Abstract
Background
Type 2 diabetes is commonly characterized by insulin deficiency and decreased sensitivity of insulin receptors, leading to a chronic state of hyperglycemia in individuals. Disease progression induces changes in the immune profile that engenders a chronic inflammatory condition. Thiazolidinedione (TDZ) drugs, such as Pioglitazone (Pio), aid in controlling disease symptoms. While the mechanisms by which Pio controls hyperglycemia are beginning to be understood, relatively little is known about the effects of Pio on suppression of the systemic immune phenotype, attributed to visceral adipose tissue and macrophages.
Methods
Here, we utilize the recently developed BBDZR/Wor type 2 diabetes rat model to test our hypothesis that a selective in vivo growth of CD3+T cells in the spleen contributes to the increase in T lymphocytes, including Tregs, independent of visceral adipose tissue. We investigated the systemic effects of Pio on multifactorial aspects of the disease-induced immune phenotype both in vivo and in vitro in normal, non-diabetic animals and in disease.
Results
Our work revealed that Pio reversed the lymphopenic status of diabetic rats, in part by an increase in CD3+ T lymphocytes and related subsets. Moreover, we found evidence that Pio caused a selective growth of newly differentiated T lymphocytes, based on the presence of recent thymic emigrants in vivo. To investigate effects of Pio on the inflammatory milieu, we examined the production of the signature cytokines TNF-α and IL-1β and found they were reduced by Pio-treatment, while the levels of IL-4, an anti-inflammatory mediator, were significantly increased in a Pio-dependent manner. The increase in IL-4 production, although historically attributed to macrophages from visceral adipose tissue under other conditions, came also from CD3+ T lymphocytes from the spleen, suggesting splenocytes contribute to the Pio-induced shift towards an anti-inflammatory phenotype.
Conclusions
We show for the first time that Pio treatment significantly suppresses the systemic inflammatory status in the BBDZR/Wor type 2 diabetes rat model by the selective growth of newly differentiated CD3+ T cells and by increasing CD3+IL-4 production in immigrant spleen lymphocytes.
Keywords: Pioglitazone, Type 2 diabetes, Immunomodulation, Recent thymic emigrants
Background
Due to a persistent rise in incidence, type 2 diabetes affects 29.1 million Americans, or 9.3 % of the US population, and the disease ranks as the 7th leading cause of death in 2010 [1–3]. In 2012, diabetes and its complications accounted for over $245 billion in direct medical costs and indirect expenses related to wages and lost work. Type 2 diabetes represents 90 % of the total cases diabetes mellitus nationwide, and current treatment options range from exercise and diet modification to pharmaceutical interventions that augment insulin sensitivity and alleviate hyperglycemia [1]. While many of the pathological consequences of type 2 diabetes are related to chronic hyperglycemia, the onset and progression of this disease also leads to a dysregulation of the immune response, resulting in chronic inflammation [4]. Therefore, in addition to combating the effects of glucose toxicity, effective treatments must concurrently mitigate the activation of inflammatory mediators in order to prevent immune-mediated pathology [4].
Thiazolidinediones (TZDs) are a common class of drugs prescribed to patients with type 2 diabetes. TZDs, such as rosiglitazone and pioglitazone, are used to lower systemic glucose concentration by insulin sensitivity [5, 6]. These compounds function as peroxisome proliferator-activated receptor gamma (PPARγ) agonists [7]. Activation of PPARγ in tandem with the retinoid X receptor modulates regulatory effects on inflammatory target genes [8, 9]. The cumulative result is decreased insulin resistance [10–13], modification of adipocyte differentiation [11, 12, 14, 15], decreased leptin levels [16], and decreased mean arterial pressure [13].
The present study aimed to assess the mechanisms responsible for systemic effects of Pioglitazone (Pio) on the immunoregulation of lymphocytes and monocytes. For these studies, we analyzed secondary immune organs in the recently developed BBZDR/Wor type 2 diabetes rat model, thus allowing us to examine effects of Pio on altered systemic immune function associated with the disease status. Past studies have examined anti-inflammatory mechanisms of PPARγ agonists, such as Pio and Ciglitazone [17–19], and report down-regulation of macrophages [20, 21] and modulation of T cell activation [22–24]. However, most of these studies focus on analyses of the macrophage and regulatory T cell population in adipose tissue and not in immune organs such as the spleen and liver. Here, we demonstrate a selection for in vivo growth of T lymphocytes, indicated by an increase in recent thymic emigrants, as a result of Pio treatment in BBDZR/Wor rats. Furthermore, to investigate extra-splenic contributions, we studied the in vitro effects of Pioglitazone in normal, non-diabetic, splenocytes and demonstrate an increase in T cells, including regulatory T cells, and in monocytes, independent of the presence of visceral adipose tissue.
Methods
Animals
Male obese and lean (non-diabetic) littermates BBZDR/Wor rats were purchased from Biomedical Research Models (Worchester, MA). Obese: diabetic model; lean littermates: non-diabetic, control group. Any obese rat that did not show glucose levels >250 mg/dL were excluded from the study. Five rats from each group received a daily intraperitoneal (i.p., 1 mL final volume) injection of 25 mg/kg Pioglitazone or vehicle (5 % DMSO) for 2 months, as described in [19], followed by sacrifice for analyses. All animal experiments were approved by The University of Tennessee Health Science Center Institutional Animal Care and Use Committee.
Glucose measurements
Rats underwent glucose measurements by glucose strip readings, as we previously described [25]. Rats glucose levels >250 mg/dL were considered diabetic [19].
Tissue culture experiments
Splenocytes and hepatocytes were isolated by physical disruption using the back end of a syringe on a 70 μM nylon strainer. Cell suspension was washed and Red Blood Cell (RBC) contaminants were eliminated using RBC Lysis Buffer (BioLegend, San Diego, CA, USA) following manufacturer’s instructions. Cells (5.0 × 105 cells/mL) were cultured in a 24-well plate in RPMI/10 % FBS/1 % Pen/Strep media. In vitro conditions included ±25 μM Pioglitazone (Pio; Sigma-Aldrich, St. Louis, MO, USA) for 24 h. Ex vivo cell activation was done using Phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) at a concentration of 100 ng/mL for 6 h. Golgi Stop® was added (1 μL per 1 mL of culture) for the last 4 h of incubation prior to harvest to inhibit cytokine transport.
Surface molecule labeling
Cell suspensions were washed in Staining Buffer (PBS/1 % FBS) prior to labeling. Cells were labeled for 30 min protected from light on ice bucket. Once labeled cells were washed multiple times with Staining Buffer and analyzed immediately. The following anti-rat antibodies were used: anti-CD3 AF-647, anti-CD4 PE-Cy7, anti-CD11b/c APC, anti-CD25 FITC, and anti-CD31 Biotin/Streptavidin Brilliant Violet 421 (SA-bv421), all from BioLegend (San Diego, CA, USA). For each antibody we used the respective Immunoglobulin G (IgG) isotype control. In addition, we used single label control Abc™ Capture Beads (Life Technologies). Data acquired in BD Biosciences LSRII Flow Cytometer and analyses were done using FlowJo vX.10.0.6 software (Tree Star, OR).
Intracellular assay
Following cell surface labeling, cells were fixed using eBioscience FoxP3 Fixation/Permeabilization Buffer (eBiosciences), followed by intracellular labeling for 30 min with either anti-rat FoxP3 PE or isotype control PE. Cells were washed multiple times prior acquisition and analysis.
Western blot
Cells were lysed in 25 μL of Lysis Buffer as previously described [26]. Protein concentrations were determined using Pierce™ BCA Protein Assay Kit (Thermo Scientific). A total of 30 μg of denatured protein was used for each sample loaded in a Novex® 4–12 % Tris–Glycine Gel (Life Technologies). Membrane was blocked in 20 mL of 5 % BSA in TBS-Tween 20 and incubated overnight at 4 °C in 1:1000 PPARγ rabbit monoclonal antibody (Cell Signaling Technologies) followed by 1:1000 anti-rabbit secondary antibody solution for 1 h at RT. Once completed, the membrane was washed and probed for β-actin as control (Cell Signaling Technologies). SuperSignal West Pico Chemiluminiscent Substrate (Thermo Scientific) was used. Densitometry analysis was done using Kodak Molecular Imager.
Statistics
Mann–Whitney U tests were used for Western Blot data and student t tests were used for flow cytometry analysis. Data was analyzed using GraphPad software (GraphPad Software, Inc., La Jolla, CA, USA). Values of p < 0.05 were considered significant.
Results
Pio eliminates hyperglycemia without a reduction in adipose tissue
Recently, we provided evidence that Pio improved retinal insulin signaling in the BBZDR/Wor type 2 diabetes rat model in a PPARγ-dependent manner [19]. Pio reduced TNF-α and SOCS3-activated insulin resistance pathways in the retina and protected against diabetic retinopathy [19]. As a next step, we wanted to assess the systemic immunomodulatory effects of Pio on BBZDR/Wor rats under the same treatment conditions.
First, we confirmed the efficacy of Pio in reducing hyperglycemia in this model. Ten obese diabetic BBZDR/Wor rats and ten lean non-diabetic littermates were randomized into treatment or control, yielding four groups (diabetic, diabetic + Pio, non-diabetic, non-diabetic + Pio). For the five rats in each of the two treatment groups, 25 mg/kg of Pio was injected intraperitoneally each day for 2 months. Figure 1a shows a dramatic reduction in blood glucose levels in the diabetic rats after Pio treatment (p < 0.05). Consistent with established mechanisms of Pio action, glucose reduction was not associated with loss of body mass (diabetic versus non diabetic: 383 ± 14.8 versus 105.3 ± 20.8; p = 0.0002; diabetic + Pio versus diabetic: 93.4 ± 28.1 versus 383 ± 14.8; p = 0.0140). Moreover, Pio-treated rats gained weight compared to untreated diabetic rats (Fig. 1b, diabetic versus non diabetic: 623 ± 12.3 versus 457 ± 29.7; p = 0.0002; diabetic + Pio versus diabetic: 820.8 ± 75.9 versus 623 ± 12.3; p = 0.0018). These results confirm that the effect of Pio on glucose normalization and the immunophenotype is independent of loss of adipose tissue.
Fig. 1.

Pioglitazone eliminates hyperglycemia and mitigates lymphopenia in BBZDR/Wor diabetic rats. a, b Pioglitazone-dependent reduction in glucose levels is independent of weight. BBDZR/Wor diabetic and non-diabetic (littermates) rats were injected daily intraperitoneally with either 25 mg/kg pioglitazone or vehicle (5 % DMSO) for 2 months. Each group had 5 rats (a *p < 0.05, # p < 0.0002; b # p = 0.0002, *p = 0.0018). c Pioglitazone restores diabetic-induced lymphopenic status. Splenocytes from all four groups were examined for the percentage of CD3+CD4+ T cells using rat anti-CD3 Alexa Fluor 647 and anti-CD4 PE-Cy7 antibodies. Upper representative pseudocolor plot of 1 rat per condition. Lower quantitation of the flow cytometry analysis (n = 3; *p = 0.0087, # p = 0.0043). d In vivo increase in percentage of T lymphocytes is partly attributed to increase in recent thymic emigrants. Gated CD3+ T cells were examined for CD4 and CD31 immunoreactivity by using rat anti-CD4 PE-Cy7 and rat anti-CD31 Biotin/Streptavidin bv421, respectively. Upper representative plot of 1 rat per condition. Lower quantitation analysis (n = 3; # p < 0.005, *p = 0.0011)
Pio mitigates lymphopenia in BBZDR/Wor rats
In addition to hyperglycemia and obesity, diabetic BBZDR/Wor rats have reduced numbers of circulating T lymphocytes compared to their non-diabetic lean littermates. Using splenocytes as an indicator of the systemic immunophenotype, we next determined differences in the relative levels of CD3+ and CD3+CD4+ splenocyte subtypes between the groups. As shown in Table 1 and Fig. 1c, the percentage of CD3+ and CD3+CD4+ T cells among splenocytes is lower in the untreated diabetic rats compared to untreated non-diabetic lean controls [CD3+: 10.7 % ± 3.0 versus 86.4 % ± 2.8, respectively, p = 0.0087; CD3+CD4+: 11.0 % ± 8.4 versus 53.1 % ± 10, respectively, p = 0.0043; (mean ± SD)]. Treatment of non-diabetic rats with Pio had no significant effect on the percentage of CD3+ T cells (86.2 % ± 0.5, p = 0.4474) and a marginally, but not significant increase on the percentage of CD3+CD4+ T cells (p = 0.05). However, treatment of the diabetic rats with Pio significantly increased the percentage of both CD3+ (64.3 % ± 3.3 versus 10.7 % ± 3.0, p = 0.0135) and CD3+CD4+ T cells (27.3 % ± 3.9 versus 11.0 % ± 8.4, p = 0.0302). This increase reversed the lymphopenia observed in the diabetic obese rats compared to their non-diabetic littermates.
Table 1.
Splenocyte immunophenotypes after treatment with Pioglitazone in vitro and in vivo
| In vitro | In vivo | |||||
|---|---|---|---|---|---|---|
| Non-diab. | Non-diab. + Pio | Non-diab. | Non-diab. + Pio | Diabetic | Diabetic + Pio | |
| CD3+ | 14.4 % ± 0.4 | 17.8 % ± 1.0* | 86.4 % ± 2.8 | 86.2 % ± 0.5 | 10.7 % ± 3.0* | 64.3 % ± 3.3* |
| CD3+CD4+ | 12.7 % ± 0.7 | 3.7 % ± 0.3# | 53.1 % ± 10 | 50.0 % ± 8.8 | 11 % ± 8.4# | 27.3 % ± 3.9# |
| CD3+CD4+CD25+ | 6.6 % ± 0.4 | 33.1 % ± 3.4¶ | 12.4 % ± 1.8 | 19.0 % ± 0.5 | 4.6 % ± 2.1¶ | 36.2 % ± 7.1¶ |
Splenocytes were labeled with rat anti-CD3 AF-647, anti-CD4 PE-Cy7, and anti-CD25 FITC. Results are % ± SD, n = 3
In vitro non-diabetic + Pio versus non-diabetic: * p = 0.0085, # p = 0.0006, ¶ p = 0.0022
In vivo diabetic versus non-diabetic (1): * p = 0.0087, # p = 0.0043, ¶ p = 0.0040
Diabetic + Pio versus Diabetic (2): * p = 0.0135, # p = 0.0302, ¶ p = 0.0178
In vivo increase of T lymphocytes by Pio correlates with increase in recent thymic emigrants
To investigate the origin of this Pio-stimulated population of lymphocytes, we labeled splenocytes in each group with anti-CD31 monoclonal antibody. Positivity of CD31 among CD3+CD4+ T lymphocytes is a marker of recent thymic emigration (reviewed in [27]). Figure 1d demonstrates a significant increase in the percentage of CD3+CD4+CD31+ splenocytes after Pio treatment in both diabetic (13.2 % ± 1.0 versus 34.2 % ± 2.3, p = 0.0011) and non-diabetic rats (34.1 % ± 2.6 versus 47.4 % ± 0.6, p < 0.005). These results suggest a Pio-dependent amelioration of lymphopenic status in the BBZDR/Wor diabetic rats due, at least in part, to lymphocyte emigration from the thymus after Pio treatment.
In vivo decrease in pro-inflammatory cytokines (TNF-a and IL-1β) and increase in anti-inflammatory cytockine, IL-4, after Pio treatment
As has been extensively shown and reviewed [28–34], states of obesity and hyperglycemia upregulate pro-inflammatory cytokines both locally and systemically. To confirm that the diabetic BBZDR/Wor rats exhibit these pathologic changes, we investigated the percentage of CD3+ splenocytes producing the pro-inflammatory cytokines TNF-ɑ and IL-1β. As expected, diabetic rats showed an increase in the percentage of CD3+TNF-ɑ-producing cells compared to the non-diabetic (11.7 % ± 1.2 versus 4.4 % ± 1.3, p < 0.005, Fig. 2a). Furthermore, our data suggests splenic lymphocytes contribute, at least part, to the pro-inflammatory cytokine milieu. Our results suggest that at least part of that action, in the case of Pioglitazone, is due to reduction of the CD3+ T cells that produce TNF-α and IL-1β. Diabetic rats treated with Pio had a lower percentage of CD3+TNF-α-producers (1.8 % ± 1.7 versus 11.7 % ± 1.2 in controls; p < 0.05, Fig. 2a). Similarly, CD3+IL-1β-producers were reduced from 5.0 % ± 1.4 to 1.3 % ± 1.6 (p < 0.05, Fig. 2b).
Fig. 2.
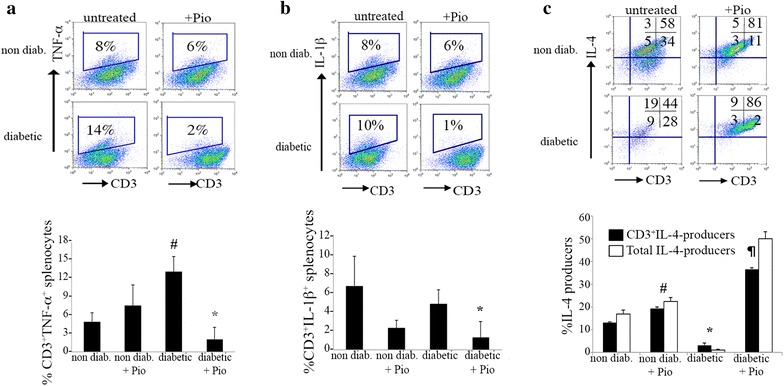
Reduced inflammatory phenotype in pio-treated diabetic BBDZR/Wor rats. Splenocytes from all four groups were cultured for 6 h ex vivo in the presence of Phorbol 12-myristate 13 acetate (PMA) at a concentration of 100 ng/mL. Golgi Plug was added for the last 4 h of treatment to inhibit protein transport. Cells were harvested and analyzed for different cytokines. Gates were established by using respective anti-cytokine isotype control. Upper panels are representative from n = 3 and lower panels (bar graphs) represent % ± SD. a Results for CD3+TNF-α-producers (# p < 0.005, p < 0.05); b CD3+IL-1β-producers (* p < 0.05); and c CD3+IL-4 and total IL-4 producers (# p < 0.0005, *p < 0.005, ¶ p < 0.05)
Next, we addressed if the reduction in pro-inflammatory mediators was accompanied by an increase in anti-inflammatory cytokines, such as IL-4. First, we show BBZDR/Wor diabetic rats have significantly diminished levels of CD3+IL-4+ splenocytes compared to non-diabetics (1.1 % ± 0.1 versus 13.5 % ± 1.3, respectively p < 0.005, Fig. 2c). As expected, there was a shift towards an anti-inflammatory phenotype with a significant increase in CD3+IL-4-producers in the spleen in both the non-diabetic and the diabetic treated groups (non-diabetic: 13.5 % ± 1.3 to 22.2 % ± 1.9, p < 0.0005; diabetic: 1.1 % ± 0.1 to 30.4 % ± 15, p < 0.05; Fig. 2c). Previous reports showed the source of IL-4 production was from a macrophage population within adipose tissue, termed M2 [35, 36]. To investigate the relative contribution of the CD3+ T cell population to the total IL-4 production, we analyze for the total IL-4-producer cells, comprising both CD3+ and CD3neg cells. Our results revealed that CD3+ T cells are large contributors, in both the non diabetic and diabetic groups, as shown in Fig. 2c. CD3+ contribution to the IL-4-producers in the non-diabetic + Pio group was 43.9 versus 80.2 % in the diabetic + Pio group. Together, these results implicate CD3+ splenocytes as contributors to the loss of anti-inflammatory signaling associated with diabetes. Furthermore, Pio treatment is able to recover critical regulatory balance in the immunophenotype.
Pio reduces CD11b/c+ population in BBZDR/Wor rats
Recent literature has placed a significant emphasis on macrophages, particularly those in adipose tissue, as drivers of obesity-related inflammation and subsequent insulin resistance [37, 38]. Specifically, there is a decrease in anti-inflammatory M2 macrophages and a reciprocal increase in pro-inflammatory M1 macrophages. These M1 macrophages are defined as CD11c+, which have been shown to increase in adipose tissue with obesity [39]. We labeled Pio-treated and untreated splenocytes with an anti-CD11b/c antibody that reacts with a common epitope within the CR3 complement receptor (C3bi) shared by CD11b and CD11c. This epitope is non-exclusive, as it is present in monocytes, dendritic cells, macrophages, and other immune cells. The percentage of splenocytes expressing this marker was higher in diabetic rats compared to non-diabetic littermates (19 versus 8 %; 2.4-fold increase, Fig. 3a). After Pio treatment, the percentage of CD11b/c+ splenocytes decreased to levels comparable to non-diabetic controls (diabetic: 10 %, non-diabetic: 9 %). Interestingly, Pio treatment did not significantly affect levels of this marker among treated and untreated non-diabetic rats (9 and 8 %, respectively). Next, we analyzed these CD11b/c+ cells by examination of CD4 positivity. Although the percentage of CD11b/c+ cells was higher in diabetic rats, a much smaller percentage of these cells were CD4+ (23 % in diabetic versus 41 % in non-diabetic, Fig. 3b). Pio treatment partially reversed this imbalance by increasing the percentage of CD4+ among the CD11b/c+ population in the diabetic rats from 23 to 33 %.
Fig. 3.

Pioglitazone alters CD11b/c+ population in splenocytes of BBZDR/Wor rats. a Splenocytes from all groups were labeled with rat anti-CD11b/c APC. b Gated population in panel a examined for expression of CD4. Figures are representative of 2-pooled splenocytes per condition
Reduction of CD4+ T lymphocytes, increase in regulatory T cells (CD3+CD4+CD25+FoxP3+) and modulation of CD11b/c+ cells after Pio treatment in vitro
After establishing that Pio treatment of BBZDR/Wor diabetic rats mitigates several aspects of the pro-inflammatory immunophenotype, we investigated which specific effects were mediated by extra-splenic mechanisms and which were due to direct effects by splenocytes. Splenocytes from non-diabetic rats were cultured in vitro for 24 h in the presence or absence of 25 μM Pio. Western blot analysis showed no statistical difference (p = 0.35; n = 3) in the levels of PPARγ protein relative to β-actin in splenocytes cultured with or without Pio (Fig. 4a, left). For comparison, we cultured hepatocytes from the same rats under the same conditions. Figure 4a, right demonstrates that levels of PPARγ protein levels in hepatocytes treated with Pio although higher, are not significant (p = 0.05, n = 3).
Fig. 4.
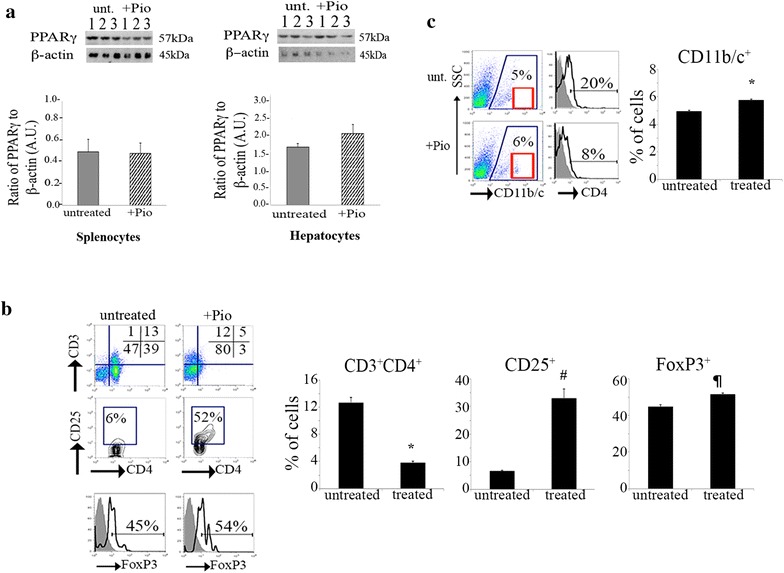
In vitro effects of Pioglitazone treatment in non-diabetic splenocytes. a Left, Similar levels of PPARγ in splenocytes with and without Pioglitazone treatment. Right, Increase in PPARγ levels in Pio-treated hepatocytes compared to untreated. Splenocytes or hepatocytes were cultured in the presence or absence of Pioglitazone 25 μM for 24 h followed by cell lysis treatment for Western blot analysis. Results are shown as bar graphs, dark gray represent untreated, white with black stripes represent Pio-treated. Graphs show the ratio of PPARγ to β-actin as arbitrary units (A.U.); n = 3. Upper part shows blot results in presence (+Pio) or absence (untreated) of treatment. b Top panels show the percentage of CD3+ and CD3+CD4+ T lymphocytes in untreated and Pio-treated splenocytes; n = 3. Cultured splenocytes were harvested and surface labeled with anti-CD3 Alexa Fluor 647, -CD4 PE-Cy7 and -CD25 FITC antibodies prior fixation and permeabilization for intracellular FoxP3-PE labeling. Mid panel shows percentage of CD3+CD4+CD25+ T cells followed by FoxP3 analysis of the gated population. Histogram shows percentage of FoxP3+ cells from the CD3+CD4+CD25+ gate; n = 3. Gray histogram depicts isotype control; black line depicts anti-rat FoxP3 labeling. Figures are representation of n = 3. At the right there is quantitation of each panel in bar graph: *p = 0.0006, # p = 0.002, ¶ p = 0.0237
Next, we examined the percentage of total CD3+ T lymphocytes in splenocytes cultured in the presence of Pio. As we found in vivo, the percentage of CD3+ cells present in splenocyte cultures increased after treatment (17.8 % ± 1.0 treated versus 14.4 ± 0.4 control, p = 0.0085) (shown in Table 1 and Fig. 4b). Furthermore, there was a prominent down-regulation of the CD4 co-receptor among CD3+ cells, as 3.7 % ± 0.3 of Pio-treated splenocytes exhibited a CD3+CD4+ phenotype compared to 12.4 % ± 0.6 in untreated cells (p = 0.0006). These results suggests that the in vivo increase in CD3+CD4+ cells in the diabetic rat spleens was in large part due to recent thymic emigration after Pio treatment. Additionally, Pio treatment exerted significant effects without upregulating PPARγ protein levels. We further characterized the CD3+CD4+ subset to evaluate the percentage of CD3+CD4+ T lymphocytes immunoreactive against CD25. Upregulation of the IL-2Rα chain, CD25, is associated with an activated and immunoregulatory phenotype. We hypothesized that CD25 expression would be increased and partially responsible for the anti-inflammatory transition after Pio treatment. Figure 4b shows the percentage of CD3+CD4+CD25+ T lymphocytes in untreated versus Pio-treated cells. In the untreated samples, an average of 6.6 % ± 0.4 of CD3+CD4+ cells co-expressed CD25 (Table 1). However, Pio-treated splenocytes showed an increased percentage of CD3+CD4+CD25+ T cells: 33.1 % ± 3.4 (p = 0.0022). To further explore this immunomodulation we monitored the percentage of cells that were FoxP3 positive, a well-established marker for immunoregulatory phenotype associated with relative immunosuppression [40–42]. While both groups showed a large population of CD3+CD4+CD25+FoxP3+ cells, this subpopulation increased from 44.7 % ± 2.5 to 51.5 % ± 2.1 (p = 0.0237) after Pio treatment. Collectively, this data suggests treatment with Pio favors an anti-inflammatory phenotype.
Lastly, we determined the effects of Pio on other immune populations by labeling Pio-treated and untreated splenocytes with anti-CD11b/c as before. Figure 4c shows a significant increase in the percentage of cells with a CD11b/c+ phenotype in untreated versus Pio-treated cells (4.92 % ± 0.09 versus 5.73 % ± 0.11, respectively; p = 0.0007).
Discussion
Two decades ago, Lehmann et al. [6], identified thiazolidinedione (TZD) derivatives as potential anti-diabetic agents in animal models of type 2 diabetes. These drugs exhibit high affinity for the peroxisome proliferator-activated receptor gamma (PPARγ) ligand. PPARγ belongs to a family of nuclear receptors that play central roles in the regulation of metabolic homeostasis, including modulation of lipids, glucose, and bone remodeling [43–45]. This class of receptor is considered a regulator of adipose tissue inflammation and its activation increases the expression of adiponectin enhancing insulin sensitivity. Pioglitazone (Pio) is a PPARγ agonist used for the treatment of type 2 diabetes [46, 47]. While Pio has extensively been studied in resident cells within adipose tissue, the effects of this drug are poorly understood in secondary immune organs, such as the spleen and liver. Our work demonstrates that Pio may reverse some of the type 2 diabetes-associated pathogenesis by a de novo increase of CD3+CD4+CD31+ T lymphocytes, with a concomitant reduction of pro-inflammatory mediators (TNF-α, IL-1β) and augmentation of anti-inflammatory cytokines (IL-4) in the BBDZR/Wor type 2 diabetes rat model.
Obesity, implicated in the etiology of type 2 diabetes, causes a chronic low-grade systemic inflammation contributing to metabolic dysfunction (reviewed in [48]). Clinically, type 2 diabetes is evidenced by a rise in blood glucose due to faulty insulin-dependent signaling mechanisms. Pio-dependent glucose normalization did not require weight loss in the BBZDR/Wor type 2 diabetes rat model (Fig. 1a, b), suggesting that PPARγ regulates additional mechanisms besides adipose tissue and body weight. The increase in adipose tissue observed in type 2 diabetes has led to focus investigations of the pro-inflammatory mediators associated with visceral adipose tissue (VAT). Two major cell populations have been investigated in the past, VAT regulatory T cells [49–53] and macrophages [23, 35, 48, 54–56]. First, Cipolleta et al., recently showed regulatory T cells expressing PPARγ suppress inflammation and regulates insulin resistance [49, 50]. Clark and colleagues [57] demonstrated Ciglitazone, another PPARγ agonist, enhances regulatory T cells in vitro. Accordingly, our work shows upregulation of CD25 in Pio-treated splenocytes in vitro. A large portion of the CD25+ cells were positive for the FoxP3 transcription factor, considered a master regulator of natural regulatory T cells [40–42]. We and others, have previously shown enhancement of the FOXP3 transcription factor in human cells correlates with increases in suppressive capacity of regulatory cells [58, 59]. Thus we speculate this population makes a major contribution to dampening of inflammation. We also speculate that the remaining FoxP3neg population exhibits an activated or induced regulatory T cell phenotype.
A number of reports have established that monocytes recruited to adipose tissue become resident macrophages [60, 61]. Macrophages accumulate in adipose tissue in both obese patients and rodents [35]. This prompted the studies to establish the linkage between an inflammatory phenotype characterized by increased levels of TNF-α and other pro-inflammatory cytokines and insulin resistance. Resident macrophages in adipose tissue of lean rodents showed an anti-inflammatory phenotype, or M2, whereas those of obese rodents showed a pro-inflammatory, or M1, phenotype. M1 macrophages displayed high levels of CD11c in their surface. While the mechanisms responsible for recruitment and establishment of subtypes of macrophages in VAT in diabetes are beginning to be understood, there is relatively little understanding of comparable mechanisms in other immune organs. Similar to the VAT findings, we observed a higher percentage of CD11b/c+ cells in splenocytes from diabetic rats compared to non-diabetics (Fig. 3a). This population decreased in the Pio-treated rats. Moreover, the population was not altered in the Pio-treated non-diabetic rats. These results suggests that diabetes-dependent inflammation causes an increase in the CD11b/c+ in splenocytes and that Pio-treatment reduces this population to baseline levels, as evidenced by the lack of effects in the untreated and treated non-diabetic rats.
Much of the pro-inflammatory cytokine milieu is attributed to the M1 population in VAT [39, 54, 62]. Our work revealed that non-VAT components are also contributors to the inflammation. We found CD3+ T lymphocytes in diabetic rats produced higher levels of TNF-α and IL-1β, both pro-inflammatory mediators, compared to non-diabetic counterparts (Fig. 2a, b). Both pro-inflamatory cytokines were significantly reduced in splenocytes of Pio-treated rats, suggesting that Pio suppresses cell immune responses in non-adipose tissue. Of particular note is the reduction in IL-1β, as this cytokine is implicated in the destruction of pancreatic β-cells [63, 64]. Reduction of pro-inflammatory cytokines may not be sufficient to control the disease. This may also be accompanied by an increase in anti-inflammatory cytokines, such as IL-4. Classically, IL-4 serves as an immunoregulatory cytokine and as a mitogen for the proliferation of cells with an anti-inflammatory phenotype [65–67]. It is important to determine the source of the IL-4 production. Our work revealed that in the spleen, CD3+ T lymphocytes, not CD3neg cells, were a major source of IL-4 (Fig. 2c). Together, this work suggests splenic components are contributors to the control of inflammation in Pio-treated diabetic rats.
Using our BBZDR/Wor rat model, we found Pio treatment in vivo caused an increase in recent thymic emigrants, which may contribute to the increase in percentage of total T lymphocytes, resulting in alleviation of lymphopenia observed in untreated BBZDR/Wor rats. Previous work in type 1, not type 2, diabetes rat models showed reduction of recent thymic emigrants is due, in part to defective intrathymic selection leading to autoimmunity [68]. Subsequent reports by Ramanathan and colleagues [69] confirmed these results. Rodent models of obesity based on mutations in leptin-coding gene (Lepob/Lepob) have shown thymic involution that reduces T cell responses and the number of T cell emigrants to the periphery [70]. This suggests that there is a compound effect of diabetes coupled with obesity. Here, we show first, type 2 diabetic BBZDR/Wor rats have reduced frequency of recent thymic emigrants and second, a Pio-dependent increase in recent thymic emigrants in diabetic rats, independent of their body weight. Our work suggests the effects are Pio-dependent as we observed an increase in recent thymic emigrants in non diabetic rats with normal blood glucose levels. Still, we cannot rule out there might be compound effects by the reduction in blood glucose in addition to Pio-treatment, as shown in the diabetic + Pio group. Recent work suggests Pio and other TZDs can act in PPARγ dependent and independent manner (reviewed in [71]). This new data provides an additional complexity level to our study and others as it demonstrated reduction in the pro-inflammatory milieu could happen independent of PPARγ signaling in the presence of Pio and other TZDs. Our work shows the presence of PPARγ in the rats hepatocytes and splenocytes. Interestingly, our study opens new investigations to integrate multiple signaling pathways that could facilitate type 2 diabetes therapeutics. Some provocative scientific concerns are if other blood glucose lowering drugs will exhibit similar in vivo results; and how does insulin receptor signaling is affected by Pio. There is a need for more biochemical studies to understand the basis of Pio’s actions.
Our investigation suggests there is promising potential for immune modulation therapy in type 2 diabetes using the TZD-derivative, Pioglitazone. More work is required to elucidate how the PPARγ-signaling pathway, via Pio, interacts with multiple cellular pathways, including surface receptors, signals driving proliferative and suppressive responses, and transcriptional regulators. Future studies aimed at investigating how to improve immune surveillance and control the complications of type 2 diabetes could provide new strategies for improving the quality of life of diabetic patients.
Authors’ contributions
BTG performed experiments, interpreted data, wrote part of the manuscript; RPL contributed to data interpretation and wrote part of the manuscript; JY performed experiments; JJS contributed to study design and provided financial support; VMT contributed to study design, performed experiments, interpret data, provided financial support and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Authors would like to thank members of Dr. Jena Steinle Lab for helpful discussions and Dr. Dianna Johnson for helpful comments.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests. VMT is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Bradley T. Gao, Email: bgao4@uthsc.edu
Ryan P. Lee, Email: rlee39@uthsc.edu
Youde Jiang, Email: Youde.jiang@wayne.edu.
Jena J. Steinle, Email: jsteinle@med.wayne.edu
Vanessa M. Morales-Tirado, Email: Vmorale1@uthsc.edu
References
- 1.Association AD. Diabetes statistics: data from the 2012 National Diabetes Fact Sheet., Alexandria, Virginia. 2012. http://www.diabetes.org/diabetes-basics/diabetes-statistics/. Accessed 1 Dec 2014.
- 2.Adebayo O, Willis GC. The changing face of diabetes in America. Emerg Med Clin North Am. 2014;32(2):319–327. doi: 10.1016/j.emc.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Organization. WH. World Health Organization: 10 facts about diabetes. 2011.
- 4.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 5.Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diab Rep. 2013;13(3):329–341. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 7.Sinha B, Ghosal S. Pioglitazone—do we really need it to manage type 2 diabetes? Diabetes Metab Syndr. 2013;7(4):243–246. doi: 10.1016/j.dsx.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Waltenberger B, Pferschy-Wenzig EM, Blunder M, Liu X, Malainer C, et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): a review. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann C, Lorenz K, Braithwaite SS, Colca JR, Palazuk BJ, Hotamisligil GS, et al. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994;134(1):264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda H, Taketomi S, Sugiyama Y, Shimura Y, Sohda T, Meguro K, et al. Effects of pioglitazone on glucose and lipid metabolism in normal and insulin resistant animals. Arzneimittelforschung. 1990;40(2 Pt 1):156–162. [PubMed] [Google Scholar]
- 11.Sugiyama Y, Shimura Y, Ikeda H. Effects of pioglitazone on hepatic and peripheral insulin resistance in Wistar fatty rats. Arzneimittelforschung. 1990;40(4):436–440. [PubMed] [Google Scholar]
- 12.Sugiyama Y, Taketomi S, Shimura Y, Ikeda H, Fujita T. Effects of pioglitazone on glucose and lipid metabolism in Wistar fatty rats. Arzneimittelforschung. 1990;40(3):263–267. [PubMed] [Google Scholar]
- 13.Kemnitz JW, Elson DF, Roecker EB, Baum ST, Bergman RN, Meglasson MD. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes. 1994;43(2):204–211. doi: 10.2337/diab.43.2.204. [DOI] [PubMed] [Google Scholar]
- 14.Hallakou S, Doare L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, et al. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46(9):1393–1399. doi: 10.2337/diabetes.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Yasuno T, Kojo H, Hirosumi J, Mutoh S, Notsu Y. Alteration in expression profiles of a series of diabetes-related genes in db/db mice following treatment with thiazolidinediones. Jpn J Pharmacol. 2000;84(2):113–123. doi: 10.1254/jjp.84.113. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto M, Tsuneyama K, Fujimoto T, Selmi C, Gershwin ME, Shimada Y. Spirulina improves non-alcoholic steatohepatitis, visceral fat macrophage aggregation, and serum leptin in a mouse model of metabolic syndrome. Dig Liver Dis. 2012;44(9):767–774. doi: 10.1016/j.dld.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Spencer M, Yang L, Adu A, Finlin BS, Zhu B, Shipp LR, et al. Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLoS One. 2014;9(7):e102190. doi: 10.1371/journal.pone.0102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchiyama M, Shimizu A, Masuda Y, Nagasaka S, Fukuda Y, Takahashi H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol Vision. 2013;19:2135–2150. [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Thakran S, Bheemreddy R, Ye EA, He H, Walker RJ, et al. Pioglitazone normalizes insulin signaling in the diabetic rat retina through reduction in tumor necrosis factor alpha and suppressor of cytokine signaling 3. J Biol Chem. 2014;289(38):26395–26405. doi: 10.1074/jbc.M114.583880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchimura K, Nakamuta M, Enjoji M, Irie T, Sugimoto R, Muta T, et al. Activation of retinoic X receptor and peroxisome proliferator-activated receptor-gamma inhibits nitric oxide and tumor necrosis factor-alpha production in rat Kupffer cells. Hepatology. 2001;33(1):91–99. doi: 10.1053/jhep.2001.21145. [DOI] [PubMed] [Google Scholar]
- 21.Majai G, Sarang Z, Csomos K, Zahuczky G, Fesus L. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol. 2007;37(5):1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa H, Takano H, Zou Y, Qin Y, Hizukuri K, Odaka K, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance. J Mol Cell Cardiol. 2005;38(2):257–265. doi: 10.1016/j.yjmcc.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Thorp E, Kuriakose G, Shah YM, Gonzalez FJ, Tabas I. Pioglitazone increases macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions of nondiabetic low-density lipoprotein receptor-null mice. Circulation. 2007;116(19):2182–2190. doi: 10.1161/CIRCULATIONAHA.107.698852. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W, Berthier CC, Lewis EE, McCune WJ, Kretzler M, Kaplan MJ. The peroxisome-proliferator activated receptor-gamma agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus. Clin Immunol. 2013;149(1):119–132. doi: 10.1016/j.clim.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, Zhang Q, Steinle JJ. Intravitreal injection of IGFBP-3 restores normal insulin signaling in diabetic rat retina. PLoS One. 2014;9(4):e93788. doi: 10.1371/journal.pone.0093788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker RJ, Steinle JJ. Role of beta-adrenergic receptors in inflammatory marker expression in Muller cells. Invest Ophthalmol Vis Sci. 2007;48(11):5276–5281. doi: 10.1167/iovs.07-0129. [DOI] [PubMed] [Google Scholar]
- 27.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113(4):769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 28.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13(3):435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 29.Imai Y, Dobrian AD, Weaver JR, Butcher MJ, Cole BK, Galkina EV, et al. Interaction between cytokines and inflammatory cells in islet dysfunction, insulin resistance and vascular disease. Diabetes Obes Metab. 2013;15(Suppl 3):117–129. doi: 10.1111/dom.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Carvalho Vidigal F, Guedes Cocate P, Goncalves Pereira L, De Cassia Goncalves Alfenas R. The role of hyperglycemia in the induction of oxidative stress and inflammatory process. Nutr Hosp. 2012;27(5):1391–1398. doi: 10.3305/nh.2012.27.5.5917. [DOI] [PubMed] [Google Scholar]
- 31.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 32.Pereira FO, Frode TS, Medeiros YS. Evaluation of tumour necrosis factor alpha, interleukin-2 soluble receptor, nitric oxide metabolites, and lipids as inflammatory markers in type 2 diabetes mellitus. Mediators Inflamm. 2006;2006(1):39062. doi: 10.1155/MI/2006/39062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bano G. Glucose homeostasis, obesity and diabetes. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):715–726. doi: 10.1016/j.bpobgyn.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 39.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 + CD25 + regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 41.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 42.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 43.Subramani PA, Reddy MC, Narala VR. The need for physiologically relevant peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands. Endocr Metab Immune Disord Drug Targets. 2013;13(2):175–183. doi: 10.2174/18715303113139990003. [DOI] [PubMed] [Google Scholar]
- 44.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferroni P, Della-Morte D, Pileggi A, Riondino S, Rundek T, Ricordi C, et al. Pleiotropic effects of PPARgamma agonist on hemostatic activation in type 2 diabetes mellitus. Curr Vasc Pharmacol. 2013;11(3):338–351. doi: 10.2174/1570161111311030008. [DOI] [PubMed] [Google Scholar]
- 46.Hofmann CA, Colca JR. New oral thiazolidinedione antidiabetic agents act as insulin sensitizers. Diabetes Care. 1992;15(8):1075–1078. doi: 10.2337/diacare.15.8.1075. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann CA, Edwards CW, 3rd, Hillman RM, Colca JR. Treatment of insulin-resistant mice with the oral antidiabetic agent pioglitazone: evaluation of liver GLUT2 and phosphoenolpyruvate carboxykinase expression. Endocrinology. 1992;130(2):735–740. doi: 10.1210/endo.130.2.1733721. [DOI] [PubMed] [Google Scholar]
- 48.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Cipolletta D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology. 2014;142(4):517–525. doi: 10.1111/imm.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486(7404):549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARgamma effects. Proc Natl Acad Sci USA. 2015;112(2):482–487. doi: 10.1073/pnas.1423486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+ CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23(6):431–437. doi: 10.1016/j.smim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011;6(1):e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol. 2014;5:470. doi: 10.3389/fimmu.2014.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagy ZS, Czimmerer Z, Szanto A, Nagy L. Pro-inflammatory cytokines negatively regulate PPARgamma mediated gene expression in both human and murine macrophages via multiple mechanisms. Immunobiology. 2013;218(11):1336–1344. doi: 10.1016/j.imbio.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms. J Immunol. 2007;178(7):4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 58.Morales-Tirado V, Wichlan DG, Leimig TE, Street SE, Kasow KA, Riberdy JM. 1alpha,25-dihydroxyvitamin D3 (vitamin D3) catalyzes suppressive activity on human natural regulatory T cells, uniquely modulates cell cycle progression, and augments FOXP3. Clin Immunol. 2011;138(2):212–221. doi: 10.1016/j.clim.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasow KA, Morales-Tirado VM, Wichlan D, Shurtleff SA, Abraham A, Persons DA, et al. Therapeutic in vivo selection of thymic-derived natural T regulatory cells following non-myeloablative hematopoietic stem cell transplant for IPEX. Clin Immunol. 2011;141(2):169–176. doi: 10.1016/j.clim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53(5):1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 61.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 62.Haase J, Weyer U, Immig K, Kloting N, Bluher M, Eilers J, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57(3):562–571. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 63.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110(6):851–860. doi: 10.1172/JCI200215318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wachlin G, Augstein P, Schroder D, Kuttler B, Kloting I, Heinke P, et al. IL-1beta, IFN-gamma and TNF-alpha increase vulnerability of pancreatic beta cells to autoimmune destruction. J Autoimmun. 2003;20(4):303–312. doi: 10.1016/S0896-8411(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 65.Severinson E, Naito T, Tokumoto H, Fukushima D, Hirano A, Hama K, et al. Interleukin 4 (IgG1 induction factor): a multifunctional lymphokine acting also on T cells. Eur J Immunol. 1987;17(1):67–72. doi: 10.1002/eji.1830170112. [DOI] [PubMed] [Google Scholar]
- 66.Fowell DJ, Magram J, Turck CW, Killeen N, Locksley RM. Impaired Th2 subset development in the absence of CD4. Immunity. 1997;6(5):559–569. doi: 10.1016/S1074-7613(00)80344-1. [DOI] [PubMed] [Google Scholar]
- 67.Mosmann TR, Coffman RL. Two types of mouse helper T-cell clone Implications for immune regulation. Immunol Today. 1987;8(7–8):223–227. doi: 10.1016/0167-5699(87)90171-X. [DOI] [PubMed] [Google Scholar]
- 68.Zadeh HH, Greiner DL, Wu DY, Tausche F, Goldschneider I. Abnormalities in the export and fate of recent thymic emigrants in diabetes-prone BB/W rats. Autoimmunity. 1996;24(1):35–46. doi: 10.3109/08916939608995355. [DOI] [PubMed] [Google Scholar]
- 69.Poussier P, Ning T, Murphy T, Dabrowski D, Ramanathan S. Impaired post-thymic development of regulatory CD4+ 25+ T cells contributes to diabetes pathogenesis in BB rats. J Immunol. 2005;174(7):4081–4089. doi: 10.4049/jimmunol.174.7.4081. [DOI] [PubMed] [Google Scholar]
- 70.Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol. 2006;177(1):169–176. doi: 10.4049/jimmunol.177.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, et al. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70(2):177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]


