Abstract
Traditional Tibetan medicine (TTM) is an old traditional medical system, which is an effective and natural method of improving physical and mental health, and has been widely spread in the western part of China for centuries. Halenia elliptica (H. elliptica) D. Don, known as “Jiadiranguo” (Tibetan medicine name) is one of the most important herbal medicine in TTM that is from the genus Halenia (family: Gentianaceae). The whole herb can be used as a medicine to treat hepatobiliary diseases and xeransis, and possesses many biological and pharmacological activities including heat clearing, bile benefiting, liver soothing, digestion promoting, blood nursing, detoxification activities, and so on. In modern research, H. elliptica can be used to treat acute or chronic hepatitis, especially hepatitis B. In addition, the chemical compounds of the herb have potent antihepatitis B virus (anti-HBV) activity in vitro. As an important TTM, further studies on H. elliptica can lead to the development of new drugs and therapeutics for various diseases, and more attention should be paid on the aspects of how to utilize it better.
Keywords: Chemistry, ethnopharmacology, Halenia elliptica, pharmacology
INTRODUCTION
As a well-known traditional medicine, Halenia elliptica (H. elliptica) D. Don (family: Gentianaceae) is customarily used to treat hepatitis and cholecystitis, which is widely distributed across the Qinghai-Tibetan Plateau and the western part of China. It has been officially recorded in “Tibetan medicine standards” since 1995 and more than 100 prescriptions containing H. elliptica have been used to treat all kinds of diseases in Tibet and Qinghai.[1] Modern pharmacological studies have shown that H. elliptica and its active constituents have a wide range of pharmacological properties including hepatoprotective, anticancer, cardiovascular, and antidiabetic activities, most of which seriously matched with its traditional uses. Meanwhile, it was also served as tea for the people in the Qinghai-Tibetan Plateau.[2]
In this review, the advances in ethnopharmacological, phytochemical, biological, and pharmacological activities; and the toxicology of H. elliptica will be revealed, and the increasing data supports further exploration of H. elliptica and its active constituents.
Botany and ethnopharmacology
Botany
According to the description of the flora of China, H. elliptica is about 15-90 cm tall. The stems are erect, subquadrangular, striate, simple, or branched. The petiole of the basal leaves are flattened and 1-3 cm in length. The stem leaves are sessile or have a short petiolate; the leaf blades are oblong, elliptic, ovate-lanceolate, or ovate with a dimension of 1.5-7 cm × 0.5-3.5 cm. The number of leaf veins is five. The calyx lobes are elliptic to ovate in shape and the apex is acuminate. The corolla is campanulate, varies from blue to purple in color,1-2.5 cm in length, basal spurs are 5-14 mm, lobes elliptic to ovate, apex obtuse and apiculate [Figure 1]. According to the size of the basal spurs, two varieties of species were separated from H. elliptica, namely, H. elliptica var. elliptica and H. elliptica var. grandiflora. However, due to their similar appearance they are called by the same name, “Jiadiranguo” in traditional Tibetan medicine (TTM).
Figure 1.

The habitat and its flower of Halenia elliptica D. Don
Resource distribution
There are about 100 species of plants of Halenia genus around the world, mainly distributed in Northern Hemisphere and South America. In China, there are two species including H. elliptica and H. Corniculata (L.) Cornaz, found in asymmetric geographical distribution. H. elliptica has a huge reserve and is widely distributed over Tibet, Sichuan, Yunnan, Guizhou, Qinghai, Xinjiang, Hunan, and Hubei while H. Corniculata (L.) Cornaz is mainly distributed in North China such as Inner Mongolia, Hebei, Liaoning, Jilin, and Heilongjiang.[3] Plants of Halenia genus grow in regions with altitudes between 800 and 4600 meters above sea level, mostly in the mountains having an altitude of 1200-3500 m and seldom in areas with elevation higher than 3500m. They mainly inhabit in hillside meadows, roadsides, and thickets, and even in waterside or arbor forests.[4]
Ethnopharmacology
H. elliptica was first recorded in Tibetan Medical Thangka of The Four Medical Tantras, a classic Tibetan medical book.[5] It was also documented in the Jing Zhu Ben Cao that “Jiadiranguo” grows in waterside marshes with iron chopstick-like stems, glaucous leaves, and slightly curly base. The herbs are bitter in taste and can be used in the treatment of pathopyretic ulcer.[6] The Yue Wang Yao Zhen recorded that it can clear liver heat and gallbladder heat.[7] The Gan Lu Ben Cao Ming Jing stated that it tastes bitter and acrid, and is a cool-natured herb with the ability to clear pulse heat and treat blood and gallbladder diseases.[8] The Zang Yao Pei Fang Xin Bian recorded that it is a bitter, cool-natured herb, and effective in treating gallbladder heat, headache, stomach heat, and liver heat. H. elliptica has also been used in Mongolian medicine for a long time, which is called “Huridige” and belongs to Rosette saxifrage. Tu Jian accounted that “Huridige” grows in the shade of forest, possesses round and thick leaves, short stems clustered as trotters, and light yellow flowers in closed form. It is a bitter, cool-natured herb, neutral in nature, and effective in clearing heat.[9]
PHYTOCHMISTRY RESEARCH
More than 30 compounds have been isolated and identified from the whole plant of H. elliptica, which abounds with xanthones and xanthone glycosides, chromones as well as flavonoids, secoiridoid glycosides, and triterpenoid alkaloids.[10] Some of them display many bioactivities in vivo or in vitro; and the different chemical compositions of H. elliptica serve its different pharmacological activities.
Xanthones and xanthone glycosides
The main chemical components of H. elliptica are xanthones and xanthone glycosides. Sun isolated five kinds of free liposoluble xanthones of H. elliptica D. Don plants such as 1,7-dihydroxy-2,3,4,5-tetramethoxyxanthone (I), 1,5-dihydroxy-2,3,7-trimethoxyxanthone (II), 1,2-dihydr-oxy-3,4,5-trimethoxyxanthone (III), 1,5-dihydroxy-2,3-dimethoxyxanthone (IV), 1,7-dihydroxy-2,3-dimethoxyxanthone (V) were obtained.[11] Three new xanthone dual glucosides, 1-O-[β-D-xylopyranose-(1-6)-β-D-glucopyranose]-2,3,5,7-tetramethoxyxanthone (VI), 1-O-[β-D-xylopyranose-(1-6)-β-D-glucopyranose]-2,3,5-trimethoxyxanthone (VII), and 1-O-[β-D-xylopyranose-(1-6)-β-D-glucopyranose]-2,3,4,5-tetramethoxyxanthone (VIII) were obtained from a water extract of H. elliptica D. Don herbs.[4] VI and VII, named haleniaside and demethoxyhaleniaside, respectively, are the two main active components of antihepatitis. Xanthone-O-glucosides isolated from H. elliptica D. Don plants by Dhasmana were identified as 2,3,7-trimethoxyxanthone-1-O-glucoside (IX) and 2,3,5-trimethoxyxanthone-1-O-glucoside (X).[12,13] Shi got access to two xanthones, 1-hydroxy-2,3,7-trimethoxyxanthone and 1-hydroxy-2,3,4,7-tetramethoxyxanthone.[14] For the first time, Zhang isolated 1-hydroxy-3,7,8-trimethoxyxanthone (XIII) and 1,7-dihydroxy-3,8-dimethoxyxanthone (XIV) from H. elliptica D. Don plants.[15] Gao got 1,7-dihydroxy-2,3,5-trimethoxyxanthone (XV) from the ethanol extract of H. elliptica D. Don plants;[16] and another new xanthone, 1,5-dihydroxy-2,3,4-trimethoxyxanthone, was isolated in 2011 (XVI).[17,18,19,20,21] The structures of xanthones and xanthone glycosides listed above are shown in Figure 2.
Figure 2.
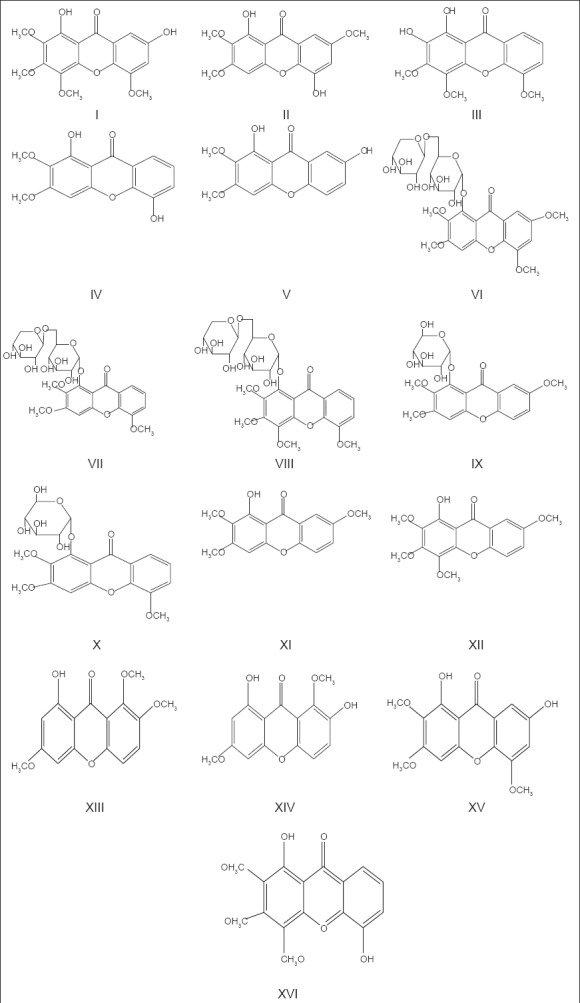
Xanthones and xanthone glycosides structures from H. elliptica
Chromones
Nine water-soluble compounds were isolated from the water soluble parts of H. elliptica, six of which were elucidated structurally as chromone derivatives (1-6, halenic acid A-C, halenichromone A-C) and three were known as 2-methylchromones (7-9). Compounds 7-9 were isolated from natural sources for the first time.[22] The structures of compounds 7-9 are shown in Figure 3.
Figure 3.
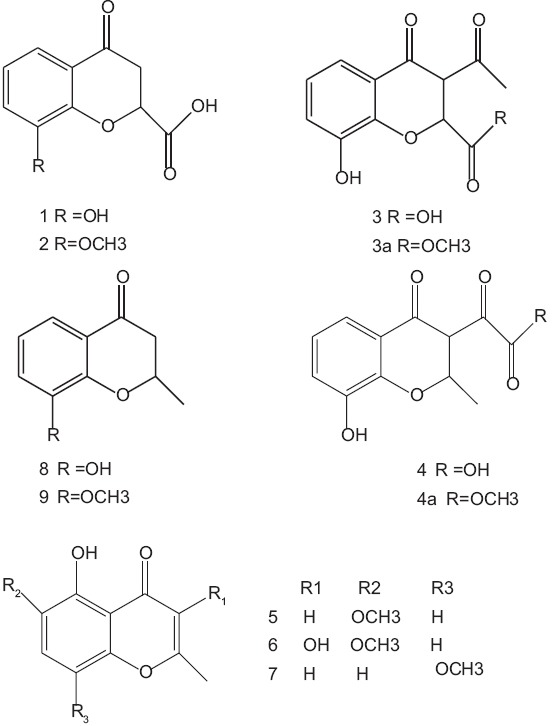
Chromones structures from H. elliptica
Other ingredients
Some flavonoids have been obtained from the whole plant of H. elliptica including luteolin-O-β-D-glucopyranoside, 7-O-primeverosylluteolin, 7-O-glucosylluteolin, luteolin, and apigenin.[19] Dhasmana isolated fat-soluble ingredients with antihepatitis activity from the plants such as pentacyclic triterpenoid oleanolic acid and sitosterol glucoside.[20] Gao obtained secoiridoid glycoside, swertiamarin and sweroside.[23]
Pharmacological effects
Protective effects against liver injury
Hepatoprotective activity, as another important property of H. elliptica, has been comprehensively studied. The hepatoprotective effects against carbon tetrachloride (CCl4)-induced liver toxicity in rats was observed by Zhang.[24] The rats were randomly divided into four groups, three experimental groups and a normal control group. The experimental groups were intraperitoneally injected Halenia decoction (0.5 mg/g amount to crude drug 0.5 g), total xanthone glycosides (5 mg/100 g), and haleniaside (3 mg/100 g), respectively, and the normal control group was intraperitoneally given water for 11 days. All the groups except the normal control group were injected with CCl4 to induce acute hepatic injury. Before injecting the drugs, the outline of liver lobules was vague, hepatic cells around the central vein were obviously necrotic, and the cytoplasm of liver lobule reduced extremely or even disappeared in the test groups. After injecting the drugs, most of the liver loboule outline was distinct, the necrotic area around the central vein decreased, and integral karyon in the central area increased. From the liver histochemistry of the treated groups it was found that the RNA content increased, especially in the haleniaside group. It suggested that haleniaside may be the main active ingredient to increase the RNA content.
Gao compared the inhibition action of six xanthones in Fe+2 -cystine-induced rat liver microsomal lipid peroxidation at concentrations of 1 μg/mL, 10 μg/mL, and 100 μg/mL, and found that C1 -hydroxyl-substituted xanthones have no antioxidant activity that may be caused by the stable hydrogen bonds between the C1 -hydroxyl group and the carbonyl group in the structure, which prevents the dissociation of the hydrogen ion. A stronger antioxidation of C7 -hydroxyl-substituted compound demonstrated that C7 hydroxyl group is an important part of the activity, so the antioxidant role of 1,7-dihydroxy-2,3,4,5-tetramethoxyxanthone is especially powerful; it is stronger than the classic antioxidant vitamin E. The presence of active hydrogen in the structure of xanthones is an important factor of the hepatoprotective action of H. elliptica.[16]
Nonspecific immunomodulatory effects
Zhang has researched the immunopharmacology of H. elliptica dry extract to clarify their effect on phagocytic function of the mononuclear macrophage on the basis of their influence on charcoal clearance rate in mice.[25] The results showed that the dry extract could enhance the mononuclear macrophage phagocytic function. But the dry extract had no effect on the hemolytic activity of serum hemolysin and splenocyte in normal mice. In the kidney-yang deficiency model, the dry extract can improve the humoral immunity of the hydrocortisone-induced mice.
Antioxidant properties[26,27]
The antioxidant properties of different extracts of H. elliptica were investigated by several established in vitro and in vivo models. The methanol extract of H. elliptica revealed strong antioxidant activity in vitro. The CCl4 -induced liver toxicity experiment showed that rats treated with the methanol extract of H. elliptica (100 mg/kg and 200 mg/kg) and silymarin (50 mg/kg) as the standard treatment, had lower levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bilirubin than those of the CCl4 group (P < 0.01). The results of the methanol extract at 100 mg/kg were comparable to those of silymarin at 50 mg/kg (P > 0.05). The methanol extract did not show any mortality at doses up to 2000 g/kg body weight. These results seem to support the traditional use of H. elliptica in pathologies involving hepatotoxicity, and the possible mechanism of this activity may be due to the strong free radical-scavenging and the antioxidant activities of the methanol extract.
Vasodilation effect[28,29]
Xanthones from H. elliptica are considered to be vasoactive substances, which exhibit either endothelium-dependent or endothelium-independent mechanisms in rat coronary artery. Six xanthones including 1-Hydroxy-2,3,5-trimethoxyxanthone (1), 1-hydroxy-2,3,4,7- tetramethoxyxanthone (2), 1-hydroxy-2,3,4,5-tetramethoxyxanthone (3), 1,7-dihydroxy-2,3,4,5-tetramethoxyxanthone (4), 1,5-dihydroxy-2,3-dimethoxyxanthone (5), and 1,7-dihydroxy-2,3-dimethoxyxanthone (6) caused vasodilation in the coronary artery precontracted with 1 μm 5-hydroxytryptamine. Removal of endothelium of the coronary artery led to decreases in the vasorelaxant effects of 1 and 6 but not of 2, 3, 4, and 5. The mechanism of the vasorelaxant effects of these xanthones may be relevant to the structure-activity differences in the level and the position of the substituent groups with the primary xanthone structure.
Antihepatitis B virus (anti-HBV) activity[22]
Chromone derivatives isolated from H. elliptica have been tested for their potencies toward inhibiting the secretion of HBV antigens in the human HBV-transfected liver cell line HepG2. Lamivudine was used as a positive control, which can suppress HbsAg secretion by 20.1% and HBeAg secretion by 19.7%, at 100 μg/mL (436 μm). The results demonstrated that 2-methylchromones compounds exhibited strong anti-HBV activities, inhibiting HBsAg secretion by 36.8% at a noncytotoxic concentration of 50 μg/mL (284 μm) for 8-hydroxy-2-methyl-4H-1-benzopyran-4-one and by 70.9% at a noncytotoxic concentration of 100 μg/mL (526 μm) for 8-methoxy-2-methyl-4H-1-benzopyran-4-one. While halenic acid and halenia chromone were slightly active or totally inactive at low concentrations. Further investigations are necessary to explore the values of 2-methylchromones as anti-HBV agents.
Other effects
H. elliptica also showed an inhibition effect on contraction of frog heart in vivo and in vitro. The chloroform extract of the whole plant has antiamoeba effect.[10]
Metabolic pathways of xanthone
Feng has studied the in vitro metabolic pathways of 1-hydroxyl-2,3,5-trimethoxyxanthone, the main constituent purified from H. elliptica; identified three metabolites (M1-M3), which demonstrated that demethylation and hydroxylation were the major phase I metabolic reactions for 1-hydroxyl-2,3,5-trimethoxyxanthone in human liver microsomes; and illustrated that CYP3A4 and CYP2C8 were the primary CYP450 isoforms responsible for its metabolism, and CYP1A2, CYP2A6, CYP2B6, CYP2C9, and CYP2C19 were also involved, especially in the formation of M3.[30] 1-hydroxyl-2,3,5-trimethoxyxanthone revealed moderate inhibitory effects on CYP1A2 (IC50 = 1.06 μm) and CYP2C9 (IC50 = 3.89 μm), minimal inhibition on CYP3A4 (IC50 = 11.94 μM), and no inhibition on CYP2D6 (dextromethorphan) and probe substrates CYP2E1 (chlorzoxazone).[31] In vitro metabolic transformations of five xanthones of H. elliptica have been evaluated by metabolic transformation in rat liver microsomes in vitro. The results showed that the metabolic transformation occurred mainly at 2-, 4-, 5-, and 7-carbonic positions on their structures. The metabolites could be considered as the new vasoactive substances.[32]
Toxicology
The results of acute toxicity test show that LD50 of H. elliptica decoction intraperitoneally injected in mice is 27.4 g/kg, and no death is observed in mice after intragastric administration at dose of 100 g/kg. The subacute oral toxicity of H. elliptica was investigated in rats. Comparison between the normal control group and the treated group two months after oral administration revealed that the latter were more active with increased food consumption and had more lustrous fur. There were no significant differences in body weight, hemogram, hepatic function, activity of glutamic pyruvic transaminase (GPT), and histological observations of the heart, the liver, and the kidney between the two groups. The results indicated that the medicine was safe in the given dose range.[25]
Future perspectives
H. elliptica is one of the most representative Tibetan medicine, which have been recorded in many traditional ancient books. In recent years, phytochemical and pharmacological studies of H. elliptica have attracted considerable interest. Large amount of extracts and active constitutes have been isolated and proved to have hepatoprotective, antiviral, and antioxidant properties, and enhance the immune response effects, etc. However, many challenges such as poor quality control and failed development of H. elliptica still exist. In the future, it is necessary to carry out further study on the structure-activity relationships and action mechanisms of major xanthones. Research should pay attention to the anti-HBV and other antihepacivirus activities of H. elliptica in vitro/in vivo. Further studies on H. elliptica can lead to the development of new drugs.
ACKNOWLEDGMENTS
The research was supported by the funds of the Basic Research for Application of Sichuan province of China (2014JY0233), Macao Science and Technology Development Fund (052/2013/A2), Macau University of Science and Technology Fund (0321), and Youth Foundation of Chengdu University (2014XQCKS10).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Li J, Yi T, Lai HS, Xue D, Jiang H, Peng HC, et al. Application of microscopy in authentication of traditional Tibetan medicinal plant Halenia elliptica. Microsc Res Tech. 2008;71:11–9. doi: 10.1002/jemt.20518. [DOI] [PubMed] [Google Scholar]
- 2.Rui G, Zhong GY, Zhang Y, Feng GR. Advance in Tibet herb of Halenia ellipitica D. Don. Trad Chin Drug Res and Clin Pharm. 2009;20:397–400. [Google Scholar]
- 3.He TN, Liu SW, Wu QR. Vol. 62. Beijing: Science Press; 1988. Flora of China; pp. 291–344. [Google Scholar]
- 4.Jiang H, Zhang H, Peng HC, Li J, Wang JG, Xia CL. The geographic distribution and plants characteristics of the Tibetan medicine Dida's original plants in Sichuan. West China J Pharm Sci. 2008;23:81–3. [Google Scholar]
- 5.Yu S, Yuan DGB. Shanghai: Science and Technology Press; 1987. Tibetan Medical Thangka of the Four Medical Tantras; p. 175. [Google Scholar]
- 6.Dimaer D. Shanghai: Science and Technology Press; 1986. JingZhu Herbal; p. 142. [Google Scholar]
- 7.Ma SL, Wang ZH, Mao JZ. Lanzhou: Gansu Nationalities Publishing House; 1993. Yue Wang Yao Zheng; p. 45. [Google Scholar]
- 8.Ga MQP. Lasa: Tibet People's Publishing House; 1993. Gan Lu Ben Cao Ming Jing (Tibetan) p. 27. [Google Scholar]
- 9.Beijing: People’ s Medical Publishing House; 1984. National Institute for the Control of Pharmaceutical and Biological Products. Ethnic drugs of China; p. 403. [Google Scholar]
- 10.Bao BQ, Sun QS, Bao GN. Advance in the study of Halenia plants: Chemical constituents and biological activities. Zhong Yao Cai. 2003;26:382–5. [PubMed] [Google Scholar]
- 11.Sun HF, Hu BL, Fan SF, Ding JY. Three new xanthones from Halenia elliptica D. Don. Acta Bot Sin. 1983;25:460–7. [Google Scholar]
- 12.Wang H, Chen H, Geng C, Zhang X, Ma Y, Jiang Z, et al. Chemical constituents of Halenia elliptica. Zhongguo Zhong Yao Za Zhi. 2011;36:1454–7. [PubMed] [Google Scholar]
- 13.Dhasmana H, Garg HS. Two xanthone glucosides from Halenia elliptica. Phytochem. 1989;28:2819–21. [Google Scholar]
- 14.Shi GF, Lu RH, Yang YS, Li CL, Yang AM, Cai LX. Isolation and crystal structure of xanthones from Swertia chirayita. Chinese J Struc Chem. 2004;23:1164–68. [Google Scholar]
- 15.Zhang D, Zhu YF, Lin SK. Identification of new chemical constituents of Tibetan medicinal herb Halenia elliptica. Chin Tradit Herbal Drugs. 2003;34:9–11. [Google Scholar]
- 16.Gao J, Wang SJ, Fang F, Si YK, Yang YC, Liu GT, et al. Xanthones from Tibetan medicine Halenia elliptica and their antioxidant activity. Acta Acad Med Sin. 2004;26:364–7. [PubMed] [Google Scholar]
- 17.Sun HF, Hu BL, Ding JY. Three new glycosides from Halenia elliptica. Acta Bot Sin. 1987;29:422–8. [Google Scholar]
- 18.Recio-Iglesias MC, Marston A, Hostettmann K. Xanthones and secoiridoid glucosides of Halenia campanulata. Phytochemistry. 1992;31:1387–9. [Google Scholar]
- 19.Rodriguez S, Wolfender JL, Odontuya G, Purev O, Hostettmann K. Xantones, Secoiridoids and Flavonoids from Halenia corniculat. Phytochemistry. 1995;40:1265–62. [Google Scholar]
- 20.Dhasmana H. Xanthones of Halenia elliptica. Phytochemistry. 1990;29:961. [Google Scholar]
- 21.Sun YW, Sun ZH, Yu PZ. A new xanthone from Halenia elliptica D. Don. J Asian Nat Prod Res. 2011;13:88–92. doi: 10.1080/10286020.2010.544254. [DOI] [PubMed] [Google Scholar]
- 22.Sun YW, Liu GM, Huang H, Yu PZ. Chromone derivatives from Halenia elliptica and their anti-HBV activities. Phytochemistry. 2011;75:169–76. doi: 10.1016/j.phytochem.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Gao GY, Li M, Feng YX, Tan P. Determination of effective constituents in 11 Swertia and related plants by HPLC. Acta Pharm Sin. 1994;29:910–2. [Google Scholar]
- 24.Zhang JM, Bao WL, Gao HP. Study of anti-hepatic injury and toxicity by Halenia elliptica and xanthone glycosides. Chin Tradit Herb Drugs. 1984;15:34–6. [Google Scholar]
- 25.Zhang J, Wang ZP, Tang RJ, Min ZH. Immuno pharmacological studies of Halenia elliptica and Halenia elliptica compound. Qinghai Medical Journal. 1986:317–8. [Google Scholar]
- 26.Huang B, Ke HB, He JS, Ban XQ, Zeng H, Wang YW. Extracts of Halenia elliptica exhibit antioxidant properties in vitro and in vivo. Food Chem Toxicol. 2011;49:185–90. doi: 10.1016/j.fct.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Ban XQ, He JS, Zeng H, Zhang P, Wang YW. Hepatoprotective and antioxidant effects of the methanolic extract from Halenia elliptica. J Ethnopharmacol. 2010;131:276–81. doi: 10.1016/j.jep.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Shi JG, Wang MZ, Che CT, Yeung JH. Vasodilatory actions of xanthones isolated from a Tibetan herb, Halenia elliptica. Phytomedicine. 2009;16:1144–50. doi: 10.1016/j.phymed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Shi JG, Wang MZ, Che CT, Yeung JH. Mechanisms of the vasorelaxant effect of 1-hydroxy-2,3,5-trimethoxy-xanthone, isolated from a Tibetan herb, Halenia elliptica, on rat coronary artery. Life Sci. 2007;81:1016–23. doi: 10.1016/j.lfs.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Feng R, Zhou XL, Tan XS, Or PMY, Hu T, Fu J, et al. In vitro identification of cytochrome P450 isoforms responsible for the metabolism of 1-hydroxyl-2,3,5-trimethoxy-xanthone purified from Halenia elliptica D. Don. Chem Biol Interact. 2014;210:12–9. doi: 10.1016/j.cbi.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Feng R, Zhou XL, Or PMY, Ma JY, Tan XS, Fu J, et al. Enzyme kinetic and molecular docking studies on the metabolic interactions of 1-hydroxy-2,3,5-trimethoxy-xanthone, isolated from Halenia elliptica D. Don, with model probe substrates of human cytochrome P450 enzymes. Phytomedicine. 2012;19:1125–33. doi: 10.1016/j.phymed.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Feng R, Zhang YY, Chen X, Wang Y, Shi JG, Che CT, et al. In vitro study on metabolite profiles of bioactive xanthones isolated from Halenia elliptica D. Don by high performance liquid chromatography coupled to ion trap time-of-flight mass spectrometry. J Pharm Biomed Anal. 2012;62:228–34. doi: 10.1016/j.jpba.2012.01.014. [DOI] [PubMed] [Google Scholar]


