Abstract
Basal lamina is present in many stem cell niches, but we still have a poor understanding of the role of these and other extracellular matrix components. Here we review current knowledge regarding extracellular matrix expression and function in the neural stem cell niche, focusing on the subependymal zone of the adult CNS. An increasing complexity of extracellular matrix molecules has been described, and a number of receptors expressed on the stem cells identified. Experiments perturbing the niche using genetics or cytotoxic ablation of the rapidly dividing precursors, or using explant culture models to examine specific growth factors, have been influential in showing how changes in these extracellular matrix receptors regulate neural stem cell behaviour. However the role of changes in the matrix itself remains to be determined. The answers will be important, as they will point to the molecules required to engineer niches ex-vivo so as to provide tools for regenerative neuroscience.
Introduction
The brain, like many tissues in the body, contains a population of stem cells. These cells have two defining properties; continual proliferation and the ability, by undergoing asymmetrical divisions, to both self renew and generate daughter cells committed to differentiation. These divisions provide the cells required for growth and maintenance of the CNS, with the numbers expanded by a finite number of further symmetrical divisions of the committed daughter cells prior to their final differentiation. For this reason, these daughters are called transit-amplifying precursors. While these differentiated progeny (neurones and glia in the CNS) migrate away to their final destinations, the stem cells remain fixed within specialized microenvironments that maintain them in a multipotent and undifferentiated state. These microenvironments are termed niches, and the concept of the niche remains highly influential in stem cell biology and medicine. A myriad of signals within the niche regulate stem cell behaviour including those from neighbouring cells (including the transit amplifying precursors), humoral factors and the extracellular matrix (ECM)[1,2]. However despite major advances in our understanding of signalling pathways that regulate potency[3], mechanisms to reprogram somatic cells to a pluripotent state (induced pluripotent cells or iPS cells)[4] and mechanisms by which stem cells undergo asymmetric divisions[5,6], we still have an inadequate understanding of how the niche regulates stem cell behaviour.
Much of what we do know comes from studies on Drosophila, where the gonadal stem cells reside in a niche containing a support cell (or niche cell). Here, regulation of the angle of cell cleavage during mitosis allows partitioning of fate determinants and asymmetric divisions[7,8] and/or ensures that only one of the daughter cells is held next to the niche cell by adhesive interactions and therefore exposed to short range signals produced in the niche (such as bone morphogenic protein (Bmp) receptor ligands) that inhibit activation of differentiation genes[9]. However, studies of vertebrate niches are also making an increasingly significant contribution to this field, and amongst these the best-studied niches are those in the CNS that contain neural stem cells (NSC). These form the focus of this review, which will discuss one set of signals thought to play a major role in niche signalling, the ECM. In the Drosophila ovariole germline stem cell niche ECM contributes to the short range of Bmp signalling, with type IV collagens limiting the diffusion of the ligand Dpp while heparan sulfate glycoproteins localized in the niche stabilize Dpp and enhance its signalling to the germline stem cells[9]. In vertebrate niches, however, the role of the ECM remains very poorly defined.
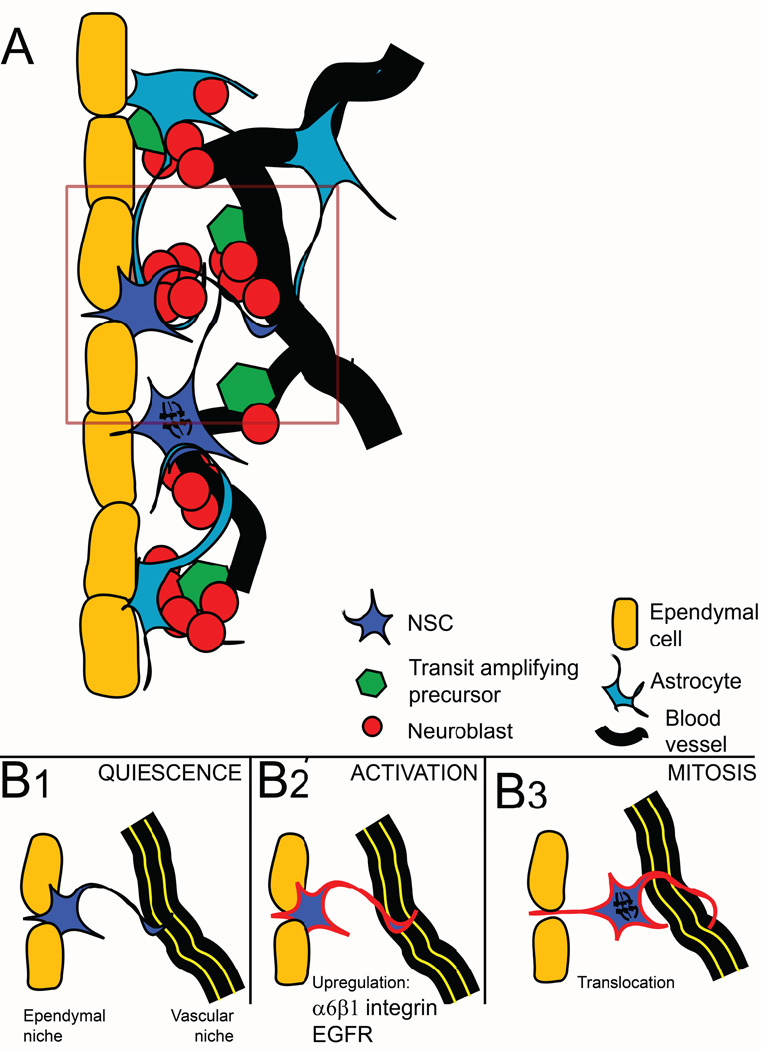
The best-described stem cell population in the adult CNS is that in the subependymal zone (SEZ) of the lateral ventricle of the rodent [10–12]. Here new neurones are generated throughout life that migrate through the rostral migratory stream (RMS) to the olfactory bulb[13]. Pioneering studies from the Alvarez-Buylla laboratory, and more recently those of Doetsch and Temple, have defined the architecture of the SEZ niche in mice in some detail[14–16](Figure 1). Stem cells are found in association with the ependymal cells that line the ventricular space of the CNS, with their apical processes protruding through the ependymal layer to contact the cerebrospinal fluid (CSF) of the ventricular space. Stem cell processes also establish direct contact with blood vessels within the SEZ – these are likely to represent the basal processes of the cell. Division of these stem cells generates a population of rapidly dividing transit amplifying cells that in turn differentiate into the neuroblasts that migrate out of the SEZ to form the olfactory neurones[17]. The close anatomical relationship of stem cells with ependymal cells and blood vessels, and the direct contact with the CSF, implicates signalling from all these sources as potential regulators of stem cell behaviour. There is also evidence that ependymal cells can generate neurones after ischaemic injury in the CNS (stroke)[18] although any such role during normal ‘homeostatic” function of the SEZ remains unproven.
Figure 1.
A) Graphic illustration of the cytoarchitecture of the subependymal zone neurogenic niche. Note that actively dividing transit amplifying precursors reside next to blood vessels, as do a fraction of neuroblasts. Quiescent NSC (in blue) are located adjacent to ependymal cells and extend a process towards the ventricle, thus receiving signals from these ependymal cells and from the CSF within the ventricular space, and only translocate near blood vessels upon mitotic activation. (Panel B) The concept of the two niches is illustrated here. NSC remain quiescent in the adult neurogenic niche, where they are positioned next to ependymal cells (B1). Upon activation, NSC express alpha6beta1 integrin, CXCR4 and EGFR (red outline in B2). This molecular machinery allows them to respond to additional signals on neighbouring blood vessels (laminins, SDF1; depicted as yellow lines on the blood vessel) and results in their translocation near the vasculature where they undergo mitosis (B3).
In the primate and human SEZ, the structure differs in that the ependymal cell layer is separated from the underlying cells by a hypocellular (gap) zone, filled with ependymal and astrocytic processes and (in the anterior-most parts) proliferating astrocytes[19,20]. Adjacent to the gap layer a ribbon of cells, including astrocytes and newborn neurones, is observed. The function of the niche however appears to be conserved, with chains of migrating cells found in the non-human primates[21,22] and in humans[23].
Adult NSC of the SEZ develop from a population of stem cells present in the embryonic telencephalon (cortex) that generate the neurones of the different cortical layers during development. These embryonic neural stem cells have a radial glial phenotype, with an apical process contacting the ventricular space and a basal process contacting the basal lamina of the overlying pial basement membrane. Initially they divide symmetrically to increase their numbers, after which they initiate self-renewing asymmetrical divisions that generate neurones via an intermediate or basal progenitor cell [24]. The great majority then undergo a final terminal symmetrical division to generate two neurones or glia, but a minority become relatively quiescent adult NSC and/or generate the ependymal cells that form the neighbours of the NSC in the adult SEZ[25].
Two other putative stem cell populations have been described in the adult CNS. First, the cells of the dentate gyrus that generate the neurones of the hippocampal granule cell layer[26]. Here, again, new neurones are born throughout life and integrate into an existing network, in this case with a potential role in learning and memory[27]. Second, a population of glial cells widespread throughout the CNS and previously described as oligodendrocyte precursors. These divide throughout life, contributing to myelin repair in white matter following demyelinating lesions[28] and also generating neurones in some parts of the CNS[29]. However, as the definition of the ECM in the microenvironment of these stem cells and the receptors they express remains poorly defined, this review will focus on the SEZ and its embryonic precursor, the ventricular zone of the developing telencephalon.
Extracellular matrix in the SEZ and embryonic telencephalic niche
As might be expected given the presence of basal lamina in the pial basement membrane or around the blood vessels of the SEZ, the best described ECM component of the niche is laminin, or more correctly the laminins. These are a family of trimeric proteins made from combinations of five alpha, three beta and three gamma subunits, with one alpha, one beta and one gamma chain making up the trimer of a specific laminin. The current nomenclature describes each laminin by its chain composition ie alpha1, beta1 and gamma1 is laminin 111[30]. Multiple alpha, beta and gamma chains have been described in the SEZ in association with the ependymal cells, the blood vessels and within the intervening parenchyma of this region[31], suggesting significant laminin trimer heterogeneity within the niche. In addition, elegant electron microscopy studies of the SEZ have revealed an unexpected but interesting complexity of the ECM in this region. Fingerlike processes of basal lamina called fractones extend from blood vessels to contact each stem cell in the niche[32]. These structures can also be seen in immunofluorescence studies as dots of laminin immunoreactivity, and such studies reveal the presence of laminin beta1 and gamma1 chains, collagen IV, nidogen and perlecan[33]. Consequently, each stem cell receives at least three different sources of laminin signals; from interstitial laminins, from their process attached to blood vessels and from the contacting fractones. Taken together with the heterogeneity of laminin expression, this generates the hypothesis that each source may contain different laminin trimers, each of which may have different functions. In keeping with this, the laminin alpha1 chain is present on blood vessels but not fractones[33], while Laminin 511 has been shown to promote epidermal and pancreatic stem cell maintenance[34,35] and alpha5 laminins used as a culture substrate are more effective than other laminins in maintaining ES cells in an undifferentiated state[36–39]. However the lack of purified laminins of each trimer combination, and the lack of trimer specific antibodies, has meant that at present the contribution of this heterogeneity to the function of the SEZ niche (or in any other stem cell niche) remains undefined. Evidence from studies of a neural stem cell line that differentiation is associated with a fall in laminin gamma1 expression[40] emphasizes just how important analysis of the functional role of this heterogeneity will be in future work.
Other ECM molecules identified in multiple studies of the SEZ include the glycoprotein tenascin C (TnC)[41–43], chondroitin sulfate proteoglycans (CSPG)[44–46], and heparan sulfate proteoglycans (HSPG)[33,47]. Here too there is potential for considerable heterogeneity, either by alternative splicing in TnC or by the addition of distinct side chains to proteoglycan core proteins. Thus, for example, in situ hybridization studies show that many different chondroitin/dermatan sulphotransferases are expressed in the adult SEZ[44]. However once again the lack of available tools at the present time to define exactly the location of each TnC or CSPG isoform limits the extent to which the biological consequences can be explored.
Two other ECM molecules have been shown to play a role in regulating the migration of newly-born neurones in the RMS after they leave the SEZ - netrin-4 and reelin. The netrins are a family of laminin-like secreted molecules known to regulate axon guidance during development, and expression by the astrocytes bordering the rostral migratory stream has been described[40]. Reelin plays an important role in controlling the positioning of newborn neurones during embryonic cortical development[48,49] and also regulates the departure of the neurones from the RMS when they reach the target olfactory bulb[50]. It is unclear whether either of these ECM molecules contributes to signaling within the SEZ niche - over-expression of reelin had no effect on neurogenesis in the SEZ even though it did increase neurogenesis in the subgranular zone of the hippocampus[51].
Extracellular matrix in the embryonic telencephalic niche
Studies of the embryonic CNS are complicated by the question of where exactly is the niche? Indeed, given that the cells are rapidly proliferating, there may be no niche in the adult sense of the word, but rather a microenvironment specialised for the rapid proliferation of NSC and the generation of neurones in the developing cortex. In the embryonic CNS, the stem cells span the entire depth of the telencephalon[24], with signals therefore likely to arise from both apical and basal surfaces of the CNS. However, the cell bodies remain close to the ventricular surface in the so-called ventricular zone (VZ) where the nucleus shuttles up and down a distance of approximately 80µm in a process termed interkinetic nuclear migration. Mitosis occurs on the ventricular surface whilst S-phase (DNA synthesis) takes place at the abventricular location. Given this, signals within the VZ are of particular interest although they will not, as explained above, be the only signals in this niche. As in the adult, many different laminin chains are expressed[52]. Interestingly the alpha1 chain that forms part of laminin 111, the classic laminin isoform associated with basal lamina, is only present at low levels while the alpha2 and 4 chains are much more highly expressed. Here again, as hypothesized for the adult SEZ, different laminins will signal from different niche components – in this case overlying basal lamina and the interstitium of the VZ. Other ECM proteins are also expressed in the VZ; TnC is abundant as in the adult SEZ[46,53] and low levels of fibronectin[54], nidogen, perlecan[55] and collagen IV[56] have also been described. In addition, high levels of proteoglycan chondroitin sulfotransferases[44], chondroitin sulfate glycosaminoglycans[57] and chondroitinsulfates[58] have been identified in the embryonic proliferative zones.
The very different behaviour of NSC within adult and embryonic niches, being quiescent in the former but rapidly proliferative in the latter, requires that the signals received in the two niches differ. Given the similarities in the ECM composition it is the expression of different receptors, rather than variation in the ECM, that is likely responsible for any contribution made by ECM signaling to these different NSC behaviours. As will be discussed next, there is evidence for this hypothesis from studies of integrin expression. However, it is important to note that the niche itself is not a static structure and the stem cells themselves could also alter the local ECM microenvironment and so regulate their own behaviour. Two such mechanisms have been described. First, proteases that cleave ECM molecules. Matrix metalloprotease 2 (MMP-2) is produced by human neuroepithelial cells, and the levels of a tissue inhibitor of this protease (TIMP-4) falls during differentiation[59]. Second, by the generation of ECM which then feeds back on stem cell behaviour. Evidence from the Drosophila ovary follicle stem cell niche shows that stem cells can regulate their proliferation via the cell autonomous production of laminins[60]. Further evidence consistent with this latter mechanism comes from studies showing that NSC of different developmental stages or commitment (oligodendrogenic versus multipotent) express different patterns of ECM components[61].
ECM receptor expression
Given the extensive evidence for laminins in the SEZ and the VZ, and in many niches elsewhere, it is unsurprising that the studies of ECM receptors in the embryonic and adult CNS have largely focused on laminin receptors; integrins, syndecans and dystroglycan. All are expressed[52], with the laminin receptor alpha6 beta1 integrin present at high levels as evidenced by colocalisation of the alpha6 and beta1 chains on embryonic NSC and on proliferating adult NSC and transit amplifying precursors[31] [16,31,40]. Interestingly, however, beta1 integrin is not expressed on quiescent adult NSC[31](Figure 2) and is upregulated when these cells activate either following depletion of daughter cell transit amplifying precursors[31] or administration of the chemokine SDF-1[62]. This suggests that changes in receptor expression, rather than in the ECM, are responsible for any changes in ECM signaling that contribute to stem cell activation. The high levels of beta1 expression seen on the embryonic NSC[52] would then be predicted, as these cells are all rapidly proliferating as they generate the many neurones required for cortical development.
Figure 2.
Quiescent NSC do not express beta1 integrin. Panel A shows a label retaining NSC in the SEZ of the adult rat. Note that the cell is still bromodeoxyuridine (BrdU) labelled 40 days after administration of the agent, and is therefore a slowly dividing stem cell rather than a rapidly dividing transit amplifying precursor cell that would dilate out the label. It is GFAP positive (a marker of adult NSC) and beta1 negative. B) Confirmation that NSC are beta1 negative is shown by FACS separation and C) neurosphere assays of beta1 high and beta1 low Sox2 expressing cells. As expected given that Sox2 is expressed in the stem and precursor cell population all neurosphere forming activity is present in the Sox2 high population from Sox2-GFP mice. A much higher level of sphere formation is then seen in the beta1 low than the beta1 high subpopulations. D) A schematic diagram of the changes in laminin receptor expression following activation of the neural stem cells by depletion of the transit amplifying precursors. Note that two receptors are upregulated; syndecan-1 and integrins. NB - Neuroblast; NSC - neural stem cell; TaP - transit amplifying precursor; E - ependymal cell; BV - blood vessel.
In the embryonic CNS, a recent report also describes beta3 integrin on the basal processes of progenitors arising from NSC in the ferret[63] – processes that, unlike those of rodent precursors, retain contact with the overlying pial basal lamina. Beta3 integrin heterodimerizes with the alphaV subunit, generating a rather promiscuous receptor binding fibronectin, vitronectin, osteopontin and other ECM molecules[64]. This is interesting, as these beta3 integrins therefore represent a different class of receptors to those discussed above that bind laminins. Multiple integrin-ECM interactions might regulate stem and precursor cell behaviour, with beta3 integrins possibly binding to fibronectin expressed in the embryonic CNS. Whether these additional integrins are only seen on the progenitor cells of higher mammals such as the ferret, where the considerable expansion of the cortex may require that the progenitors have different properties so as to generate sufficient neurons, or whether they will also be present on rodent progenitors remains to be determined.
The signaling pathways activated by these receptors remain largely undefined, despite our increasingly sophisticated understanding of the intracellular pathways that maintain pluripotency in embryonic stem (ES) cells[3]. There may however be significant differences in NSC and ES cell signaling. Inhibition of ERK signaling promotes ES cell maintenance[3]. In contrast, inhibition of ERK suppresses proliferation of early NSC [65] and neurospheres[66], while its enhancement induces proliferation of adult SEZ and hippocampus progenitors[40,67,68].
Experimental approaches to ECM function in CNS stem cell niches
A number of approaches have been taken towards establishing the function of extracellular matrix and receptors in the NSC niche: i) genetic manipulation of specific molecules ii) blocking antibodies injected in vivo iii) perturbation of the niche by drugs or cytokines and iv) cell culture studies. Each has strengths and weaknesses but together they have generated some exciting hypotheses as to the regulation of NSC by ECM.
i) Genetic manipulation of specific molecules
Two sets of integrin knockouts have revealed phenotypes in the embryonic CNS; alpha6 and beta1. These were examined as alpha6 beta1 integrin is a principal laminin receptor in the CNS and elsewhere. Alpha6 knockouts reveal areas of disrupted pial basal lamina and abnormal migration of the neurones generated by the telencephalic NSC[69]. In a more detailed examination of NSC in these mice, no abnormalities of proliferation or neurogenesis was observed despite disruption of basal process attachment[70]. The beta1 knockout studies (using a cre/lox approach to remove the integrin only in the CNS) also disrupt the basal process attachment to the overlying basal lamina. These experiments showed that detachment was followed by apoptosis of the NSC, suggesting that the integrin provides essential survival signals for the NSC[71]. The same detachment and apoptosis phenotype was seen with laminin alpha2 and alpha4 chain knockouts[71]. Perhaps surprisingly, these studies revealed that beta 1 was not required for the regulation of NSC proliferation. Two other relevant integrin knockouts have been examined – alpha3 that heterodimerises with beta1 to form a laminin/collagen receptor, and alphaV that forms a fibronectin receptor. Abnormalities of neuronal migration and axon integrity were seen in the alpha3 and alphaV knockouts respectively, but no NSC abnormalities were reported in either[72,73].
In contrast to the architectural and survival abnormalities associated with the integrin knockouts, removal of TnC affects NSC development. As shown in elegant single cell clonal studies by the Temple lab, embryonic NSC generate neurones and then glia in a predefined sequence[74]. The neuronal to glial transition is regulated, at least in part, by upregulation of the EGF receptor in response to FGF signalling[75,76]. TnC deficient animals show a delay in EGF receptor expression and a reduced ability of NSC to generate glia[77]. TnC amplifies FGF signalling, and loss of this amplification has therefore been suggested to explain the phenotype[77]. Whether amplification requires integrin signalling (as is the case for up-regulation of PDGF signalling by TnC on oligodendrocyte precursor cells[78]) or simply sequestration and concentration of the growth factor by extracellular matrix, as occurs with HSPG[79], remains to be determined.
Integrin and ECM knockouts have not yet contributed to our understanding of the adult SEZ as much as might be expected due to i) the early lethality of many of the knockouts and ii) the lack of NSC, transit amplifying precursor, neuroblast and other niche cell-specific cre lines to enable conditional knockout approaches. One exception is the integrin beta8 knockout, where loss of alphaV beta8 leads to reduced stem and/or precursor cell proliferation in the SEZ[80]. Here a paracrine role has been proposed. A principal function of alphaVbeta8 is to activate latent TGFbeta by enabling matrix-metalloprotease mediated release of the active cytokine from the latent complex[81], which could then act on the endothelial cells to stimulate the release of factors that promote stem/precursor cell proliferation[80]. The existence of such factors has been shown by studies on embryonic NSC[82]. The cell type expressing alphaVbeta8 and activating TGFbeta is likely to be astrocytes[83], but this role in the SEZ remains to be confirmed by cell type-specific knockouts.
Expression of receptor constructs by electroporation represents an alternative approach to analysis of ECM receptor function. This technique has been used extensively in the embryonic CNS and, given that techniques for electroporation in the adult CNS are now available[84], should provide powerful tools for the studies of the adult SEZ. However caution is required in interpreting what are essentially over-expression experiments, as mutant receptors may activate pathways not normally regulated by that receptor in a physiological setting. One valuable use of electroporation will be lineage tracing, enabling the effect of knockouts or other perturbations to be analysed at the level of individual cell clones in vivo, an analysis routine in studies of invertebrate stem cell biology and essential to decipher NSC niche interactions in detail.
ii) Blocking antibodies injected in vivo
A caveat in the interpretation of knockout studies is that compensatory mechanisms may hide the true role of the target. Antibody blocking experiments provide an alternative strategy and a solution to this, with acute loss of function making compensation less likely. This approach has been taken to alpha6 and beta1 integrins in both embryonic telencepalon and adult SEZ niches[16,62,85]. In the embryo, an alteration in the plane of cell cleavage was observed following intraventricular injections of beta1 blocking antibodes, with loss of NSC dividing with a cleavage plane oblique to the ventricular surface but no loss in the number of cells dividing with a plane perpendicular to the surface[85]. The significance of this is that the perpendicular plane of division splits the apical membrane between daughter cells while an oblique plane will partition the apical membrane of the cell to one or other daughter. As this apical membrane has been implicated in fate determination[86], such a change in cell cleavage could potentially alter the balance between symmetric and asymmetric divisions. Interpretation of studies such as this, however, is made more difficult by structural abnormalities also induced by the perturbation of adhesion molecule function, in this case disruption of the apical process in many of the NSC. Whilst interesting in that it reveals an unexpected role for integrins in apical process retention in the VZ, the disruption of the apical cell-cell interactions complicates analysis of any effects on cell division. A similar concern exists for the studies of blocking antibodies in the adult SEZ; while such studies have reported an increase in precursor cell proliferation and migration[16,31], the disruption of ependymal cell architecture that follows the antibody injection (Kazanis, unpublished observations) may generate unexpected secondary effects on the NSC.
iii) Perturbation of the niche by drugs or cytokines
An alternative approach to reveal the function of ECM receptors is to study their expression in association with activation of quiescent NSC. However, while many types of lesion might activate NSC (eg stroke[87]), changes in the ECM itself in response to cytokine and other signals associated with tissue damage would make interpretation difficult. These effects of tissue damage can be minimized by specific ablation of the transit amplifying precursors in the adult SEZ using the cytotoxic drug AraC. This leads to NSC activation and the rapid reconstitution of the cells of the transit amplifying and neuroblast populations of the niche[88]. This experimental technique can be used in knockout mice, as shown by studies in TnC-deficient mice showing this ECM molecule is not required for regeneration[41]. In studies using this technique to examine receptor expression during activation, analysis of beta1 integrin revealed a striking upregulation[31]. This suggests that integrin signalling is associated with activation i.e. re-entry into the cell cycle and the generation of transit amplifying precursors. This effect may be direct on the intracellular signaling pathways that promote mitosis, but an alternative mechanism is provided by a recent study examining the effect of the chemokine SDF1 on SEZ explants. As shown in Figure 1, the cell body of NSC in the SEZ are found in two locations - immediately next to the ependymal cells and next to blood vessels. As proliferating cells are located close to blood vessels, this suggests the hypothesis that there are two niches in the SEZ - one for the quiescent cells next to the ependymal cells and one for activated cells in which blood vessel derived signals are responsible for activation.
This hypothesis raises the question as to what factors regulate entry of the NSC in the “active” niche? The chemokine SDF1 plays a key role in attracting haematopoietic stem cells into their bone marrow niche[89] and, in light of this, Kokovay and colleagues[62] examined the effect of SDF1 in the SEZ. They showed that NSC cell bodies relocated from the ependymal niche to a blood vessel niche in response to this chemokine (Figure 1). Beta1 integrin was also upregulated by SDF1 and beta1 blocking antibodies prevented this movement between niches[62], arguing that one function of the integrin is to promote NSC relocation and enable NSC activation by other signals in the blood vessel niche. This relocation could be achieved either by migration or by a form of interkinetic nuclear migration, as the cell already spans the ependyma-blood vessel space with its processes[14–16].
Experiments administering cytokines have also revealed an important possible role for HSPGs localized in fractones in the adult SEZ. These proteoglycans capture biotinylated FGF-2 after injection[33]. FGF-2 is a well-described NSC mitogen[90], which suggests the hypothesis that HSPGs in fractones “present” FGF-2 to NSC and so stimulate their division. A role for HSPG in the presentation of FGF-2 is well established in other cell types and, in keeping with such a role in the fractones, HSPG-degrading enzymes abolish FGF-2 binding to the fractones[33].
iv) Cell culture studies
Cell culture is a powerful tool for establishing the role of extracellular cues in the regulation of NSC behaviour. It can be used for analysis of extracellular signals, as exemplified by recent studies showing that a netrin-4/laminin complex promotes proliferation and migration of a NSC cell line[40]. Cell culture studies have also shown that primary NSC proliferate and differentiate on laminin substrates[91], and high levels of integrins have been reported in the cell populations from such cultures[92]. However, the extent to which NSC recapitulate their developmental program in vitro is remarkable. Embryonic NSC generate the different types of cortical neurones found in each layer in the correct sequence[93,94] while adult SEZ NSC generate neurones via transit amplifying and final neurogenic divisions just as they do in vivo[95]. These results suggest that control of timing and cell fate in the divisions of NSC and their daughters is regulated by intrinsic mechanisms, and that extracellular signals from the niche ECM are not required. However it is possible that the transcription factor activity that underpins these intrinsic mechanisms also changes the secretion of ECM, which then contributes to NSC regulation by autocrine/paracrine signalling. Studies of the function of ECM on NSC differentiation in these culture systems and in other models of adult SEZ differentiation[95,96] are required to examine this possibility. The availability of recombinant laminins will enable the role of each specific laminin to be tested in isolation or in specific combinations and will increase the power of these studies.
An important culture technique is the use of neurospheres. These are 3D aggregates that grow from individual stem cells and create a mixture of stem cells, precursor cells and more differentiated cell types over multiple passages in culture. These have been used to demonstrate the existence of stem cells in CNS tissue[90] although, as primary spheres can also be derived from transit-amplifying precursors[97] it is important to show that the ability to form spheres is seen in secondary passages. Neurospheres have used to quantify the number of such stem cells and examine their response to signals in the extracellular environment[98]. They can also be used to study growth factor responses; for example, it has been shown that removal of the carbohydrate side chains from CSPGs reduces the sensitivity of the stem/precursor cells to FGF-2[99]. Their utility for more detailed cell signaling studies is however limited by the heterogeneity of the spheres, making it hard to distinguish direct effects from those mediated indirectly via astrocytes or other cell types in the sphere. Another area where spheres may be useful is in niche modeling. Histological analysis has shown that these spheres form an architecture resembling an inside out cortex[66] and the presence of stem cells and laminins around the edge suggests that they may provide an in vitro model of some aspects of the niche[100],.
Conclusions
It is clear from the above that we still have a very limited understanding of the role of ECM signalling in the niches of NSC. In this regard, the CNS is not unique; our understanding of ECM function in any stem cell niche is poor despite the long-standing recognition of the presence of basal lamina in these niches. This lack of knowledge is a significant impediment to our understanding of NSC biology, and three areas of research may offer new insights. First, techniques that enable a comprehensive analysis of the ECM components of the niche. The discovery using micro-array profiling that a ECM protein, nephronectin, is present in the epidermal niche of the hair follicle[101] illustrates the potential power of unbiased strategies towards ECM identification. While the sensitivity of current proteomic strategies and the difficulty of isolating intact niches from tissue currently prevents protein-based approaches, the rapid technical advances in this area may make this feasible in the future. Second, the use of live imaging to analyse NSC and precursor cell behaviour with respect to the ECM components of the niche. Such studies will reveal the true relationships between stem cells and the ECM, and are likely to provide new and unexpected insights. For example, studies in mouse seminiferous tubules (containing the spermatogonial stem cells) have shown that stem cell niches become reorganized as the vessels change shape[102] and are not the static structures that might be inferred from invertebrate studies. Third, analysis of matrix stiffness in the niche. It is now recognized that ECM signalling is much more complex than simply provision of ligands to cell surface receptors, and matrix stiffness is also an important instructive determinant in the stem cell microenvironment. Studies on mesenchymal stem cells show that stiff substrates promote osteogenic differentiation whilst softer substrates promote neurogenic differentiation[103]. Changes in extracellular matrix in the niche that result in a local change in the substrate stiffness may therefore have a major effect on stem cell behaviour.
Will a better understanding of the ECM signalling in the niche of the adult CNS have implications for regenerative therapies? Clearly, yes; strategies to enhance repair by endogenous stem cells or to replace lost cells by transplantation of exogenous stem cells will be improved by manipulations that mimic niche signals and so promote stem cell survival, proliferation and differentiation. Whilst recognising that integration of newly differentiated neurones and glia remains a formidable challenge, the manufacture of artificial niches that could be transplanted adjacent to regions of CNS damage represent a particularly exciting approach to future work and one that will require a much better understanding of the role of the ECM.
Acknowledgements
Work in the authors’ laboratory was supported by the National Institutes of Health– National Institute of Biomedical Imaging and Bioengineering Quantum Grant Project (1P20EB00706), the National Institute on Aging Intramural Research Program, BBSRC, MRC and the Wellcome Trust.
References
- 1.Ferraro F, Celso CL, Scadden D. Adult stem cels and their niches. Adv Exp Med Biol. 2010;695:155–168. doi: 10.1007/978-1-4419-7037-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 3.Wray J, Kalkan T, Smith AG. The ground state of pluripotency. Biochem Soc Trans. 2010;38:1027–1032. doi: 10.1042/BST0381027. [DOI] [PubMed] [Google Scholar]
- 4.Okita K, Yamanaka S. Induction of pluripotency by defined factors. Exp Cell Res. 2010;316:2565–2570. doi: 10.1016/j.yexcr.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Knoblich JA. Asymmetric cell division: Recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betschinger J, Knoblich J. Dare to be different: Asymmetric cell division in drosophila c. Elegans and vertebrates: Current Biology. 2004;14:R674–R685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: Lessons from the drosophila germline. Journal of cell science. 2005;118:665–672. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- 8.Spradling AC, Nystul T, Lighthouse D, Morris L, Fox D, Cox R, Tootle T, Frederick R, Skora A. Stem cells and their niches: Integrated units that maintain drosophila tissues. Cold Spring Harb Symp Quant Biol. 2008;73:49–57. doi: 10.1101/sqb.2008.73.023. [DOI] [PubMed] [Google Scholar]
- 9.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: A decade of discovery suggests a unified view of stem cell regulation. Developmental cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: Neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihrie RA, Alvarez-Buylla A. Lake-front property: A unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller FD, Gauthier-Fisher A. Home at last: Neural stem cell niches defined. Cell stem cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 14.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell stem cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell stem cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult svz stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell stem cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisen J. Forebrain ependymal cells are notch-dependent and generate neuroblasts and astrocytes after stroke. Nature neuroscience. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 19.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. The Journal of comparative neurology. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 20.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 21.Gil-Perotin S, Duran-Moreno M, Belzunegui S, Luquin MR, Garcia-Verdugo JM. Ultrastructure of the subventricular zone in macaca fascicularis and evidence of a mouse-like migratory stream. The Journal of comparative neurology. 2009;514:533–554. doi: 10.1002/cne.22026. [DOI] [PubMed] [Google Scholar]
- 22.Quinones-Hinojosa A, Chaichana K. The human subventricular zone: A source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 24.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. Cns-resident glial progenitor/stem cells produce schwann cells as well as oligodendrocytes during repair of cns demyelination. Cell stem cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. Pdgfra/ng2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature neuroscience. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, Eckley DM, Sasaki T, Patton B, Mattson MP, Hirschi KK, Dickinson ME, ffrench-Constant C. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: Fractones and the fibroblast/macrophage network. The Journal of comparative neurology. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 33.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird J, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem cells (Dayton, Ohio) 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 34.Paquet-Fifield S, Schluter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, Brouard N, Zannettino A, Saunders N, Thompson N, Li J, Kaur P. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. The Journal of clinical investigation. 2009;119:2795–2806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: Implications for beta-cell growth and differentiation. Diabetes, obesity & metabolism. 2008;10(Suppl 4):119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 36.Evseenko D, Schenke-Layland K, Dravid G, Zhu Y, Hao QL, Scholes J, Wang XC, Maclellan WR, Crooks GM. Identification of the critical extracellular matrix proteins that promote human embryonic stem cell assembly. Stem cells and development. 2009;18:919–928. doi: 10.1089/scd.2008.0293. [DOI] [PubMed] [Google Scholar]
- 37.Vuoristo S, Virtanen I, Takkunen M, Palgi J, Kikkawa Y, Rousselle P, Sekiguchi K, Tuuri T, Otonkoski T. Laminin isoforms in human embryonic stem cells: Synthesis, receptor usage and growth support. Journal of cellular and molecular medicine. 2009;13:2622–2633. doi: 10.1111/j.1582-4934.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki T, Futaki S, Hasegawa K, Kawasaki M, Sanzen N, Hayashi M, Kawase E, Sekiguchi K, Nakatsuji N, Suemori H. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochemical and biophysical research communications. 2008;375:27–32. doi: 10.1016/j.bbrc.2008.07.111. [DOI] [PubMed] [Google Scholar]
- 39.Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not-332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem cells (Dayton, Ohio) 2008;26:2800–2809. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- 40.Staquicini FI, Dias-Neto E, Li J, Snyder EY, Sidman RL, Pasqualini R, Arap W. Discovery of a functional protein complex of netrin-4, laminin gamma1 chain, and integrin alpha6beta1 in mouse neural stem cells. Proc Natl Acad Sci U S A. 2009;106:2903–2908. doi: 10.1073/pnas.0813286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazanis I, Belhadi A, Faissner A, Ffrench-Constant C. The adult mouse subependymal zone regenerates efficiently in the absence of tenascin-c. J Neurosci. 2007;27:13991–13996. doi: 10.1523/JNEUROSCI.3279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Chevigny A, Lemasson M, Saghatelyan A, Sibbe M, Schachner M, Lledo PM. Delayed onset of odor detection in neonatal mice lacking tenascin-c. Molecular and cellular neurosciences. 2006;32:174–186. doi: 10.1016/j.mcn.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. The Journal of comparative neurology. 2005;487:407–427. doi: 10.1002/cne.20576. [DOI] [PubMed] [Google Scholar]
- 44.Akita K, von Holst A, Furukawa Y, Mikami T, Sugahara K, Faissner A. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem cells (Dayton, Ohio) 2008;26:798–809. doi: 10.1634/stemcells.2007-0448. [DOI] [PubMed] [Google Scholar]
- 45.Thomas LB, Gates MA, Steindler DA. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 46.Gates MA, Thomas LB, Howard EM, Laywell ED, Sajin B, Faissner A, Gotz B, Silver J, Steindler DA. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. The Journal of comparative neurology. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 47.Fuxe K, Chadi G, Tinner B, Agnati LF, Pettersson R, David G. On the regional distribution of heparan sulfate proteoglycan immunoreactivity in the rat brain. Brain Res. 1994;636:131–138. doi: 10.1016/0006-8993(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 48.Frotscher M. Role for reelin in stabilizing cortical architecture. Trends Neurosci. 2010;33:407–414. doi: 10.1016/j.tins.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Gaiano N. Strange bedfellows: Reelin and notch signaling interact to regulate cell migration in the developing neocortex. Neuron. 2008;60:189–191. doi: 10.1016/j.neuron.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Hack I, Bancila M, Loulier K, Carroll P, Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nature neuroscience. 2002;5:939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- 51.Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, de Lecea L, Martinez A, Delgado-Garcia JM, Soriano E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30:4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lathia JD, Patton B, Eckley DM, Magnus T, Mughal MR, Sasaki T, Caldwell MA, Rao MS, Mattson MP, ffrench-Constant C. Patterns of laminins and integrins in the embryonic ventricular zone of the cns. The Journal of comparative neurology. 2007;505:630–643. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- 53.Gotz M, Bolz J, Joester A, Faissner A. Tenascin-c synthesis and influence on axonal growth during rat cortical development. Eur J Neurosci. 1997;9:496–506. doi: 10.1111/j.1460-9568.1997.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 54.Pearlman AL, Sheppard AM. Extracellular matrix in early cortical development. Prog Brain Res. 1996;108:117–134. [PubMed] [Google Scholar]
- 55.Giros A, Morante J, Gil-Sanz C, Fairen A, Costell M. Perlecan controls neurogenesis in the developing telencephalon. BMC developmental biology. 2007;7:29. doi: 10.1186/1471-213X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali SA, Pappas IS, Parnavelas JG. Collagen type iv promotes the differentiation of neuronal progenitors and inhibits astroglial differentiation in cortical cell cultures. Brain research. 1998;110:31–38. doi: 10.1016/s0165-3806(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 57.Sirko S, von Holst A, Wizenmann A, Gotz M, Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727–2738. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 58.von Holst A, Sirko S, Faissner A. The unique 473hd-chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci. 2006;26:4082–4094. doi: 10.1523/JNEUROSCI.0422-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frolichsthal-Schoeller P, Vescovi AL, Krekoski CA, Murphy G, Edwards DR, Forsyth P. Expression and modulation of matrix metalloproteinase-2 and tissue inhibitors of metalloproteinases in human embryonic cns stem cells. Neuroreport. 1999;10:345–351. doi: 10.1097/00001756-199902050-00025. [DOI] [PubMed] [Google Scholar]
- 60.O'Reilly AM, Lee HH, Simon MA. Integrins control the positioning and proliferation of follicle stem cells in the drosophila ovary. The Journal of cell biology. 2008;182:801–815. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin S, Sun Y, Liu Y, Khaner H, Svant S, Cai J, Xu QX, Davidson BP, Stice SL, Smith AK, Goldman SA, Reubinoff BE, Zhan M, Rao MS, Chesnut JD. Whole genome analysis of human neural stem cells derived from embryonic stem cells and stem and progenitor cells isolated from fetal tissue. Stem cells (Dayton, Ohio) 2007;25:1298–1306. doi: 10.1634/stemcells.2006-0660. [DOI] [PubMed] [Google Scholar]
- 62.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult svz lineage cells home to and leave the vascular niche via differential responses to sdf1/cxcr4 signaling. Cell stem cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. Osvz progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nature neuroscience. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 64.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 65.Li BS, Ma W, Zhang L, Barker JL, Stenger DA, Pant HC. Activation of phosphatidylinositol-3 kinase (pi-3k) and extracellular regulated kinases (erk1/2) is involved in muscarinic receptor-mediated DNA synthesis in neural progenitor cells. J Neurosci. 2001;21:1569–1579. doi: 10.1523/JNEUROSCI.21-05-01569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a mapk signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- 67.Shioda N, Han F, Morioka M, Fukunaga K. Bis(1-oxy-2-pyridinethiolato)oxovanadium(iv) enhances neurogenesis via phosphatidylinositol 3-kinase/akt and extracellular signal regulated kinase activation in the hippocampal subgranular zone after mouse focal cerebral ischemia. Neuroscience. 2008;155:876–887. doi: 10.1016/j.neuroscience.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 68.Matsumoto J, Morioka M, Hasegawa Y, Kawano T, Yoshinaga Y, Maeda T, Yano S, Kai Y, Fukunaga K, Kuratsu J. Sodium orthovanadate enhances proliferation of progenitor cells in the adult rat subventricular zone after focal cerebral ischemia. The Journal of pharmacology and experimental therapeutics. 2006;318:982–991. doi: 10.1124/jpet.106.104562. [DOI] [PubMed] [Google Scholar]
- 69.Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 70.Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Gotz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 71.Radakovits R, Barros CS, Belvindrah R, Patton B, Muller U. Regulation of radial glial survival by signals from the meninges. J Neurosci. 2009;29:7694–7705. doi: 10.1523/JNEUROSCI.5537-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 73.Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 74.Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of cns cell generation: A programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 75.Ciccolini F, Svendsen CN. Fibroblast growth factor 2 (fgf-2) promotes acquisition of epidermal growth factor (egf) responsiveness in mouse striatal precursor cells: Identification of neural precursors responding to both egf and fgf-2. J Neurosci. 1998;18:7869–7880. doi: 10.1523/JNEUROSCI.18-19-07869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lillien L, Raphael H. Bmp and fgf regulate the development of egf-responsive neural progenitor cells. Development. 2000;127:4993–5005. doi: 10.1242/dev.127.22.4993. [DOI] [PubMed] [Google Scholar]
- 77.Garcion E, Halilagic A, Faissner A, ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin c. Development. 2004;131:3423–3432. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 78.Garcion E, Faissner A, ffrench-Constant C. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-c to neural precursor proliferation and migration. Development. 2001;128:2485–2496. doi: 10.1242/dev.128.13.2485. [DOI] [PubMed] [Google Scholar]
- 79.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 80.Mobley AK, Tchaicha JH, Shin J, Hossain MG, McCarty JH. Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. Journal of cell science. 2009;122:1842–1851. doi: 10.1242/jcs.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through mt1-mmp-dependent activation of tgf-beta1. The Journal of cell biology. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 83.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: An angiogenic control switch. The American journal of pathology. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Vry J, Martinez-Martinez P, Losen M, Temel Y, Steckler T, Steinbusch HW, De Baets MH, Prickaerts J. In vivo electroporation of the central nervous system: A non-viral approach for targeted gene delivery. Progress in neurobiology. 2010;92:227–244. doi: 10.1016/j.pneurobio.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Loulier K, Lathia JD, Marthiens V, Relucio J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, Patton B, Colognato H, Rao MS, Mattson MP, Haydar TF, Ffrench-Constant C. Beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS biology. 2009;7:e1000176. doi: 10.1371/journal.pbio.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 87.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends in molecular medicine. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 91.Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol. 2001;19:475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- 92.Hall PE, Lathia JD, Caldwell MA, Ffrench-Constant C. Laminin enhances the growth of human neural stem cells in defined culture media. BMC neuroscience. 2008;9:71. doi: 10.1186/1471-2202-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, Gaillard A, Vanderhaeghen P. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 94.Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nature neuroscience. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 95.Costa MR, Ortega F, Brill MS, Beckervordersandforth R, Petrone C, Schroeder T, Gotz M, Berninger B. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development. 2011;138:1057–1068. doi: 10.1242/dev.061663. [DOI] [PubMed] [Google Scholar]
- 96.Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 97.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell stem cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hall PE, Lathia JD, Caldwell MA, Ffrench-Constant C. Laminin enhances the growth of human neural stem cells in defined culture media. BMC neuroscience. 2008;9:71. doi: 10.1186/1471-2202-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sirko S, von Holst A, Weber A, Wizenmann A, Theocharidis U, Gotz M, Faissner A. Chondroitin sulfates are required for fibroblast growth factor-2-dependent proliferation and maintenance in neural stem cells and for epidermal growth factor-dependent migration of their progeny. Stem cells (Dayton, Ohio) 2010;28:775–787. doi: 10.1002/stem.309. [DOI] [PubMed] [Google Scholar]
- 100.Campos LS. Neurospheres: Insights into neural stem cell biology. Journal of neuroscience research. 2004;78:761–769. doi: 10.1002/jnr.20333. [DOI] [PubMed] [Google Scholar]
- 101.Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 103.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]