Abstract
Objective
To assess the efficacy and safety of combination varenicline/bupropion sustained-release (SR) treatment for smokers who are unlikely to achieve abstinence using nicotine patch treatment based on an assessment of initial smoking reduction prior to the quit date.
Method
A randomized double-blind, parallel group, adaptive treatment trial identified 222 cigarette smokers showing ≤50% reduction in smoking after 1 week of nicotine patch treatment. Smokers were randomized to receive 12 weeks of varenicline/bupropion treatment vs. varenicline plus placebo. The primary outcome was continuous smoking abstinence at weeks 8–11 after the target quit date.
Results
Combination varenicline/bupropion treatment increased the abstinence rate relative to varenicline: 39.8% vs. 25.9% (odds ratio, 1.89; 95% CI, 1.07–3.35; P=0.029). Male smokers showed a greater effect of combination treatment than female smokers: 50.9% vs. 19.6% for males (odds ratio, 4.26; 95% CI, 1.73–10.49; P=0.002), and 29.3% vs. 30.6% for females (odds ratio, 0.94; 95% CI, 0.43–2.05; P=0.87). Highly dependent smokers also showed a greater effect than smokers with lower levels of dependence: 44.4% vs. 18.6% for highly dependent smokers (odds ratio, 3.51, 95%CI, 1.64–7.51, P=0.001), and 31.7% vs. 39.5% for smokers with a lower level of dependence (odds ratio, 0.71, 95% CI, 0.28–1.80, P=0.47). Both treatments were well tolerated.
Conclusions
Combination varenicline/bupropion treatment proved significantly more efficacious than varenicline alone for male smokers or for smokers with a high degree of dependence who did not show a sufficient initial response to pre-quit nicotine patch treatment.
Trial Registration
ClinicalTrials.gov identifier: NCT01303861
Keywords: Smoking cessation, Nicotine patch, Varenicline, Bupropion, Biomarkers, Adaptive treatment, Carbon monoxide
Cigarette smoking remains a leading cause of premature death and disease in the U.S. and developing countries throughout the world (1). According to recent studies, smoking is even more lethal than previously believed, with continuing smokers experiencing nearly three times the death rate of nonsmokers (2). Smoking cessation demonstrably reduces the risk of smoking-related mortality, with the greatest benefit occurring the younger a smoker is when he or she quits (3). Unfortunately, with current pharmacotherapies, which include nicotine replacement therapy, varenicline and bupropion sustained-release (SR), success rates remain low, with less than 25% of smokers remaining abstinent 1 year after treatment (4).
To increase the efficacy of smoking cessation treatment, we have developed and validated an adaptive treatment approach designed to provide each smoker with the treatment most likely to succeed, while minimizing risks and side effects. According to this strategy, smokers initially receive nicotine patch treatment starting 2 weeks before a target quit date. Although not yet FDA approved in the U.S., the initiation of nicotine replacement therapy before the quit date has been shown to be well tolerated and several studies have found it to enhance continuous smoking abstinence (5–7). Moreover, it allows the early identification of positive responders to nicotine replacement based on the extent to which ad lib smoking declines in the first week of pre-quit nicotine patch treatment. In prior studies, smokers who did not decrease their smoking by >50% in the first week showed a much lower abstinence rate after the quit date than smokers who did show this extent of smoking reduction (8, 9). Using this early marker of nicotine patch response to guide subsequent treatment, we were able to prevent approximately 10% of treatment failures among patch non-responders by modifying the treatment before the target quit date, either by switching to varenicline or augmenting nicotine replacement therapy with bupropion (9).
Given that varenicline and bupropion have different mechanisms of action, it is reasonable to hypothesize that their therapeutic effects might be additive. This rationale was supported by recent open-label investigations suggesting that combination varenicline/bupropion treatment was well tolerated and potentially highly efficacious (10, 11). Inasmuch as combination treatment with two drugs that carry “black box” FDA warnings may face significant hurdles in becoming a first line therapy, a more feasible approach may be to evaluate the usefulness of this combination treatment for smokers who are not likely to succeed using nicotine patch alone. The present study evaluated whether combination varenicline/bupropion treatment is more efficacious than varenicline alone as a rescue treatment for nicotine patch non-responders.
Method
Study Design
The study was a double-blind, parallel arm adaptive treatment trial, in which early response to nicotine patch treatment was assessed in a pre-quit phase, and subsequently nicotine patch “non-responders,” who failed to show a >50% decrease in ad lib smoking (assessed using expired air carbon monoxide (CO)) were randomly assigned to receiving either varenicline plus placebo treatment (n=109) or varenicline plus bupropion (n=113). The nicotine patch pre-quit responders were entered into a separate study to explore combination nicotine replacement therapy treatment, the results of which will be reported elsewhere.
Study Procedures
The study was approved by the Duke University Medical Center Institutional Review Board. Adult smokers expressing a desire to quit smoking were recruited through newspaper, radio and television advertisements. Those eligible were 18–65 years of age, reported smoking an average of ≥ 10 cigarettes/day for 3 cumulative years, displayed end-expired air CO ≥ 10 ppm, and did not display any exclusionary feature on history, physical exam or laboratory evaluation (see Supplemental Data). After complete description of the study, written informed consent was obtained from subjects, who were compensated up to $330 for study participation.
After screening and enrollment in the study, participants were seen at the research center weekly for 2 weeks before the quit date, and at 4 sessions held 1, 3, 7 and 11 weeks after the quit date. Brief (<15 min) support was provided at each session, and clinical trial materials were dispensed. Smoking diaries and measures of expired air CO were also collected at each session.
After the first week of pre-quit nicotine patch treatment, all patch non-responders received varenicline pills, and in addition were randomized either to taking bupropion sustained-release (SR) tablets or placebo tablets that were identical in appearance. The recommended dosing titration schedule was used for both varenicline (0.5 mg once daily on days 1–3, 0.5 mg twice daily on days 4–7, followed by 1 mg twice daily through 12 weeks) and for bupropion (150 mg daily for 3 days, followed by 150 mg twice daily through 12 weeks).
Initial nicotine patch dosing patches was based on initial expired air CO reading; participants with CO>30 ppm at baseline received 42 mg/day (two 21 mg/day patches) and the remaining participants wore a single 21 mg/day patch daily. In the 21 mg condition, an active patch was applied each morning; in the 42 mg/day dose condition, an active 21 mg patch was applied each morning and a second patch at noon (which has been found to reduce the likelihood of nausea). This personalized dosing regimen was based on previous research suggesting that heavy smokers may not receive adequate nicotine replacement with a 21 mg patch (12, 13).
Dose reductions for medications were allowed in the event of adverse effects. Adverse effect ratings were collected using 7-point rating scales.
Statistical analyses
Treatment groups were initially compared on demographic and smoking history variables, using ANOVA, in order to identify potential confounding factors.
To evaluate the hypothesis that combination varenicline/bupropion treatment would enhance abstinence rates compared to varenicline alone, logistic regression was used to compare the two treatment groups on the primary outcome of continuous abstinence at end of treatment (weeks 8–11 after the target quit date). Abstinence was assessed based on self-reports of no smoking confirmed by end-expired CO levels ≤ 10 ppm. A secondary outcome was point (7-day) abstinence at 6 months (self-reported abstinence confirmed by CO at the follow-up).
In an intent-to-treat analysis, participants who withdrew from the study or were lost to follow-up were classified as non-abstinent, consistent with prior reports suggesting that dropouts are very likely to be smoking (14). However, if not all dropouts are smoking, then this failure imputation for study dropouts might lead to reduced estimates of variance, biased conclusions, and/or elevated rates of Type I error (16). Similar problems occur with standard imputation methods that erroneously assume data are missing at random (MAR) with respect to treatment outcome. In the current instance, a high proportion of dropouts reported smoking on the session immediately prior to withdrawal, suggesting that the MAR assumption was tenuous. Accordingly, we estimated two logistic regression models to model the probabilities of dropping out and of smoking based on individual subject characteristics and smoking status at prior timepoints, carrying the associated coefficients forward into separate imputation models (SAS 9.2, PROC MI). A third set of imputation models was estimated based on procedures described by Hedeker et al. (16), using a variety of assumed odds ratios to model the probability of smoking among study dropouts. Results from these and the latter two imputations were combined into single estimates as described by Schafer (17) (SAS 9.2, PROC MIANALYZE). A sensitivity analysis was then conducted to determine how robust the conclusions were to the different assumptions underlying these various imputation regimes (including last observation carried forward (LOCF) and missing = smoking).
Demographic variables, including sex, were examined for potential moderating effects on treatment outcome. Additionally, we sought to replicate two specific findings reported in a recent article published since the completion of the present study. Ebbert et al. (15) reported that dependence and level of smoking moderated the efficacy of combined varenicline/bupropion treatment, with more highly dependent (Fagerström Test for Nicotine Dependence score>5) and heavier smokers (≥ 20 cigarettes/day) showing a greater therapeutic response to the combination treatment. The same baseline variables (and their interaction with treatment condition) were therefore included in our logistic regression models to assess their potential association with treatment outcome in the present study.
Adverse effects were tabulated according to severity and compared between treatments using chi-square tests.
Results
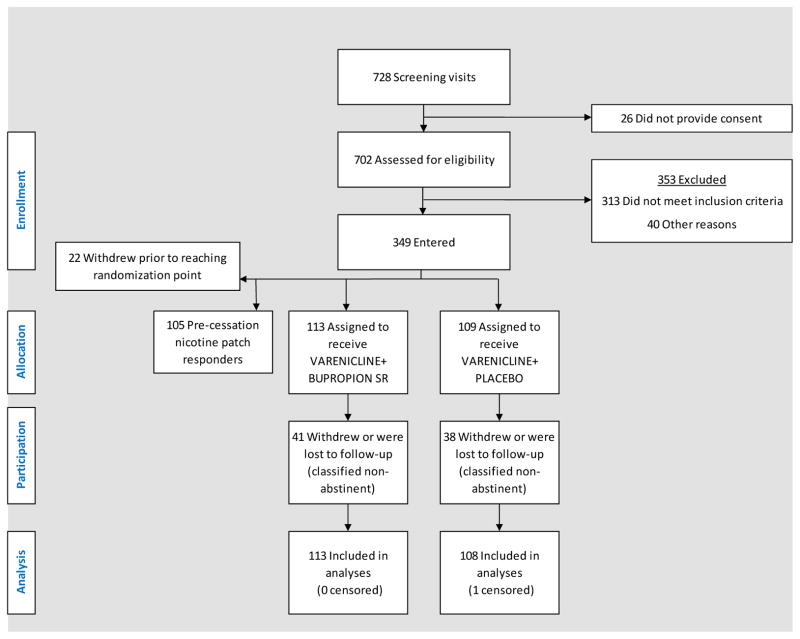
Of 728 smokers screened, 349 participants were entered into the study and 222 nicotine patch non-responders at week 1 were randomly assigned to the two rescue treatment conditions (Fig. 1). 221 of these 222 participants were included in the analyses; one participant was censored due to a positive pregnancy test reported on the first day of treatment (which was promptly discontinued). In all, 35.6% of participants dropped out after randomization; main reasons cited for discontinuation were scheduling conflict or other personal circumstance (10.4%), loss of interest (5.8%), inability to quit smoking (1.4%), and 12.2% could not be contacted. Treatment related adverse effects accounted for 7 discontinuations (3.2%).
Figure 1.
CONSORT diagram depicting participant recruitment, eligibility assessment, allocation to treatment conditions, and disposition.
Participant characteristics
The demographic characteristics and smoking histories of participants were similar across the two randomized treatment conditions (Table 1). Of the overall sample, 45.7% were men and 62.4% were White. Participants’ mean age was 44.1 years old. They smoked on average 20.7 cigarettes per day and had smoked for 26 years. On average they had made 7.2 prior quit attempts and the mean score on the Fagerström Test for Nicotine Dependence was 6.1, indicating a moderate level of dependence.
Table 1.
Baseline participant characteristics
| Varenicline | Varenicline+Bupropion | |||
|---|---|---|---|---|
| (n=108) | (n=113) | |||
| Characteristics (Continuous) | Mean | SD | Mean | SD |
| Age | 44.5 | 12.6 | 43.7 | 10.5 |
| No. of years smoked | 26.9 | 11.5 | 25.2 | 10.3 |
| No. of cigarettes/day | 20.6 | 8.8 | 20.7 | 8.5 |
| FTND Dependence Score | 6.0 | 1.8 | 6.2 | 2.0 |
| No. of prior attempts to quit | 6.8 | 12.7 | 7.5 | 14.5 |
| Expired air CO (ppm) | 24.8 | 10.8 | 24.6 | 9.6 |
| Characteristics (Categorical) | N | % | N | % |
|---|---|---|---|---|
| Men | 46 | 42.6 | 55 | 48.7 |
| Race | ||||
| White | 70 | 64.8 | 68 | 60.2 |
| Black | 32 | 29.6 | 41 | 36.3 |
| Asian | 2 | 1.9 | 0 | 0 |
| Other | 4 | 3.7 | 4 | 3.5 |
| Having CO >30 ppm | 38 | 35.2 | 35 | 31.0 |
| (42 mg patch dose wk 1) | ||||
Efficacy
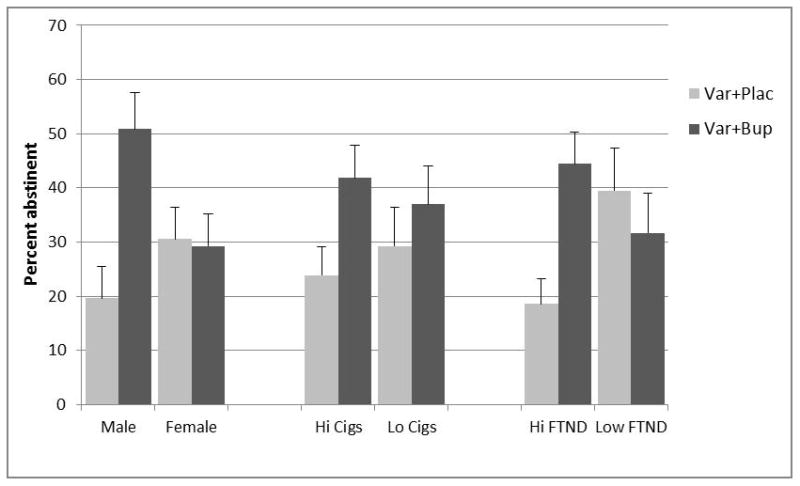
Using an intent-to-treat analysis that included dropouts as well as study completers, the primary outcome of 4-week smoking abstinence for weeks 8–11 showed a significant effect of combination varenicline/bupropion treatment relative to varenicline alone: 39.8% vs. 25.9%, (odds ratio, 1.89; 95% CI, 1.07–3.35; P=0.029). However, the therapeutic effect of the combination was confined to male smokers (Table 2), who showed an enhancement of abstinence from 19.6% to 50.9%. In female smokers there was no difference between treatment conditions: 29.3% for varenicline/bupropion vs. 30.6% for varenicline alone. In a logistic regression model including treatment, sex and their interaction, the sex by treatment interaction was significant, P=0.013.
Table 2.
Smoking Abstinence at the End of Treatment (Weeks 8–11) According to Sex, Baseline Smoking Rate and Level of Nicotine Dependence
| Sex | Treatment | n | Proportion completing treatment (n, %) | Proportion abstinent (n, %) | Odds ratio (95% CI) | P value |
|---|---|---|---|---|---|---|
| Male | Varenicline+Bupropion | 55 | 36 (65.5%) | 28 (50.9) | 4.26 (1.73–10.49) | 0.002 |
| Varenicline+Placebo | 46 | 31 (67.4%) | 9 (19.6) | |||
| Female | Varenicline+Bupropion | 58 | 36 (62.1%) | 17 (29.3) | 0.94 (0.43–2.05) | 0.87 |
| Varenicline+Placebo | 62 | 40 (64.5%) | 19 (30.6) | |||
| Baseline Smoking Rate | ||||||
| ≥20 cigs/day | Varenicline+Bupropion | 67 | 46 (68.7) | 28 (41.8) | 2.29 (1.09–4.81) | 0.03 |
| Varenicline+Placebo | 67 | 42 (62.7) | 16 (23.9) | |||
| <20 cigs/day | Varenicline+Bupropion | 46 | 26 (56.5) | 17 (37.0) | 1.42 (0.58–3.49) | 0.45 |
| Varenicline+Placebo | 41 | 29 (70.7) | 12 (29.3) | |||
| Level of Nicotine Dependence | ||||||
| High | Varenicline+Bupropion | 72 | 51 (70.8) | 32 (44.4) | 3.51 (1.64–7.51) | 0.001 |
| Varenicline+Placebo | 70 | 24 (60.0) | 13 (18.6) | |||
| Low | Varenicline+Bupropion | 41 | 21 (51.2) | 13 (31.7) | 0.71 (0.28–1.80) | 0.47 |
| Varenicline+Placebo | 38 | 29 (76.3) | 15 (39.5) |
In order to determine to what extent the sex by treatment interaction could be accounted for by other baseline demographic and smoking history variables (Table 1), these variables were examined for sex differences. None were found with the exception of number of prior quit attempts, which was higher for men than women (mean, 9.8 vs. 4.9, F(1, 217)=7.04, P=0.008). However, this variable did not account for the sex by treatment interaction, which remained significant after number of quit attempts was entered as a covariate into the logistic regression model.
Based on findings reported by Ebbert et al. (15), the interactions of treatment with baseline nicotine dependence level and cigarette consumption were examined. The dependence by treatment interaction was significant (P=0.009), with the combination treatment increasing abstinence in highly dependent smokers but not in smokers with lower levels of dependence (see Table 2).
Both of the above interactions remained significant when included in the same logistic regression model (P=0.005 for the sex by treatment interaction and P=0.003 for the dependence x treatment interaction). Both interactions were also found to be relatively robust when subjected to the above-described sensitivity analysis using multiple imputations for study dropouts (P values ranging from 0.06–0.0001).
The interaction of treatment with baseline cigarette consumption was not significant (P=0.35), but the pattern of abstinence rates was nonetheless consistent with the interaction reported by Ebbert et al. (15), inasmuch as the combination treatment was found to be more efficacious than varenicline in heavy smokers but not in lighter smokers (Table 2).
Six month follow-up
Point abstinence at 6 months showed a similar pattern of findings to abstinence at the end of treatment: interaction of treatment with sex, P=0.06; interaction of treatment with baseline cigarette consumption, P=0.43; and interaction of treatment with baseline nicotine dependence, P=0.006. The combination treatment was more efficacious than varenicline in male smokers (29.1% vs. 10.9%, simple effect odds ratio, 3.36, 95% CI, 1.12–10.06, P=0.03), heavier smokers (25.4% vs. 13.4%, simple effect odds ratio, 2.19, 95% CI, 0.90–5.35, P=0.08), and more highly dependent smokers (29.2% vs. 10.0%, simple effect odds ratio, 3.71, 95% CI, 1.46–9.41, P=0.006). No significant differences were detected for female smokers (22.4% vs. 21.0%, simple effect odds ratio, 1.09, 95% CI 0.46–2.60), lighter smokers (26.1% vs. 22.0%, simple effect odds ratio, 1.26, 95% CI, 0.47–3.38, P=0.65), and less dependent smokers (19.5% vs. 28.9%, simple effect odds ratio, 0.60, 95% CI, 0.21–1.69, P=0.33).
Safety, Tolerability and Compliance
Adverse events
A few serious adverse events occurred during the study: 1 participant in the varenicline condition (who had also been receiving lisinopril for hypertension) was hospitalized with kidney failure and subsequently discharged; in the varenicline/bupropion condition, 1 participant was involved in a non-fatal auto accident and 1 was hospitalized with an MRSA cellulitis of the leg originating from a bite. None of these adverse events could clearly be attributed to the treatments received.
Tolerability and compliance with treatments
Nicotine patches, administered in week 1, were well tolerated overall, with only 0.7% of participants requiring patch dose reductions in the 21 mg condition and 11% in the 42 mg patch condition: chi-square (df=1, N=221)=13.33, Fisher’s exact P=0.0007. In the overall sample, however, there were no significant differences in adverse effect reports between the 21 mg and 42 mg doses. Adverse effects rated more than moderate in severity and occurring with a frequency >5% included: vivid dreams (20.8%); itching/burning at the patch application sites (16.1%); insomnia (9.4%); thirst (8.8%); and dry mouth (5.2%). Based on daily diaries, 93.6% of the total patch doses were received in the 21 mg condition and 87.4% in the 42 mg condition. One participant in the 42 mg patch condition dropped out in the first week due to nausea and vomiting.
Varenicline and bupropion SR treatments were also generally well tolerated. Overall 11.5% of participants required dose reductions in the varenicline/bupropion condition and 24.8% in the varenicline condition: chi-square (df=1, N=221)=6.61, Fisher’s exact P=0.014). In the overall sample, however, there was no significant difference in the incidence of adverse effects between the varenicline/bupropion and varenicline treatment conditions. Adverse effects rated more than moderate in severity and occurring with a frequency >5% included: headache (9.3%); diaphoresis (8.8%); nasal/sinus irritation (5.9%); change in taste perception (17.2%); dry mouth (10.8%); thirst (15.7%); cough (8.8%); muscle/joint pain/aches (7.4%); heartburn (5.8%); nausea (5.8%); constipation (6.8%); irritability (11.3%); vivid dreams (18.1%); insomnia (13.7%); and anxiety (8.8%). Based on daily diaries, 77.2% of the total number of oral medication doses were taken, which did not differ significantly between conditions (78.4% in the varenicline/bupropion condition vs. 76.0% in the varenicline alone condition).
Seven participants (4 in the varenicline/bupropion condition and 3 in the varenicline condition) dropped out due to adverse events that were likely to be related to treatment (e.g., nausea, dizziness, sleep disturbances, anxiety, and depression).
Compliance and adverse effect ratings were also examined for treatment related sex differences and differences between smokers with high and low dependence. However, none accounted for the interactive effects of sex or dependence on treatment efficacy, which remained significant when each adverse effect was included as a covariate in a logistic regression model.
By the end of treatment, abstinent smokers gained more weight on average than non-abstainers (2.84 kg vs. 1.02 kg; F(1,138)=11.56, P=0.0009), with no significant sex or treatment differences. Weight gain in successful quitters was 3.05 kg for varenicline/bupropion vs. 2.5 kg for varenicline.
Discussion
The study results showed an overall benefit of adding bupropion treatment to varenicline for nicotine patch non-responders. Moreover, male smokers and more highly dependent smokers appeared to show a greater therapeutic response. For these smokers, end-of-treatment abstinence rates were more than doubled in the combination treatment condition. This difference observed at the end of treatment was sustained, although to a lesser extent, at the 6-month follow-up point. In contrast, no statistically significant difference between the two treatments was shown in female smokers or less dependent smokers, although the 95% confidence intervals for the odds ratios cannot preclude an effect in these subgroups.
Since submission of the present article for publication, Ebbert et al. (15) published a study evaluating the efficacy of combination varenicline/bupropion treatment. That study also reported a significantly higher end-of-treatment abstinence rate for combination varenicline/bupropion treatment than for varenicline alone, as well as a greater therapeutic effect in more dependent smokers. No statistically significant sex by treatment interaction was detected. However, the design of that study differed in an important respect from the current study: all subjects were randomized to the two treatments, in contrast opposed to randomizing only the nicotine patch non-responders.
What potential mechanisms might explain why male smokers who do not obtain sufficient smoking reduction during pre-quit nicotine patch treatment benefit from receiving combination varenicline/bupropion treatment? In animal models, some studies have found that females do not show the extent of nicotinic receptor upregulation that males show after chronic nicotine administration (18). Moreover, a recent brain imaging study in human smokers found that men, but not women, showed an upregulation of nicotinic receptors in the striatum, a brain region important for drug addiction and reward (19). The extent of nicotinic receptor upregulation, in turn, has recently been shown to predict smoking relapse (20). Therefore, it is conceivable that bupropion and its metabolites, acting as nicotinic receptor antagonists (21, counteract the adverse effects of nicotinic receptor upregulation in male smokers.
Additionally, sex differences have been reported in striatal dopamine D2/D3 receptor availability (22), with male smokers showing a downregulation compared with male nonsmokers or female smokers. Males have also been found to exhibit greater dopamine release to drugs of abuse (23) and may be more dependent on the dopaminergic stimulation obtained from smoking. Varenicline has been shown to influence striatal dopaminergic transmission, both by enhancing dopamine release (24) and upregulating dopamine D2/D3 receptors (25). However, varenicline, acting upon the same receptors as nicotine itself, may provide insufficient dopamine release for nicotine patch non-responders. Bupropion, in contrast, enhances dopamine transmission through a distinct mechanism, by blocking dopamine re-uptake (26). Thus, male smokers who do not respond adequately to nicotine patch treatment might benefit from the action of bupropion on dopamine transmission to a greater extent than female smokers.
The interaction of treatment with baseline nicotine dependence is difficult to attribute to the selection of nicotine patch non-responders, given that the interaction was also observed in the Ebbert et al. (15) study that included nicotine patch responders. The interaction is also difficult to explain in terms of the two component treatments, as nicotine dependence has not generally been reported as a moderating factor in studies that have evaluated varenicline or bupropion individually (28, 29). However, the index of efficacy in these studies has been the comparison of each agent to placebo rather than comparing combination treatment to varenicline alone. Mechanisms underlying the interaction of combination varenicline/bupropion treatment with dependence will require further investigation.
In addition to showing superior efficacy of combination treatment in specific subpopulations of smokers, the present results further validate an adaptive treatment model, in which smokers receive a week of pre-quit nicotine patch treatment, and based on their reduction in ad lib smoking, either remain on the patch or receive adaptive modifications of treatment. The previous randomized controlled trial in our center cited above (9) showed that approximately 10% of smokers who showed a poor initial response to pre-quit nicotine patch treatment could be rescued by adaptive changes in treatment. In that study, the rescue treatments comprised varenicline alone or augmentation of nicotine patch treatment with bupropion. The present study extends these results by showing that combination varenicline/bupropion treatment is an efficacious rescue treatment for male smokers and for highly dependent smokers.
The study design had several strengths, including the randomized, placebo-controlled design, and the use of an adaptive treatment regimen. The study also had limitations in that it did not evaluate whether varenicline/bupropion treatment might prove more efficacious than varenicline as an initial treatment for nicotine patch responders; however, our rationale was to avoid the additional risks and side effects of varenicline and bupropion for nicotine patch responders, who have a reasonable chance of quitting using nicotine replacement alone (98). Another limitation of the study was the use of a tailored dose of pre-cessation nicotine patch therapy that is not in accordance with current product labeling in the U.S. However, the FDA has recently issued more liberal guidelines regarding concurrent use of nicotine replacement by individuals who continue to smoke (30). Additionally, pre-cessation nicotine patch use is approved in several other countries.
In summary, based on the magnitude of enhancement in abstinence rate, and excellent safety/tolerability profile, the present results support the use of combination varenicline/bupropion treatment for male or highly dependent smokers who do not respond with sufficient smoking reduction during the first week of pre-quit nicotine patch. Future research should investigate potential biobehavioral mechanisms that may account for the differences in treatment response across subpopulations of smokers.
Supplementary Material
Figure 2.
Acknowledgments
Many members of the Duke Center for Smoking Cessation contributed to the successful completion of the research described in this report. In particular, the authors acknowledge the invaluable assistance of Susan Claerhout, Tanaia Loeback, Amanda Mitchell, Wendy Roberts, Al Salley, Michael Siernos, Kay Scime, and Eric Westman in supervising the conduct of this study. Statistical consultation from H. Ryan Wagner, Ph.D. was also greatly appreciated. Active bupropion sustained-release and placebo tablets were supplied by Murty Pharmaceuticals, Inc. (Lexington, KY), under contract from the National Institute on Drug Abuse.
Footnotes
Disclosure
This study was supported by grant 1P50 DA027840 from the National Institute on Drug Abuse and a grant from Philip Morris, USA. The sponsors had no role in the planning or execution of the study, data analysis or publication of results. The authors disclose consulting and patent purchase agreements with Philip Morris International for nicotine inhalation technology, and consulting agreements with Targacept, Inc. and Novartis, Inc.
References
- 1.Lando HA, Wilson K. Combating the global tobacco epidemic. Preventive medicine. 2010 Jan-Feb;50(1–2):11–2. doi: 10.1016/j.ypmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-year trends in smoking-related mortality in the United States. The New England Journal of Medicine. 2013 Jan 24;368(4):351–64. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. The New England journal of medicine. 2013 Jan 24;368(4):341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006 Jul 5;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Lindson N, Aveyard P. Response to Rose (2011): nicotine preloading: the importance of a pre-cessation reduction in smoking behavior. Psychopharmacology. 2011 Nov;218(2):459–60. doi: 10.1007/s00213-011-2351-z. [DOI] [PubMed] [Google Scholar]
- 6.Schuurmans MM, Diacon AH, van Biljon X, Bolliger CT. Effect of pre-treatment with nicotine patch on withdrawal symptoms and abstinence rates in smokers subsequently quitting with the nicotine patch: a randomized controlled trial. Addiction. 2004 May;99(5):634–40. doi: 10.1111/j.1360-0443.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- 7.Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res. 2009 Sep;11(9):1067–75. doi: 10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- 8.Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol Med. 2010 Jul-Aug;16(7–8):247–53. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. Am J Psych. 2013 doi: 10.1176/appi.ajp.2013.12070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009 Mar;11(3):234–9. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- 11.Issa JS, Abe TO, Moura S, Santos PCJL, Pereira AC. Effectiveness of coadministration of varenicline, bupropion, and serotonin reuptake inhibitors in a smoking cessation program in the real-life setting. Nicotine Tob Res. 2013 Jun;15(6):1146–50. doi: 10.1093/ntr/nts230. [DOI] [PubMed] [Google Scholar]
- 12.Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy. Percentage of replacement and smoking cessation. JAMA. 1995 Nov 1;274(17):1353–8. Epub 1995/11/01. eng. [PubMed] [Google Scholar]
- 13.Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, Hays T, Lewis SF, Baker TB. Varying nicotine patch dose and type of smoking cessation counseling. JAMA. 1995 Nov 1;274(17):1347–52. Epub 1995/11/01. eng. [PubMed] [Google Scholar]
- 14.Foulds J, Stapleton J, Hayward M, Russell MA, Feyerabend C, Fleming T, Costello J. Transdermal nicotine patches with low-intensity support to aid smoking cessation in outpatients in a general hospital. A placebo-controlled trial. Arch Fam Med. 1993;2(4):417–423. doi: 10.1001/archfami.2.4.417. [DOI] [PubMed] [Google Scholar]
- 15.Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, Hurt RD. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: A randomized trial. JAMA. 2014 Jan 8;311(2):155–63. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102:1564–73. doi: 10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 17.Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman & Hall; 1997. [Google Scholar]
- 18.Mochizuki T, Villemagne VL, Scheffel U, Dannals RF, Finley P, Zhan Y, Wagner HN, Jr, Musachio JL, et al. Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: gender differences. Synapse. 1998 Sep;30(1):116–8. doi: 10.1002/(SICI)1098-2396(199809)30:1<116::AID-SYN15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, Krishnan-Sarin S, Staley JK, Picciotto MR, O’Malley SS. Sex differences in availability of beta2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Archives of general psychiatry. 2012 Apr;69(4):418–27. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody AL, Mukhin AG, Mamoun MS, Luu T, Neary M, Liang L, Shieh J, Sugar CA, Rose JE, Mandelkern M. Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2014.138. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Molecular pharmacology. 2004 Sep;66(3):675–82. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- 22.Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012 Aug;15(7):989–94. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005 May 19;48(10):3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 25.Crunelle CL, de Wit TC, de Bruin K, Ramakers RM, van der Have F, Beekman FJ, van den Brink W, Booij J. Varenicline increases in vivo striatal dopamine D2/3 receptor binding: an ultra-high-resolution pinhole [123I]IBZM SPECT study in rats. Nuclear Med Biol. 2012 Jul;39(5):640–4. doi: 10.1016/j.nucmedbio.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. The European journal of neuroscience. 2007 May;25(10):3099–108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- 27.Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004 Nov;99(11):1462–9. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 28.Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Arch Intern Med. 2004 Sep 13;164(16):1797–803. doi: 10.1001/archinte.164.16.1797. [DOI] [PubMed] [Google Scholar]
- 29.Fagerström K, Hughes J. Varenicline in the treatment of tobacco dependence. Neuropsychiatr Dis Treat. 2008 Apr;4(2):353–63. doi: 10.2147/ndt.s927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of HealthHuman Services, FoodDrug Administration. Modifications To Labeling of Nicotine Replacement Therapy Products for over-the-counter human use. Federal Register. 2013 Apr;78(63):19,718–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.