and eggs collected from cattle reared in an area of endemic babesiosis was carried out using PCR and nested PCR[57] and the infectivity of B. bigemina was recorded as higher to ticks fed on calves (56.2%) than cows (15.9%). Another interesting finding of the study was the significantly higher frequency of infection with B. bigemina (56.2%) than B. bovis (4.7%) in female engorged ticks collected from calves. Using nested-PCR similar findings of frequency of B. bigemina was higher in female ticks collected from calves (24.4%) than those collected from cows (9.4%) reported.[58] The frequency of Babesia spp. infections in engorged female B. microplus ticks and eggs collected from calves showed more frequency of infections in calves than engorged ticks collected from cows. The hatching rates of B. microplus larvae has been reported to lower in samples collected from calves than from cows particularly in those where Babesia spp. was detected in egg samples.[59]
ENVIRONMENT
Climatic data such as environmental temperature, humidity, and rainfall of a particular area are responsible for transmission of babesiosis and opined that the important factors of the presence of B. bigemina in cattle in hilly region of Meghalaya might be due to the spread of infected ticks under favorable climatic conditions, which has been changed in Meghalaya as a whole due to global warming that results in the spread of ticks and babesiosis.[60] It has been mentioned that the longevity of larvae and the number of annual generations of B. microplus are influenced by the climate.[20]
PHYLOGENY
As discussed earlier, morphologically babesias are two groups – large and small depending on their size. Recent literature reported morphological classifications are also related to phylogenetic characterizations that are based on small subunit-ribosomal RNA gene (18S rRNA) sequences. As per phylogenetic clusters, small babesias are more related to Theileria spp than the large, with the exception of B.divergens which is genetically related to large babesias.[7,9] Recently, 18S rRNA gene for phylogenetic analysis of Babesia has been used[61] and accordingly piroplasms were divided into five distinct clades – (i) Babesia spp. from ungulates: B. caballi, B. bigemina, B. ovis, B. bovis, and oher Babesia spp. from cattle. (ii) B. canis and B. gibsoni from canines together with B. divergens and Babesia odocoilei, (iii) B. microti group with B. rodhaini, B. felis, Babesia leo, B. microti, and Theileria annae (iv) Western US Theileria – like group, containing Babesia conradae (v) Theileria-group containing all Theileria spp. from bovines.[2,7]
B. canis has been identified from the blood of three cats by molecular diagnosis.[61] The feline genotype of B. canis as B. canis subsp. presentii has been proposed.[62] So, it has been opined that the larger forms of Babesia in cats might be a feline genotype of canine Babesia sp.[17] Partial sequencing of 18S rRNA gene demonstrated that in cat with Babesia like symptoms was infected with B. canis canis.[63]
Worldwide there are three different sub species of B. canis named as B. canis rossi, B. canis canis, and B. canis vogeli that have been recognized on the basis of geographical distribution of vector tick, differences in pathological and clinical syndrome, antigenic property, and molecular analysis.[64] Similarly, recent molecular analyses have revealed three morphologically similar but genotypically distinct small Babesia of which B. gibsoni Asia type is endemic to Asia, North America, North and East Africa.[65] Among the three sub species of B. canis, B. canis vogeli is predominant in Brazil.[66] B. canis is considered to be virulent in dogs that are found in Europe and B. rossi that are only reported from Africa is highly virulent[35] whereas B. vogeli which is worldwide distributed are considered as mildly virulent.[67] B. canis vogeli has been reported from India using PCR.[68] Sequence and phylogenetic analysis of 18S rRNA and ITS 1 sequences of 15 small form Babesia positive canine blood samples collected from India revealed thirteen isolates shared high sequence identity with each other and with B. gibsoni Asian genotype.[69] They have also found that another two isolates were close together with B. orientalis, Babesia sp. (Kashi 1 isolate), and B. occultans of bovines. In a study, Indian isolates of B. bigemina showed genetic variation among themselves. In dogs of Northwest Spain, a B. microti-like piroplasm named as B. annae (T. annae) has been found.[70]
Two B. bigemina isolates of North India showed highest similarity (64%) whereas two South India isolates showed 50% similarity and isolates from these two region showed only 41% similarity.[71] The Indian B. bigemina sequences were found to be closely related with the cognate gene nucleotide sequences of B. bigemina from Argentina and Kenya where 99.1–99.9% and 99.0–99.7% nucleotide identity was observed, respectively. Distant relationship of these Indian organisms was observed with few cognate gene sequences from China where more than 7% divergence was observed in the distance matrix.[29] B. bigemina isolates from yak of Arunachal Pradesh has been shown to have a close relation with isolates from China, Mexico, Australia, and Zimbabwe.[72]
CONCLUSIONS
Many epidemiological factors are involved for causing babesiosis in vertebrate hosts and the study on babesiosis is complex in nature involving genetic composition and resistance of hosts, climatic conditions, tick infections, and infection rate in hosts. Monitoring of the infection with the use of modern techniques is very much needed to control the infection. The parasites Babesia have a wide host range and very little work has been done in India regarding phylogenetic relationship of Babesia spp. isolated from different hosts. More research work in this respect is needed to know the evolution of Babesia spp. of a particular area as it has great importance to study the trans boundary diseases of animals.
Acknowledgment
We are thankful to the Director, ICAR Research Complex for NEH Region, Umiam, Meghalaya, for providing facilities to prepare this review article.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Babes V. Sur 1’haemoglobinurie bacterienne duboeof. C R Hebd Seances Acad Sci. 1888;107:692–694.65. [Google Scholar]
- 2.Bock R, Jackson L, de Vos A, Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129(Suppl):S247–69. doi: 10.1017/s0031182004005190. [DOI] [PubMed] [Google Scholar]
- 3.Smith T, Kilborne FL. Washington, DC: US Department of Agriculture Bureau of Animal Industry; 1893. Investigations Into the Nature, Causation and Prevention of Texas or Southern Cattle Fever. Bulletin; pp. 1–301. [Google Scholar]
- 4.Collett MG. Survey of canine babesiosis in South Africa. J S Afr Vet Assoc. 2000;71:180–6. doi: 10.4102/jsava.v71i3.710. [DOI] [PubMed] [Google Scholar]
- 5.Kivaria FM, Ruheta MR, Mkonyi PA, Malamsha PC. Epidemiological aspects and economic impact of bovine theileriosis (East Coast fever) and its control: A preliminary assessment with special reference to Kibaha district, Tanzania. Vet J. 2007;173:384–90. doi: 10.1016/j.tvjl.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9:11–6. [PubMed] [Google Scholar]
- 7.Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: Recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–37. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters TP. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501. doi: 10.1080/00034989859465. [DOI] [PubMed] [Google Scholar]
- 9.Homer MJ, Aguilar-Delfin I, Telford SR, 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–69. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavassoli M, Tabatabaei M, Mohammadi M, Esmaeilnejad B, Mohamadpour H. PCR-based detection of Babesia spp. infection in collected ticks from cattle in west and North-West of Iran. J Arthropod Borne Dis. 2013;7:132–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Laha R, Das M, Goswami A, Singh P. Losses of milk production due to Babesia bigemina infection in a cross bred cow – A case study. J Protozool Res. 2012;; 22:6–9. [Google Scholar]
- 12.Ruprah NS. New Delhi: Oxonian Press Pvt. Ltd; 1985. Textbook of Clinical Protozoology. [Google Scholar]
- 13.Soulsby EJ. 7th ed. London, U.K: Bailliere Tindall; 1986. Helminths, Arthropods and Protozoa of Domesticated Animals. [Google Scholar]
- 14.Kumar S, Kumar R, Sugimoto C. A perspective on Theileria equi infections in donkeys. Jpn J Vet Res. 2009;56:171–80. [PubMed] [Google Scholar]
- 15.Mehlhorn H, Schein E. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol Res. 1998;84:467–75. doi: 10.1007/s004360050431. [DOI] [PubMed] [Google Scholar]
- 16.Chhabra S, Ranjan R, Uppal SK, Singla LD. Transplacental transmission of Babesia equi (Theileria equi) from carrier mares to foals. J Parasit Dis. 2012;36:31–3. doi: 10.1007/s12639-011-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabu L, Sreekrishnan R, Devada K, Rejitha TS, Lakshmanan B. Babesia species in a cat – A case report. J Vet Parasitol. 2013;27:68–9. [Google Scholar]
- 18.Mudaliar SV, Achary GR, Alwar VS. On a species of Babesia in an Indian wild cat (Felis catus) Indian Vet J. 1950;26:392–5. [PubMed] [Google Scholar]
- 19.Perry BD, Chamboko T, Mahan SM, Medley GF, Minjauw B, O’Callaghan CJ, et al. The economics of integrated tick and borne diseases control on commercial farms in Zimbabwe. Zimb Vet J. 1998;29:21–9. [Google Scholar]
- 20.Alonso M, Arellano-Sota C, Cereser VH, Cordoves CO, Guglielmone AA, Kessler R, et al. Epidemiology of bovine anaplasmosis and babesiosis in Latin America and the Caribbean. Rev Sci Tech. 1992;11:713–33. doi: 10.20506/rst.11.3.623. [DOI] [PubMed] [Google Scholar]
- 21.Reddy GG, Mishra AK, Rao JR, Tewari AK. Comparison of indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) in detecting Babesia bigemina infection in cattle. Acta Vet Hung. 1997;45:67–74. [PubMed] [Google Scholar]
- 22.Jithendran KP. Blood protista of cattle and buffaloes in Kangra Valley, Himachal Pradesh. Indian J Anim Sci. 1997;67:207–8. [Google Scholar]
- 23.Garg R, Banerjee PS, Yadav CL. Subclinical babesiosis and anaplasmosis in an organized farm in Uttaranchal. J Vet Parasitol. 2004;18:151–3. [Google Scholar]
- 24.Banerjee PS, Dabas S, Vatsya S, Bhatt P, Yadav CL. Babesiosis in a crossbred cow with clumping of parasitized red blood cells. J Vet Parasitol. 2005;19:153–4. [Google Scholar]
- 25.Ravindran R, Mishra AK, Rao JR. On the high sero prevalence of bovine babesiosis in Wayanad district of Kerala. J Appl Anim Res. 2002;22:43–8. [Google Scholar]
- 26.Ravindran R, Mishra AK, Rao JR. Slide enzyme-linked immunosorbent assay for the diagnosis of Babesia bigemina infection in bovines. Vet Res Commun. 2007;31:999–1004. doi: 10.1007/s11259-007-0033-4. [DOI] [PubMed] [Google Scholar]
- 27.Singh H, Mishra AK, Rao JR, Tewari AK. A PCR assay for detection of Babesia bigemina infection using clotted blood in bovines. J Appl Anim Res. 2007;32:201–2. [Google Scholar]
- 28.Singh H, Mishra AK, Rao JR, Tewari AK. Comparison of indirect fluorescent antibody test (IFAT) and slide enzyme linked immunosorbent assay (SELISA) for diagnosis of Babesia bigemina infection in bovines. Trop Anim Health Prod. 2009;41:153–9. doi: 10.1007/s11250-008-9170-1. [DOI] [PubMed] [Google Scholar]
- 29.Laha R, Mondal B, Biswas SK, Chand K, Das M, Sarma D, et al. Detection of Babesia bigemina infection in cattle from North-Eastern India by polymerase chain reaction and its genetic relatedness with other isolates. Trop Anim Health Prod. 2015;47:633–6. doi: 10.1007/s11250-015-0769-8. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Das Singla L, Tuli A, Kaur P, Batth BK, Javed M, et al. Molecular prevalence of Babesia bigemina and Trypanosoma evansi in dairy animals from Punjab, India, by duplex PCR: A step forward to the detection and management of concurrent latent infections. Biomed Res Int 2013. 2013 doi: 10.1155/2013/893862. 893862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh NK, Singh H, Jyoti, Haque M, Rath SS. Prevalence of parasitic infections in cattle of Ludhiana district, Punjab. J Parasit Dis. 2012;36:256–9. doi: 10.1007/s12639-012-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nair AS, Ravindran R, Lakshmanan B, Kumar SS, Tresamol PV, Saseendranath MR, et al. Haemoprotozoa of cattle in Northern Kerala, India. Trop Biomed. 2011;28:68–75. [PubMed] [Google Scholar]
- 33.Muraleedharan K, Syed Ziauddin K, Hussain PM, Pattabyatappa B, Mallikarjun GB, Seshadri SJ. Incidence of Anaplasma sp., Babesia sp. and Trypanosoma sp. in cattle of Karnataka. J Vet Parasitol. 2005;19:135–7. [Google Scholar]
- 34.Durrani AZ, Kamal N. Identification of ticks and detection of blood protozoa in friesian cattle by polmerase chain reacton test and estimation of blood parameters in district Kasur, Pakistan. Trop Anim Health Prod. 2008;40:441–7. doi: 10.1007/s11250-007-9117-y. [DOI] [PubMed] [Google Scholar]
- 35.Megat Abd Rani PA, Irwin PJ, Gatne M, Coleman GT, Traub RJ. Canine vector-borne diseases in India: A review of the literature and identification of existing knowledge gaps. Parasit Vectors. 2010;3:28. doi: 10.1186/1756-3305-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundar N, Balachandran C, Senthivelan A. Incidence of Babesia gibsoni infection in dogs in Tamil Nadu. J Vet Parasitol. 2004;18:79–80. [Google Scholar]
- 37.Chaudhuri S. Studies on Clinico-Therapeutic Aspects of Babesiosis in Dogs. In MVSC Thesis. Indian Vetrinary Research Institute. 2006 [Google Scholar]
- 38.Chaudhuri S, Varshney JP. Clinical management of babesiosis in dogs with homeopathic Crotalus horridus 200C. Homeopathy. 2007;96:90–4. doi: 10.1016/j.homp.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Senthil Kumar K, Vairamuth S, Kathiresanl D. Prevalence of Haemoprotozoans in canines in Chennai City. Tamilnadu J Vet Anim Sci. 2009;5:104–8. [Google Scholar]
- 40.Balachandran C, Sridhar R, Pazhanivel N, Anooraj R. A note on the incidence of Babesia canis in a 10 day old pup on post mortem examination in Chennai, Tamil Nadu. Indian J Anim Res. 2010;44:73–5. [Google Scholar]
- 41.Karunakaran S, Pillai UN, Sasidharan HP. Babesia gibsoni infection in a German Shepherd dog. Vet World. 2011;4:269–70. [Google Scholar]
- 42.Varshney JP, Dey S. A clinical study on haemoprotozoan infections in referral canines. J Remount Vet Corp. 1998;37:83–9. [Google Scholar]
- 43.Laha R, Bhattacharjee K, Sarmah PC, Das M, Goswami A, Sarma D, et al. Babesia infection in naturally exposed pet dogs from a North-Eastern state (Assam) of India: Detection by microscopy and polymerase chain reaction. J Parasit Dis. 2014;38:389–93. doi: 10.1007/s12639-013-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta SL, Suryanarayana C, Singh RP. Prevalence of Babesia equi infection in apparently healthy equines of Hisar by CA test. Indian J Parasitol. 1985;9:69–70. [Google Scholar]
- 45.Kumar S, Malhotra DV, Dhar S. Serodiagnosis of Babesia equi infection – A comparison of Dot-ELISA, complement fixation test and capillary tube agglutination test. Vet Parasitol. 1997;69:171–6. doi: 10.1016/s0304-4017(96)01124-7. [DOI] [PubMed] [Google Scholar]
- 46.Malhotra DV, Banerjee DP, Gautam OP. Prevalence of latent cases of Babesia equi infection in some parts of North West India as measured by the capillary agglutination test. Equine Vet J. 1978;10:24–6. doi: 10.1111/j.2042-3306.1978.tb02209.x. [DOI] [PubMed] [Google Scholar]
- 47.Sengupta PP, Virmani I, Yadav MP. Pathological changes in the donkeys experimentally infected with Babesia equi. Indian J Anim Sci. 1999;69:169–70. [Google Scholar]
- 48.Dallwitz MJ, Young AS, Mahoney DF, Sutherst RW. Comparative epidemiology of tick-borne diseases of cattle with emphasis on modelling. Int J Parasitol. 1987;17:629–37. doi: 10.1016/0020-7519(87)90140-8. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh S, Bansal GC, Gupta SC, Ray D, Khan MQ, Irshad H, et al. Status of tick distribution in Bangladesh, India and Pakistan. Parasitol Res. 2007;101(Suppl 2):S207–16. doi: 10.1007/s00436-007-0684-7. [DOI] [PubMed] [Google Scholar]
- 50.Wadhwa DR, Pal B, Mandial RK. Epidemiological and clinico-therapeutic study of babesiosis in cattle. Indian J Vet Res. 2008;17:22–4. [Google Scholar]
- 51.Shaw SE, Day MJ, Birtles RJ, Breitschwerdt EB. Tick-borne infectious diseases of dogs. Trends Parasitol. 2001;17:74–80. doi: 10.1016/s1471-4922(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 52.Stegeman JR, Birkenheuer AJ, Kruger JM, Breitschwerdt EB. Transfusion-associated Babesia gibsoni infection in a dog. J Am Vet Med Assoc. 2003;222:959–63. doi: 10.2460/javma.2003.222.959. [DOI] [PubMed] [Google Scholar]
- 53.Fukumoto S, Suzuki H, Igarashi I, Xuan X. Fatal experimental transplacental Babesia gibsoni infections in dogs. Int J Parasitol. 2005;35:1031–5. doi: 10.1016/j.ijpara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Friedhoff KT, Soulé C. An account on equine babesioses. Rev Sci Tech. 1996;15:1191–201. doi: 10.20506/rst.15.3.972. [DOI] [PubMed] [Google Scholar]
- 55.Neitz NN. Classification, transmission and biology of piroplasms of domestic animals. Ann N Y Acad Sci. 1956;64:56–111. [Google Scholar]
- 56.Ravindran R, Rao JR, Mishra AK. Detection of Babesia bigemina DNA in ticks by DNA hybridization using a nonradioactive probe generated by arbitrary PCR. Vet Parasitol. 2006;141:181–5. doi: 10.1016/j.vetpar.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira-Sequeira TC, Oliveira MC, Araujo JP, Jr, Amarante AF. PCR-based detection of Babesia bovis and Babesia bigemina in their natural host Boophilus microplus and cattle. Int J Parasitol. 2005;35:105–11. doi: 10.1016/j.ijpara.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira MC, Oliveira-Sequeira TC, Regitano LC, Alencar MM, Néo TA, Silva AM, et al. Detection of Babesia bigemina in cattle of different genetic groups and in Rhipicephalus (Boophilus) microplus tick. Vet Parasitol. 2008;155:281–6. doi: 10.1016/j.vetpar.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira MC, Oliveira-Sequeira TC, Araujo JP, Jr, Amarante AF, Oliveira HN. Babesia spp. infection in Boophilus microplus engorged females and eggs in Sao Paulo State, Brazil. Vet Parasitol. 2005;130:61–7. doi: 10.1016/j.vetpar.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Laha R, Das M, Goswami A, Singh P. A clinical case of babesiosis in a cross bred cow of Meghalaya. Indian J Anim Res. 2012;46:302–5. [Google Scholar]
- 61.Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part II. Phylogenetic analysis and evolutionary history. Vet Parasitol. 2003;114:173–94. doi: 10.1016/s0304-4017(03)00141-9. [DOI] [PubMed] [Google Scholar]
- 62.Baneth G, Kenny MJ, Tasker S, Anug Y, Shkap V, Levy A, et al. Infection with a proposed new subspecies of Babesia canis, Babesia canis subsp. presentii, in domestic cats. J Clin Microbiol. 2004;42:99–105. doi: 10.1128/JCM.42.1.99-105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: A molecular study. Vet Microbiol. 2003;93:307–17. doi: 10.1016/s0378-1135(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 64.Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2003;33:885–904. doi: 10.1016/s0195-5616(03)00039-1. viii. [DOI] [PubMed] [Google Scholar]
- 65.Zahler M, Rinder H, Zweygarth E, Fukata T, Maede Y, Schein E, et al. ‘Babesia gibsoni’ of dogs from North America and Asia belong to different species. Parasitology. 2000;120:365–9. doi: 10.1017/s0031182099005557. [DOI] [PubMed] [Google Scholar]
- 66.Duarte SC, Parente JA, Pereira M, Soares CM, Linhares GF. Phylogenetic characterization of Babesia canis vogeli in dogs in the state of Goiás, Brazil. Rev Bras Parasitol Vet. 2011;20:274–80. doi: 10.1590/s1984-29612011000400004. [DOI] [PubMed] [Google Scholar]
- 67.Uilenberg G. Babesia – A historical overview. Vet Parasitol. 2006;138:3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 68.Kundu K, Kumar S, Maurya PS, Mandal M, Ram H, Garg R, et al. PCR based identification of Babesia canis vogeli in clinically affected dogs. J Vet Parasitol. 2012;26:167–9. [Google Scholar]
- 69.Mandal M, Banerjee PS, Garg R, Ram H, Kundu K, Kumar S, et al. Genetic characterization and phylogenetic relationships based on 18S rRNA and ITS1 region of small form of canine Babesia spp. from India. Infect Genet Evol. 2014;27:325–31. doi: 10.1016/j.meegid.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 70.Schoeman JP. Canine babesiosis. Onderstepoort J Vet Res. 2009;76:59–66. [PubMed] [Google Scholar]
- 71.Ravindran R, Sreekumar C, Saravanan BC, Udaykumar M, Tewari AK, Kumar S, et al. Genetic variation among Indian isolates of Babesia bigemina. J Vet Parasitol. 2010;24:159–63. [Google Scholar]
- 72.Saravanan BC, Das S, Siju SJ, Tewari AK, Sankar M, Kataktalware MA, et al. Babesia bigemina infection in yak (Poephagus grunniens L.): Molecular detection and characterization. Vet Parasitol. 2013;194:58–64. doi: 10.1016/j.vetpar.2012.12.024. [DOI] [PubMed] [Google Scholar]


