Abstract
Ginkgo biloba (G. biloba) is an ancient medicinal tree species that has been in existence for millennia without undergoing modifications due to its resistance to environmental stresses. Palaeobotanical history showed a wide distribution of the species across the globe but declined over geological time, becoming restricted to narrow geographical range with few surviving individuals in the modern day. The tree is slow growing, adapted to many ecological conditions and shows numerous adaptation in developmental patterns. Medicinal use of the species is attracting research interest, especially the various parts of the tree that are used in orthodox or traditional medicine to treat diseases due to the many bioactive compounds. The primary compounds receiving increasing research interest are the triterpene lactones and flavonoids; these are the target of biotechnological strategies being employed to enhance production. Many genetic and environmental factors have contributed to the endangered status of the species; conservation measures are required to protect it from extinction. In many countries, the cultivation of plantations for the supply of ginkgo leaf-based pharmaceutical formulations is in progress, and efforts to standardize ginkgo leaf extract as herbal medication for human use are being made. Microcuttings and cuttings, cryopreservation, and plant tissue culture have all aided to conserve G. biloba.
Keywords: Bilobalides, conservation, Ginkgo biloba, ginkgolides, short shoots, triterpene lactones
INTRODUCTION
The name Ginkgo is derived from a wrong transcription of the Japanese name Yin-Kwo (silver fruit), while the epithet biloba refers to the bilobed shape of leaves; the English name “maidenhair tree” is due to a resemblance of the leaf shape and veins to maidenhair fern.[1] It is regarded as a “living fossil” because of the species’ uninterrupted existence for 270 million years without changes and for being the oldest tree in the world, with no living relative in existence. Its relationship with other plants is uncertain. As a result, it is classified in its own division: Ginkgophyta, having the extant species G. biloba. For this reason, the ginkgo is considered the “missing link” between gymnosperms and angiosperms.[2] The distinguishing feature of the ginkgo from conifers is its multiflagellated sperm cells, as in cycads, on vegetative anatomy, and partial molecular analysis of its genome suggests a much closer relationship to cycads than to conifers.[3,4] Vigorous young growing trees show distinct pyramidal growth with a principal central leader and spaced whorls of lateral branches growing out in diagonal orientation to the trunk, but with progression in maturity, increase in height slows, accompanied by the formation of a spreading crown due to the filling out of the branched, juvenile structures.[5] This dioecious tree bears clusters of fan-shaped, deciduous, alternate, simple leathery leaves (2-5 cm long) with forking parallel venation; and the leaves turn bright yellow, then fall within 15 days during autumn.[6] The tree shows extremely slow growth with very poor regeneration through seeds; vegetative propagation through cuttings is the most appropriate propagation method. The ginkgo's sprouting ability enabled it to persist on mountains with eroded slopes and played a role in its survival with morphological stability.[7] Male and female plants occur in 1:1 ratio, with rare reports of monoecious individuals. The plant shows high resistance to environmental stresses, microbial diseases (fungal, viral, and bacterial), other pests, and gaseous pollutants ozone and SO2, making it suitable and relevant for planting in urban areas. Indeed, it can act as a model to study disease resistance and stress in plants.[8,9,10] Wild species native to China are believed to be members of mixed mesophytic forests that at one point covered the hill country along the Yangtze River valley border. The ginkgo has tremendous medicinal, spiritual, and horticultural importance in Chinese culture. The supplements are bestselling herbal medications with a long history of use in traditional medicine to treat blood disorders; these are known to improve memory and offer the best-known way to keep the mind sharp. Leaves and seeds of G. biloba have been used in Chinese herbal medicine for thousands of years. Modern research focuses on the standardization of G. biloba extract from the dried green leaves. The tree produces biflavones, constituents in its leaves: the terpene trilactones (ginkgolides A, B, C, J, P, and Q, and bilobalides) many flavonol glycosides, proanthocyanidins, alkylphenols, simple phenolic acids, 6-hydroxykynurenic acid, 4-O-methylpyridoxine, and polyphenols. Ginkgo leaf extract is used in medicine due to its therapeutic actions in regulating cerebral blood flow, protection against free radicals, and delaying the progress of dementia and diabetes.[11,12,13] The standard extract is developed by pharmaceutical companies in the USA and Europe, with billions of doses sold in the last 40 years.[14] This article highlights the paleobotanical history, biology, phytochemistry, propagation, and conservation strategies employed to meet the pharmaceutical demand for the raw material from G. biloba.
PALAEOBOTANICAL HISTORY
G. biloba and other species in the genus were at one point widespread throughout the world. The range declined until 2 million years ago, when the trees were restricted to a small area in China.[15] Extensive fossil records of ginkgo plants and many reports of Ginkgophyta foliage and wood from many stratigraphic regions in the northern and southern hemispheres are known. The most plausible ancestral group of the order Ginkgoales is Peltaspermales, while the closest living relatives of the clade are cycads that share the characteristics of motile sperm with the extant G. biloba.[15] The oldest proof of the genus dates back to the Early Jurassic; it diversified and spread throughout Laurasia in the Middle Jurassic and the Early Cretaceous, and the number of species is much smaller than thought, owing to the practice of form-genera nomenclature of fossil parts.[16] The genus reached its pinnacle in diversity and distribution in the Late Jurassic and the Early Cretaceous, afterward showing rapid decline before the end of the Cretaceous. Evidence indicates that the decline was due to decreased temperatures.[15] Its decline continued into the Tertiary period, being particularly striking from the Oligocene; by the Paleocene, G. adiantoides had become the only species of the genus remaining in the northern hemisphere, while a markedly different form persisted in the southern hemisphere.[7] Further fossil evidence showed a consistent relative abundance of the ginkgo's ecological tolerance since the Cretaceous, with a growth preference for warm-temperate climates characterized by moist summers and cool winters;[17,18] other evidence indicated its origin to be in the Early Permian and in 16 genera distributed across temperate forests at the height of their worldwide radiation; however, by the Oligocene, 17 out of 19 genera, having 60 species, became extinct.[15] Direct precursors of G. biloba are traced to the Early Cretaceous, a few primitive plants with aligned characteristics to the Ginkgoales in the upper Palaeozoic and during the Mesozoic, especially the Jurassic and the Early Cretaceous, when the Ginkgo attained its greatest prominence.[19] The few Ginkgo species that persisted in gradually fewer numbers became narrow in their geographic range, disappearing from all but one continent, where G. biloba survived, whereas among fossil Ginkgo species, G. yimaensis and G. adiantoides are regarded as ancestors to G. biloba.[20] Further evidence showed that Ginkgo disappeared from polar areas through the end of the Miocene, most likely due to the extensive cooling that occurred throughout the northern hemisphere, and disappeared from Europe by the end of the Pliocene as temperatures dropped and the rainfall regime shifted from wet summers to dry, and Ginkgo fossils disappeared from the records except in a small area of central China, where G. biloba survived.[17,20] G. biloba and G. gardneri from the Paleogene of Scotland are the only Ginkgo species that existed in the northern hemisphere during the Cenozoic period, and all known occurrences of Ginkgo in the Pleistocene are from southwestern Japan.[18,19] Evidence points to the natural distribution of Ginkgo forests and the potential Pleistocene refugia of G. biloba as being situated in southwestern China.[21,22] The genus originated from the remote mountainous valleys of Zhejiang province and was earlier thought to be extinct in the wild, but a large population of the species exists in Tianmushan in Zhejiang province, in eastern China.[23] Whether the population is native or not is a subject of controversy due to the lack of seedlings in Tianmushan forest and the occurrence of asexual regeneration of individuals.[23] Studies showed high genetic similarity among ginkgo trees from the area that went against the argument on natural origin of the populations and suggested that the trees in the area may have been planted and preserved by Chinese monks over a period of 1,000 years.[24] Through application of several molecular markers, this tree population was analyzed to reconstruct the phytogeographic history of G. biloba, but understanding of the population genetics and phytogeographic history is still limited due to the inadequate number of populations studied and limitations of using genetic markers.[25]
BIOLOGY
G. biloba has a long juvenile period, reaching maturity at 20-30 years of age, and bearing seeds at 30-40 years of age.[26,27] Mature trees reach a height of 20-40 m, and few individuals grow beyond this range. The vigorous young ginkgo tree is pyramidal with a principal central leader and wide-spaced whorls of lateral branches that grow out at a diagonal orientation to the trunk; increase in height slows at maturity when the tree fills out the sparse, branched juvenile structures in a spreading crown formation.[5,28] It produces long shoots with wide-spaced leaves subtending axillary buds, and short shoots with clustered leaves lacking internodes and axillary buds. The leaves are deciduous, petiolate, fan-shaped, bilobed, thickened at the margin, broader than they are long, dichotomous-veined, and arranged in an alternate or clustered fashion of 3-5 on the short shoots.[1] Long shoots build up the basic tree framework through the generation of new growing points; short shoots produce the majority of leaves and reproductive structures, but environmental and physiological conditions can reverse the growth pattern of the shoots.[28] The tree is dioecious, on occasion monoecious, with male and female sex organs produced on short shoots in the axils of bud scales and leaves. The catkins of males emerge before the leaves fall off and after pollen is shed, and pollination is facilitated by the wind, while the ovules of females are produced in pairs and borne at the ends of stalks 2-3 mm long.[29,30] The “special trees” found in Minobu-cho, Yamanashi, Japan produce ovules or microsporangia with stalks in one short shoot, ovules on the leaf surface in a “female tree,” and microsporangia on the leaf margin of a “male tree.”[31] The receptive ovules in other ginkgo populations secrete small droplets of mucilaginous fluid from the micropyle, which functions toward the capture of wind-pollinated or airborne pollen; the neck cells contribute to the opening of the archegonia through changes in the production of fertilization fluid that attracts spermatozoids to the archegonia.[32] Retraction of the fluid toward the end of the day brings pollen into the pollen chamber and the ovule. Once inside, a male gametophyte undergoes 4 months of development that result in the production of a pair of multiflagellated spermatozoids. One of the spermatozoids fertilizes the egg cell while the ovule remains on the tree.[19,33,34] Developing ovules are green-colored till maturity in the autumn, when they turn yellow due to changing temperatures and fall from the tree 1 month after fertilization occurs. Embryo maturation completes in 6-8 weeks after seeds fall, and the foul odor from the fruits indicates that they are mature.[35,36] Mature ginkgo seeds are large, comprising an embryo embedded in the female gametophyte tissue and surrounded by a thick seed coat that has soft, fleshy sarcotesta, a hard stony middle layer, and a thin membranous inner layer.[34] Several authors have suggested many different animals as seed dispersal agents in geological history and in modern times, and many mammals have been observed as dispersal agents.[37] The seeds fall from the tree in a state of dormancy before the embryo has developed, and when cleaned and placed in a warm greenhouse after dispersal, the embryo grows to full size and germinates within 8-10 weeks.[34,38] This is because the temperature of the season plays a role in setting in motion the development of the embryo in the seed and later germination into the seedling. The behavior of G. biloba seeds accounts for the species᾽ warm-temperate distribution in recent times and also for its fossil ancestors. However, in “special trees,” seeds produced on the leaves do not germinate because of the nondevelopment of the pollen chamber at the time pollen is shed, and the gametophyte in the leaf ovule does not accept sperm because of the desiccation of its cytoplasm and nucleus.[31,39]
IMPORTANCE AND PHYTOCHEMISTRY
G. biloba is the most valued and ancient among medicinal plants, its use dating back to 1505 AD.[40] Despite its existence for over 200 million years, the real value of the species was recognized again in the last 40 years due to the discovery of the increasing importance of the tree's pharmacological compounds and their therapeutic effects. The therapeutic effect and pharmacological action are due to the joint effect of multiple components, and no individual component is regarded to solely exert the effect.[41] The tree's leaves and fruit are used in Chinese traditional medicine to treat many diseases.[42] Ginkgo leaf extract has been developed for pharmaceutical purposes in Germany since 1965, but the first commercially available extract registered for human use was in France in 1974 under the name EGb761 and is among the bestselling herbal medications worldwide.[43,44,45] The extract has been shown to improve memory function and enhance motivation in a monoaminergic, in particular dopaminergic role associated with the underlying mechanism of clinical effect in G. biloba.[46] In Europe and the USA, pharmaceutical products from G. biloba are produced, and the leaf extract is one of the five top-selling herbal supplements.[45] The standardized extract is administered in Europe at a clinical daily dosage of 120-240 mg for at least 8 weeks[47] and is composed of at least 24% ginkgo flavone glycosides and 6% terpene lactones [Figure 1].[48]
Figure 1.
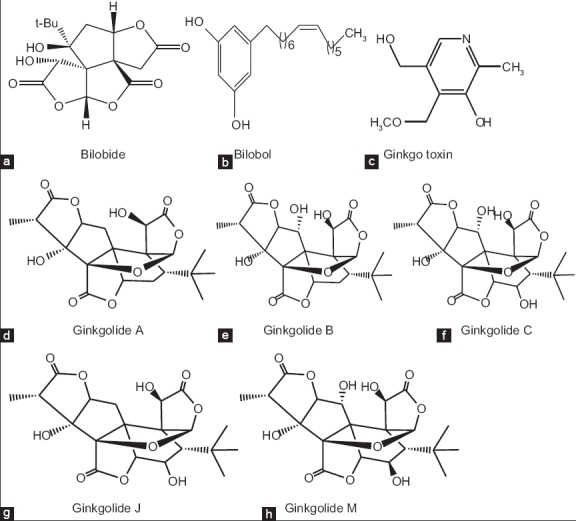
(a) Bilobide, (b) bilobol, (c) Ginkgo toxin, (d) Ginkgolide A, (e) Ginkgolide B, (f) Ginkgolide C, (g) Ginkgolide J, (h) Ginkgolide M
Phytochemical constituents in G. biloba have been investigated, with many compounds isolated and described. Novel phytochemicals are terpenes and trilactones (ginkgolides and bilobalides), flavonoids, and other compounds.[49,50] Knowledge about the metabolism and regulation is limited due to their occurrence in large numbers and variable synthesis over time with tissue; the terpene trilactones are neither polar nor nonpolar complex molecules.[51,52] Because of these properties, it is difficult to isolate pure ginkgolide and bilobalide molecules.[53,54] The complex structural framework makes it difficult to categorize them into any category of natural products.[55] First isolated from root bark, regarded as diterpenes containing spirononane carboxylic ring, 3 lactones, tetrahydrofuran ring and tetrabutyl group and make up ginkgolides A, B, C, J, M, K, and L. Apart from this, two new diterpenoid compounds, ginkgolides P and Q are isolated from leaves.[50,56,57] Among the many compounds produced by G. biloba, ginkgolide A is the most active, then bilobalide and ginkgolic acid, which showed antifeedant activity against cabbage butterfly larvae.[58] In the early stage, they are thought to be biosynthesized via the mevalonate pathway, but further studies showed biosynthesis via the deoxyerythritol phosphate pathway.[52,59] In this latter pathway, pyruvate and glyceraldehyde-3-phosphate react to produce 2-C-methyl-D-erythritol 2,4-cyclodiphosphate and dimethylallyl pyrophosphate and isopentynyl pyrophosphate. These two end products react to produce geranylgeranyl pyrophosphate, which converts to levopimaradiene, leading to the synthesis of dehydroabietane transported from plastids to the cytoplasm; this is in turn converted to ginkgolides through a series of oxidation reaction steps.[52,59] Studies support evidence of the occurrence of transcripts of levopimaradiene synthase gene (LPS) in the roots and male strobili of G. biloba, and these are believed to be biosynthesized in the root, and the product then translocated to the leaves.[57] However, others have opined that this biosynthesis occurs in the aerial parts of the tree.[60,61] Bilobalides are sesquiterpenes closely related to ginkgolides, but differ from ginkgolides by the absence of tetrahydrofuran ring, while bilobol plays a role in stress tolerance.[62] Tree gender, stage of development, age, soil, and natural variability due to the allogamous status of plant species all lead to great diversity in terpene content, but tree age is a major determinant of this content in different ways.[63] Diurnal, seasonal, and climatic factors affect biosynthesis and the resultant yield. The biosynthesis occurs in active-growing tissues and aerial parts of the ginkgo plant.[60,64,65,66,67] These factors have shown no influence on the production of flavonoid content during the complete annual vegetative cycle from early bud stage to fall of leaves.[68,69] However, production of the secondary metabolites in leaves of G. biloba in response to elevated ozone (O3) is differential; elevated O3 was shown to increase the concentration of quercetin and terpenes 23% higher than control due to the level of reactive oxygen species (ROS) but decreased isorhamnetin, condensed tannin, phenolic contents[70] The increase in terpene concentration is season-dependent, with the greatest increase occuring in September, while the concentration of bilobalide increased by 220%, ginkgolide C by 69.6%, ginkgolide A by 34.1%, and ginkgolide B by 34.3%.[70]
Trilactones in G. biloba have been extensively studied, but flavonoids have received less research attention. The flavonoids are flavones, biflavones, flavonols, tannins, and associated glycosides,[51] which have antioxidant, antifeeding, and antinutritive action against insect herbivores; significant differential levels of quercetin and kaempferol are observed when Spodoptera littoralis feed upon ginkgo compared to instances of mechanical damage.[10] Twenty flavonoid glycosides along with glucosides, quercetin, kaempferol 3-rhamnosides, 3-rutinosides, p-coumaric esters of glucorhamnosides of quercetin, kaempferol, and biflavones are known to occur in G. biloba. The biflavones include amentoflavone, 5-methoxybilobetol, bilobetol, isoginkgetin, ginkgetin, and sciadopitysin.[51] Of the flavonoids, flavonol glycosides are found more abundantly in leaves than are other flavonoids, and a majority of those are derived from quercetin, kaempferol, and isorhamnetin, while aglycones occur in low concentrations.[51]
The leaves of G. biloba contain nonphenolics such as ascorbic acid, D-glucaric acid, quinic acid, and shikimic acid[71] and phenolics such as protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic, chlorogenic acid, 6-hydroxykynurenic acid,[72,73] ginkgolic acid, cardanols, cardol, and urushiol.[74,75,76] Standardized ginkgo leaf extract obtained from dried leaves contains 3.1% ginkgolides, 2.9% bilobalides, and 24% flavonoids.[40,55] The other components of the extract are anthocyanidins and organic acids that play important roles in the water solubility of the extract, high proanthocyanidins, polyisoprenoid-derived betulaprenols, and flavan-3-ols;[51] coumaroyl flavonol glycoside;[77] 2-hexenal, ginnol, ginnon, ginkgolic, and organic acids (nonphenolics and phenolics).[51,75] The ginkgo heartwood contains monoterpenes and sesquiterpenes; the seeds contain bilobol, ginkgolic acid, 4-methoxy-pyridoxine, and ginkbilobin.[51,78] Further, the leaves and sarcotesta contain ginkgolic acid and long-chain alkylphenols bilobol or cardol.[62,79] A toxin 4-0-methyl-pyridoxine, the mechanism of action of which is to antagonize the activity of vitamin B6, is produced by raw ginkgo nuts.[80] Phytochemicals such as rutin, quercetin and kaempferol are known to be genotoxic, but yet, there are no indepth studies on the carcinogenic effect of these phytochemicals that are isolated from ginkgo leaf extract. The few recent studies on the anticancer activity of the extract in in vitro models showed inhibited cell proliferation, tumor suppression, and DNA damage repair effect of the extract.[81,82,83,84]
The last few decades saw an increase in published works on the phytochemistry, pharmacological activity, and uses of G. biloba, and the tree is regarded as a model for the study of plant senescence. Current interest focuses on the pharmacology, toxicology, and clinical research on the neuroprotective importance of ginkgo leaf extract. The pharmaceutical value of the extract is gaining increasing recognition, and efforts to standardize the extract are being made via incorporation into the pharmacopeia of many European countries. Standardization of the extract into dosage for safe human use as a herbal drug is facilitated by the regulatory agencies in these countries, and safety measures are taken. The recent and growing developments in studying the neuroprotective role of ginkgolides may, it is hoped, address the problems of clinical therapy of neurodegenerative diseases.
CONSERVATION, CULTIVATION, AND PROPAGATION
The oldest ginkgo trees are found growing near Daoist and Buddhist temples: the trees’ survival depended on Buddhist monks, who venerated the tree cultivated on the temple grounds, and the old trees played an important role in the preservation, along with the dissemination of the species.[1] For long, G. biloba has been cultivated in China, with some trees planted at temples over 1,500 years old. In Korea the ginkgo is cultivated for the beauty of its leaves, and for its edible and medicinal nuts; in parts of Japan it is widely planted according to its status in Confucianism.[1] In many of these areas, naturalization occurred, while in others, intentionally planted ginkgos are male cultivars grafted on plants propagated via seeds. Male trees do not produce malodorous seeds. Although cultivated trees exist throughout the world, there is no certainty whether G. biloba persists in the wild, but the International Union for Conservation of Nature (IUCN) listed the species under the threatened category of “endangered” due to the rapid decline in the numbers of individual trees around the world, for which conservation measures are required.[84] Among the conservation strategies employed for ginkgo plants are the selection of high-yielding individuals and developing propagation techniques. Propagation is achieved through application of in vitro microcuttings along with other tissue culture techniques. China, France, and Germany are undertaking initiatives for large-scale propagation, and such initiatives are implemented through plantations to conserve the species’ population and for herbal medicine.[85] In India, a few isolated populations exist in the Himalayan mountains and other regions, for which immediate conservation measures are needed.[1] However, seedling development of the ginkgo shows a unique mechanism in clonal regeneration, with remarkable power of survival in nature; under cultivation and because of the plant's dioecious nature, propagation from seeds is difficult.[37] Low germination rates of the seeds, recalcitrance, and a long juvenile phase are other problems in propagating G. biloba.[34] However, the high environmental adaptability of the species and its unparalleled tolerance to environmental stress make it a favorite for planting throughout the temperate and subtropical world for medicine, food, and ornamental purposes; cultivation can be traced to the nearly past thousant years, with the fruits used for food and medicine for millennia.[1] The choice of right genotype at the early stage of development and clonal propagation through in vitro techniques will play a great role in the multiplication, conservation, and production of ginkgolides and bilobalides for drug development by the pharmaceutical industry. Several selections of G. biloba have been made for ornamental purposes throughout the species’ long history, and older horticultural forms are still cultivated. These cultivated forms include ovulate trees that produce seeds attached to leaves, trees having a narrow and upright growth habit, trees with a spreading growth habit comprising exclusive horizontal branches, and an unstable form of trees having leaves striped with yellow or white.[86] These and other known cultivars are propagated in a variety of ways, depending on the season in a year, and male trees are more desirable for planting, as female trees produce foul-smelling fruits.[29] In temperate areas, G. biloba is cultivated for ornamental purposes and grows best when planted in full sun, but shows indefinite persistence under conditions of low light and low nutrients.[87] In subtropical climates or in soils over wet or dry during the season, the plants do not grow very well.[88,89,90] Several cultivars produced in China are used in large-scale cultivation to produce commercial product of ginkgo nuts, and have been in existence for over 600 years with atleast 44 cultivars selected based on size, shape of the nuts and productivity. The propagation of the cultivars is by grafting on seedling rootstocks, and nuts are produced at 5 years along with light crops in alternate years.[42,89,90] Across the globe, 25 million G. biloba trees are pruned and mechanically harvested in 1 year.[85,91] Fifty million G. biloba trees grown primarily in China, France, and the US (South Carolina), produce over 8,000 t of dried leaves each year; this cultivation is done to address commercial demands for G. biloba products.[1,67] In addition, large-scale cultivation of ginkgo leaf EGb761 extract has been in existence in the US and France since 1982, and G. biloba seedlings have been planted across over 2,000 ha in eastern China since 1992 to provide green ginkgo leaves.[91,92] Because of extremely slow growth and poor regeneration through seeds, vegetative propagation via cuttings is a possible way to augment regeneration of G. biloba trees.[29] Conventional cuttings are valuable option plans to conserve the species, and the cuttings are rooted through the use of root growth-inducing agents, with auxins such as indole-3-butyric acid (IBA) and alpha-naphthaleneacetic acid (NAA) being the most efficient.[93] Development of efficient protocols for clonal propagation have aided mass propagation in a short time, having an influence on conservation of the species. Among propagation methods, micropropagation by cuttings and microcuttings has aided the clonal propagation of Ginkgo trees.[93] Production of the trees is met with the limitation of a small propagation rate, collection labor intensity, high variability in the yield of triterpene lactones (depending on tree sex and season), and low yield of ginkgolides in leaves, especially ginkgolide B.[94] Constant and continuous commercial-scale supply of the trilactones from field-grown leaves has not received enough recognition for real-value application at the field level.[40,95,96] However, progress is being made in conserving the species for phytochemicals through the cultivation of organs and tissues to produce secondary metabolites. In vitro regeneration of the ginkgo shoot is derived from embryo and cotyledon, immature zygotic embryos, apical and nodal bud explants, and rooting performed on a medium added with endosperm extract.[97,98,99] Somatic embryogenesis, or development whereby embryoids are produced from somatic cells, is obtained from microspore, haploid protoplast, mega gametophyte, and immature zygotic embryo explants.[100,101,102,103] Despite successes in shoot regeneration and somatic embryogenesis, complete plantlet regeneration and later transfer to field condition is yet to be achieved.[94] Several strategies are employed to enhance production of the secondary compounds in the ginkgo.[104] The use of signaling molecules to enhance biosynthesis of the triterpenes in cell cultures is a choice strategy. Addition of methyl jasmonate repressed cell growth and induced browning, with damage to G. biloba cell suspension cultures, but enhanced the biosynthesis of ginkgolides and bilobalides. When cultures are treated with a combination of methyl jasmonate and salicylic acid, further enhancement of the yield of the compounds occurred; the addition of precursors increased cell growth without any effect on the elicitation of triterpene lactones, but their ratio is modified.[105] G. biloba cell cultures treated with biotic elicitor Candida ablicans showed the enhanced accumulation of bilobalide and ginkgolide compared to control.[106] Cryopreservation offers an alternative to labor- and cost-intensive in vitro culture, and samples stored in liquid nitrogen do not need periodic subcultures, and can be stored for a long time using the least space.[107] Cryopreservation of 2-year-old G. biloba callus through the desiccation method, with optimal preculture on sucrose-and-ABA amended medium for 14 days being made.[108] Adequate time for desiccation is 150 min, and profiles of calli cryopreserved for 2 years remained stable. Preculture of G. biloba callus on regrowth after desiccation without cryopreservation had an effect on callus with a water content of 20% freshwater (FW) and was not influenced by the presence of abscisic acid (ABA), but preculture with ABA resulted in a lower desiccation rate. Post-thaw regrowth of calli is occasional, regardless of sugar concentration in the medium, while pretreatment with ABA and sucrose ensured stable regrowth after cryopreservation, and are associated with changes in content and composition of endogenous soluble sugars in the calli.[109]
CONCLUSION
The worldwide sales of ginkgo leaf products are difficult to estimate but believed to be worth around half a billion USD or more, and establishing plantations to cultivate the species to meet the industrial demand for ginkgo leaf raw material is a promising approach for conserving the species. In many countries such as France, the USA, and China, plantations have been established to cultivate G. biloba for the supply of ginkgo leaves to the pharmaceutical industry. Application of biotechnology through tissue culture and genetic engineering are choice approaches for conservation of the species and to meet industrial raw material demands for production of ginkgo herbal supplements. The low yield of ginkgolides and bilobalides and the slow growth of the tree along with low yield of the compounds in undifferentiated tissue are impediments to the supply of the compounds, especially the ginkgolide B that shows promise as highly antagonist against platelet-activating factor, which is involved in the development of many respiratory, cardiovascular, renal, and central nervous system disorders. In order to overcome existing shortcomings, the selection of high-yield genotypes, optimization of culture conditions through culture medium selection, elicitation, permeabilization, and precursor feeding will be promising. Considering the recent increase in knowledge about the metabolism and regulation of key enzymes involved in the biosynthesis of the compounds, the development of transgenic ginkgo with enhanced production of those metabolites is a serious possibility. Phytochemical and population genetic studies are unraveling many threads of information regarding the medicinal importance and phytogeographic history of G. biloba. At present, no wild population of the species is in existence, and a program for the transfer of cultivated trees to the wild is yet to be established, but at the local levels the tree is conserved through plantation for ornamental and for religious or spiritual purposes. It is hoped that in the future, conservation measures to restore the population of G. biloba to earlier ranges, if possible, will save the species from extinction.
ACKNOWLEDGMENTS
This work was aided by financial support from the Department of Biotechnology, Government of India (New Delhi) and the Third World Academy of Science (Strada Costiera, Trieste, Italy) under a DBT-TWAS Postgraduate Research Fellowship. Special appreciation is owed to Hamdard University (New Delhi) for providing research facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Singh B, Kaur P, Gopichand, Singh RD, Ahuja PS. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79:401–18. doi: 10.1016/j.fitote.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Z, Zheng S. The missing link in Ginkgo evolution. Nature. 2003;423:821–2. doi: 10.1038/423821a. [DOI] [PubMed] [Google Scholar]
- 3.Fu-hsiung W, Zu-keng C. A contribution to the embryology of Ginkgo with a discussion of the affinity of the Ginkgoales. Acta Bot Sin. 1983;25:199–211. [Google Scholar]
- 4.Hasebe M. Molecular phylogeny of Ginkgo biloba: Close relationship between Ginkgo biloba and cycads. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H, editors. Ginkgo Biloba-A Global Treasure. Tokyo: Springer-Verlag; 1997. pp. 173–81. [Google Scholar]
- 5.Del Tredici P. The architecture of Ginkgo biloba L. In: Edelin C, editor. L’Arbre: Biologie et Developement. Montpellier, France: Naturalia Monspeliensia; 1991. pp. 155–68. [Google Scholar]
- 6.Gilman EF, Watson DG. Ginkgo biloba Autumn Gold, Autumn Gold Maidenhair Tree. Fact Sheet ST-274, U.S. Forest Service, Department of Agriculture. 1993. [Last accessed on 2014 Oct 20]. Available from: http://hort.ifas.ufl.edu/database/documents/pdf/tree_fact_sheets/ginbilb.pdf .
- 7.Del Tredici P, van Beek TA. The Evolution, Ecology, and Cultivation of G. biloba. In: Del Tredici P, van Beek TA, editors. Ginkgo Biloba. Netherlands: Harwood Academic Publishers; 2000. pp. 7–23. [Google Scholar]
- 8.Sinclair WA, Lyon HH, Johnson WT. Diseases of Trees and Shrubs. Cornstock Publishing Associates Ithaca. 1987:575. [Google Scholar]
- 9.Honda H, Hori T, Ridge RW, Tulecke W, Del Tredici P, Trémouillaux-Guiller J, et al. Ginkgos and insects. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H, editors. Ginkgo Biloba-A Global Treasure. Tokyo: Springer-Verlag; 1997. pp. 243–50. [Google Scholar]
- 10.Mohanta TK. Advances in Ginkgo biloba research: Genomics and metabolomics perspectives. African J Biotechnol. 2012;11:15936–44. [Google Scholar]
- 11.Cheng S, Xu F, Wang Y. Advances in the study of flavonoids in Ginkgo biloba leaves. J Med Plant Res. 2009;3:1248–52. [Google Scholar]
- 12.Weinmann S, Roll S, Schwarzbach C, Vauth C, Willich SN. Effects of Ginkgo biloba in dementia: Systematic review and meta-analysis. BMC Geriatr. 2010;10:14. doi: 10.1186/1471-2318-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Meng Q, Qian T, Yang Z. Ginkgo biloba extract enhances glucose tolerance in hyper insulinism-induced hepatic cells. J Nat Med. 2011;65:50–6. doi: 10.1007/s11418-010-0456-z. [DOI] [PubMed] [Google Scholar]
- 14.Gray DE, Upton R, Chandra A, Porter A, Harris RK. Quantitative analysis of flavonol glycosides in Ginkgo biloba: A comparison of two analytical methods. Phytochem Anal. 2006;17:56–62. doi: 10.1002/pca.886. [DOI] [PubMed] [Google Scholar]
- 15.Royer DL, Hickey LJ, Wing SL. Ecological conservatism in the “living fossil” Ginkgo. Palaeobiol. 2003;29:84–104. [Google Scholar]
- 16.Tralau H. The phytogeographic evolution of the genus Ginkgo L. Bot Not. 1967;120:409–22. [Google Scholar]
- 17.Tralau H. Evolutionary trends in the genus Ginkgo. Lethaia. 1968;1:63–101. [Google Scholar]
- 18.Uemura K, Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, et al. Cenozoic history of East Asia. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H, editors. Ginkgo biloba-A Global Treasure. Tokyo: Springer-Verlag; 1997. pp. 207–21. [Google Scholar]
- 19.Rothwell GW, Holt B. Fossils and phenology in the evolution of Ginkgo biloba. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H, editors. Ginkgo Biloba a Global Treasure: From Biology to Medicine. Tokyo, Japan: Springer-Verlag; 1997. pp. 223–30. [Google Scholar]
- 20.Samylina VA. Final stages of the history of the genus Ginkgo L. in Eurasia. Bot J. 1967;52:303–16. [Google Scholar]
- 21.Xiang Y, Xiang Z. Ancient Ginkgo biloba report 3: Investigation on ancient Ginkgo biloba remnant population in Guiyang. Guizhou Sci. 1999;17:221–30. [Google Scholar]
- 22.Hsieh L, Duhai Z. Analysis for the origin of Ginkgo population in Tianmu Mountains. Sci Silvae Sin. 2004;40:28–31. [Google Scholar]
- 23.Tredici PD, Ling H, Yang G. The Ginkgos of Tian Mu Shan. Conserv Biol. 1992;6:202–9. [Google Scholar]
- 24.Shen L, Chen XY, Zhang X, Li YY, Fu CX, Qiu YX. Genetic variation of Ginkgo biloba L. (Ginkgoaceae) based on cpDNA PCR-RFLPs: Inference of glacial refugia. Heredity (Edinb) 2004;94:396–401. doi: 10.1038/sj.hdy.6800616. [DOI] [PubMed] [Google Scholar]
- 25.Gong W, Chen C, Dobes C, Fu CX, Koch MA. Phylogeography of a living fossil: Pleistocene glaciations forced Ginkgo biloba L. (Ginkgoaceae) into two refuge areas in China with limited subsequent postglacial expansion. Mol Phylogenet Evol. 2008;48:1094–105. doi: 10.1016/j.ympev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Hadfield M. Some notes on the Ginkgo. Quart J For. 1960;54:331–7. [Google Scholar]
- 27.Ponder HG, Shumack RL, Gilliam CH. Liners the first step in shade tree production. Am Nurserym. 1981;153:10–11. 54, 64. [Google Scholar]
- 28.Gunkle JE, Thimann KV, Wetmore RH. Studies of development in long shoots and short shoots of Ginkgo biloba L., part IV. Growth habit, shoot expression and the mechanism of its control. Am J Bot. 1949;36:309–16. [PubMed] [Google Scholar]
- 29.Laurain D. Cultivation of Ginkgo biloba on a large scale. In: Van Beek TA, editor. Ginkgo Biloba. Amsterdam, Netherlands: Harwood Academic Publishers; 2000. pp. 63–79. [Google Scholar]
- 30.Echenard V, Lefort F, Calmin G, Perroulaz R, Belhahri L. A new and improved automated technology for early sex determination of Ginkgo biloba. Arboricul Urban For. 2008;34:300–7. [Google Scholar]
- 31.Soma S. Development of the female gametophyte in the ovules on the leaf blade of Ginkgo biloba. Ann Rep Fac Educ Bunkyo Univ. 1999;33:112–7. [Google Scholar]
- 32.Wang D, Lu Y, Zhang M, Lu Z, Luo K, Cheng F, et al. Structure and function of the neck cell during fertilization in Ginkgo biloba L. Trees. 2014;28:995–1005. [Google Scholar]
- 33.Friedman WE. Growth and development of the male gametophyte of Ginkgo biloba within the ovule (in vivo) Am J Bot. 1987;74:1797–815. [Google Scholar]
- 34.Holt B, Rothwell G. Is Ginkgo biloba (Ginkgoaceae) really an oviparous plant? Am J Bot. 1997;84:870. [PubMed] [Google Scholar]
- 35.Lee CL. Fertilization in Ginkgo biloba. Bot Gaz. 1955;117:79–100. [Google Scholar]
- 36.Maugini E. Anatomical and histological differences between male and female plants of Ginkgo biloba. Giorn Bot Ital. 1965;72:233–42. [Google Scholar]
- 37.Chase J. The Ginkgo-A True Living Fossil (Ginkgo biloba). Summer-Forests for Oregon. 2007. [Last accessed on 2014 Oct 17]. Available from: http://www.oregon.gov/odf/urbanforests/docs/featuredtreeginko.pdf .
- 38.Li TT, Chen SM. Temperature and the development of the Ginkgo embryo. Sci Rep Natl Tsing Hua Univ Ser B Biol Sci. 1934;2:37–9. [Google Scholar]
- 39.Sanae S. In vitro culture of the female gametophyte on the leaf: Trial to get the offspring. Bunk Univ Fac Educ. 2000;34:109–4. [Google Scholar]
- 40.Drieu K, Jaggy H, van Beek TA. Medicinal and Aromatic Plants-Industrial Profiles. Amsterdam: CRC Press; 2000. Ginkgo biloba; p. 35. [Google Scholar]
- 41.De Feudis FV. Paris: Elsevier; 1991. Ginkgo Biloba Extract (EGb 761): Pharmacological Activities and Clinical Applications; p. 1187. [Google Scholar]
- 42.Hatano K, Miyakawa T, Sawano Y, Tanokura M, Victor R, Reedy VR, et al. Antifungal and lipid transfer proteins from Ginkgo (Ginkgo biloba) Seeds. In: Reedy VR, Watson RR, Patel VB, editors. Nuts and Seeds in Health and Disease Prevention. United Kingdom: Elsevier Inc; 2011. pp. 528–34. [Google Scholar]
- 43.DeFeudis FV. Weisbaden: Ulistein Medical; 1998. Ginkgo Biloba Extract (EGb 761): From Chemistry to the Clinic; pp. 119–33. [Google Scholar]
- 44.McKenna DJ, Jones K, Hughes K. Efficacy, safety, and use of Ginkgo biloba in clinical and preclinical applications. Altern Ther Health Med. 2001;7:70–86. 88-90. [PubMed] [Google Scholar]
- 45.Cassileth B. Ginkgo (Ginkgo biloba) Oncology (Williston Park) 2011;25:971. [PubMed] [Google Scholar]
- 46.Yoshitake T, Yoshitake S, Kehr J. The Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and Ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br J Pharmacol. 2010;159:659–68. doi: 10.1111/j.1476-5381.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rister R, Klein S, Riggins C. Austin, TX: American Botanical Council; 1998. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines; pp. 136–8. [Google Scholar]
- 48.Chang JY, Chang MN. Medicinal uses of Ginkgo biloba. Today's Ther Trends. 1997;15:63–74. [Google Scholar]
- 49.Nakanishi K. The Ginkgolide. Pure Appl Chem. 1967;14:89–113. doi: 10.1351/pac196714010089. [DOI] [PubMed] [Google Scholar]
- 50.van Beek TA. Chemical analysis of Ginkgo biloba leaves and extracts. J Chromatogr A. 2002;967:21–55. doi: 10.1016/s0021-9673(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 51.Huh H, Staba EJ. The botany and chemistry of Ginkgo biloba L. J Herbs Spices Med Plants. 1992;1:91–124. [Google Scholar]
- 52.Zeng Z, Zhu J, Chen L, Wen W, Yu R. Biosynthesis pathways of Ginkgolides. Pharmacog Rev. 2013;7:47–52. doi: 10.4103/0973-7847.112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding C, Chen E, Zhou W, Lindsay RC. A method for extraction and quantification of ginkgo terpene trilactones. Anal Chem. 2004;76:4332–6. doi: 10.1021/ac049809a. [DOI] [PubMed] [Google Scholar]
- 54.Jaracz S, Malik S, Nakanishi K. Isolation of Ginkgolides A, B, C, J and bilobalide from G. biloba extracts. Phytochemistry. 2004;65:2897–902. doi: 10.1016/j.phytochem.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 55.van Beek TA, Montoro P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts and phytopharmaceuticals. J Chromatogr A. 2009;1216:2002–32. doi: 10.1016/j.chroma.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Furukawa S. Studies on constituents of the Ginkgo biloba L, leaves part I and II. Sci Papers Inst Phys Chem Res. 1932;19:27–42. [Google Scholar]
- 57.Liao HJ, Zheng YF, Li HY, Peng GP. Two new Ginkgolides from leaves of Ginkgo biloba. Planta Med. 2011;77:1818–21. doi: 10.1055/s-0030-1271153. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto T, Sei T. Antifeedant activities of Ginkgo biloba L. components against the larva of Pieris rapae crucivora. Agric Biol Chem. 1987;51:249–50. [Google Scholar]
- 59.Stømgaard K, Nakanishi K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angew Chem Int Ed Engl. 2004;43:1640–58. doi: 10.1002/anie.200300601. [DOI] [PubMed] [Google Scholar]
- 60.Carrier DJ, van Beek TA, Heijden RV, Verpoorte R. Distribution of Ginkgolides and terpenoid biosynthetic activity in Ginkgo biloba. Phytochem. 1998;48:89–92. [Google Scholar]
- 61.Kim JH, Lee KI, Chang YJ, Kim SU. Developmental pattern of Ginkgo biloba levopimaradiene synthase (GbLPS) as probed by promoter analysis in Arabidopsis thaliana. Plant Cell Rep. 2012;31:1119–27. doi: 10.1007/s00299-012-1232-1. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka A, Arai Y, Kim SN, Ham J, Usuki T. Synthesis and biological evaluation of bilobol and adipostatin A. J Asian Nat Prod Res. 2011;13:290–6. doi: 10.1080/10286020.2011.554828. [DOI] [PubMed] [Google Scholar]
- 63.Balz JP, Courtois D, Drieu J, Drieu K, Reynoird JP, Sohier C, et al. Production of Ginkgolides and bilobalide by Ginkgo biloba plants and tissue cultures. Planta Med. 1999;65:620–6. doi: 10.1055/s-1999-14088. [DOI] [PubMed] [Google Scholar]
- 64.Flesh V, Jacques M, Cosson L, Teng BP, Petiard V, Balz JP. Relative importance of growth and light level on terpene content of Ginkgo biloba. Phytochem. 1992;31:1941–5. [Google Scholar]
- 65.van Beek TA, Lelyveld GP. Concentration of Ginkgolides and bilobalide in Ginkgo biloba leaves in relation to the time of year. Planta Med. 1992;58:413–6. doi: 10.1055/s-2006-961503. [DOI] [PubMed] [Google Scholar]
- 66.Inoue H, Kamoda S, Terada T, Saburi Y. Ginkgolide production in relation to organogenesis in Ginkgo biloba. J Wood Sci. 1998;44:375–8. [Google Scholar]
- 67.Nakanishi K. Terpene trilactones from Gingko biloba: From ancient times to the 21 st century. Bioorg Med Chem. 2005;13:4987–5000. doi: 10.1016/j.bmc.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Lobstein L, Rietsch-Jako I, Haag-Berrurier M, Anton R. Seasonal variation of the flavonoid content from Gingko biloba leaves. Planta Med. 1991;57:430–3. doi: 10.1055/s-2006-960142. [DOI] [PubMed] [Google Scholar]
- 69.Sticher O, Meier B, Hasler A, Van Beek TA. The analysis of Ginkgo flavonoids. In: VanBeek TA, editor. Ginkgo biloba (Medicinal and Aromatic Plants - Industrial Profiles) Vol. 12. Amsterdam: Harwood Academic Publishers; 2000. pp. 179–202. [Google Scholar]
- 70.He X, Wang W, Chen W, Dong T, Liu C, Chen Z, et al. Changes of main secondary metabolites in leaves of Ginkgo biloba in response to ozone fumigation. J Environ Sci (China) 2009;21:199–203. doi: 10.1016/s1001-0742(08)62251-2. [DOI] [PubMed] [Google Scholar]
- 71.Hasler A, van Beek TA. Chemical constituents of Ginkgo biloba. In: VanBeek TA, editor. Ginkgo biloba (Medicinal and Aromatic Plants-Industrial Profiles) Vol. 12. Amsterdam: Harwood Academic Publishers; 2000. pp. 109–42. [Google Scholar]
- 72.Schenmen A, Hölzl J. 6-Hydroxykynurenic acid, the first N-containing compound from the Ginkgo biloba leaf. Planta Med. 1986;52:235–6. [Google Scholar]
- 73.Ellnain-Wojtaszek M, Kruczyński Z, Kasprzak J. Analysis of the content of flavonoids, phenolic acids as well as free radicals from Ginkgo biloba L. leaves during the vegetative cycle. Acta Pol Pharm. 2001;58:205–9. [PubMed] [Google Scholar]
- 74.Chung BY, Won LS, Lee BR, Lee CH. A new chemical constituents of green leaves of Ginkgo biloba L. J Korean Chem Soc. 1982;26:95–8. [Google Scholar]
- 75.Irie J, Murata M, Homma S. Glycerol-3-phosphate dehydrogenase inhibitors, anacardic acids, from Ginkgo biloba. Biosci Biotech Biochem. 1996;60:240–3. doi: 10.1271/bbb.60.240. [DOI] [PubMed] [Google Scholar]
- 76.Schötz K. Quantification of allergenic urushiols in extracts of Ginkgo biloba leaves, in simple one-step extracts and refined manufactured material (EGb 761) Phytochem Anal. 2004;15:1–8. doi: 10.1002/pca.733. [DOI] [PubMed] [Google Scholar]
- 77.Teng BP, Braquet P. Chemistry of Ginkgolides. In: Braquet P, editor. Ginkgolides-Chemistry, Biology, Pharmacology and Clinical Perspectives. Vol. 1. Barcelona: J. R. Prous Science Publications; 1998. pp. 37–42. [Google Scholar]
- 78.Wang H, Ng TB. Ginkobilobin, a novel antifungal protein from Ginkgo biloba seeds with sequence similarity to embryo-abundant protein. Biochem Biophys Res Commun. 2000;279:407–11. doi: 10.1006/bbrc.2000.3929. [DOI] [PubMed] [Google Scholar]
- 79.He X, Bernart MW, Nolan GS, Lin L, Lindenmaier MP. High-performance liquid chromatography-electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba) J Chromatogr Sci. 2000;38:169–73. doi: 10.1093/chromsci/38.4.169. [DOI] [PubMed] [Google Scholar]
- 80.Wada K, Haga M. Food poisoning by Ginkgo biloba seeds. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H, editors. Ginkgo Biloba-A Global Treasure. Tokyo: Springer-Verlag; 1997. pp. 309–21. [Google Scholar]
- 81.Jiang W, Qiu W, Wang Y, Cong Q, Edwards D, Ye B, et al. Ginkgo may prevent genetic-associated ovarian cancer risk: Multiple biomarkers and anticancer pathways induced by Ginkgolide B in BRCA1-mutant ovarian epithelial cells. Eur J Cancer Prev. 2011;20:508–17. doi: 10.1097/CEJ.0b013e328348fbb7. [DOI] [PubMed] [Google Scholar]
- 82.Marques F, Azevedo F, Johansson B, Oliveira R. Stimulation of DNA repair in Saccharomyces cerevisiae by Ginkgo biloba leaf extract. Food Chem Toxicol. 2011;49:1361–6. doi: 10.1016/j.fct.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 83.Esmekaya MA, Aytekin E, Ozgur E, Güler G, Ergun MA, Omeroðlu S, et al. Mutagenic and morphologic impacts of 1.8GHz radiofrequency radiation on human peripheral blood lymphocytes (hPBLs) and possible protective role of pre-treatment with Ginkgo biloba (EGb 761) Sci Total Environ. 2011;410-411:59–64. doi: 10.1016/j.scitotenv.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 84.Fu LG, Jin JM. Vol. 1. Beijng, China: Science Press; 1992. Chinese Plant Red Data Book: Rare and Endangered Plants; pp. 474–5. [Google Scholar]
- 85.Schmid W, Balz J. Cultivation of Ginkgo biloba L. on three continents. Acta Hort (ISHS) 2003;676:177–80. [Google Scholar]
- 86.Santamour FS, He SA, McArdle AJ. Checklist of cultivated Ginkgo. J Arboricult. 1983;9:88–92. [Google Scholar]
- 87.Handa M, Iizuka Y, Fujiwara NH, Ridge RW, Tulecke W, Del Tredici P, et al. Ginkgo landscapes. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H, editors. Ginkgo Biloba-A Global Treasure. Tokyo: Springer-Verlag; 1997. pp. 259–83. [Google Scholar]
- 88.Santamour FS, Jr, He S, Ewert TE. Growth, survival and sex expression in Ginkgo. J Arboricult. 1983;9:170–1. [Google Scholar]
- 89.Del Tredici P. Ginkgos and people: A thousand years of interaction. Arnoldia. 1991;51:2–15. [Google Scholar]
- 90.Shepperd WD. GA USA: National Seed Laboratory Riggins Mill Rd Dry Branch; 2008. Ginkgoaceae—Ginkgo family Ginkgo biloba L. ginkgo. USDA FS Agriculture Handbook 727 - The Woody Plant Seed Manual; pp. 559–61. [Google Scholar]
- 91.Balz JP, editor. Proceedings of the International Seminar on Ginkgo. China, Beijing, China: State Sci Tech Comm PR; 1997. Agronomic aspects of G. biloba leaves production; pp. 101–17. [Google Scholar]
- 92.He S, Yin GU, Pang Z. Resources and prospects of Ginkgo biloba in China. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Tremouillaux-Guiller J, Tobe H, editors. Ginkgo Biloba-A Global Treasure. Tokyo: Springer-Verlag; 1997. pp. 373–83. [Google Scholar]
- 93.Purohit VK, Phondani PC, Rawat LS, Maikhuri RK, Dhyani D, Nautiyal AR. Propagation through rooting of stem cuttings of Ginkgo biloba Linn. A living fossil under threat. J Am Sci. 2009;5:139–44. [Google Scholar]
- 94.Kayser O, Quax WJ, Sohier C, Courtois D. Ginkgo biloba and production of secondary metabolites. In: Kayser O, Quax WJ, editors. Medicinal Plant Biotechnology: From Basic Research to Industrial Applications. Weinheim, Germany: WILEY-VCH Verlag GmbH and Co. KGaA; 2007. pp. 493–514. [Google Scholar]
- 95.Sohier C. Plant biotechnology: An avant-garde research for an ancestral tree, the Ginkgo biloba. Ann Pharm Fr. 2002;60:22–7. [PubMed] [Google Scholar]
- 96.Sabater-Jara AB, Souliman-Youssef S, Novo-Uzal E, Almagro L, Belchí-Navarro S, Pedreño MA. Biotechnological approaches to enhance the biosynthesis of Ginkgolides and bilobalide in Ginkgo biloba. Phytochem Rev. 2013;12:191–205. [Google Scholar]
- 97.Camper ND, Coker PS, Wedge DE, Keese RJ. In vitro culture of Ginkgo. In Vitro Cell Dev Biol Plant. 1997;33:126–7. [Google Scholar]
- 98.Choi PS, Cho DY, Soh WY. Shoot organogenesis from immature zygotic embryo cultures of Ginkgo biloba. Biol Plant. 2003;47:309–12. [Google Scholar]
- 99.Tommasi F, Scaramuzzi F. In vitro propagation of Ginkgo biloba by using various bud cultures. Biol Plant. 2004;48:297–300. [Google Scholar]
- 100.Laurain D, Trémouillaux-Guiller J, Chénieux JC. Embryogenesis from microspores of Ginkgo biloba L., a medicinal woody species. Plant Cell Rep. 1993;12:501–5. doi: 10.1007/BF00236095. [DOI] [PubMed] [Google Scholar]
- 101.Laurain D, Chénieux JC, Trémouillaux-Guiller J. Direct embryogenesis from female haploid protoplasts of Ginkgo biloba L., a medicinal woody species. Plant Cell Rep. 1993;12:656–60. doi: 10.1007/BF00232819. [DOI] [PubMed] [Google Scholar]
- 102.Fontanel A, Serraf I, Pétiard V. Regeneration in Ginkgo biloba L: Induction of embryogenesis from megametophyte according to the development stage. CR Acad Sci Paris. 1994;317:149–55. [Google Scholar]
- 103.Laurain D, Chénieux JC, Trémouillaux-Guiller J. Somatic embryogenesis from immature zygotic embryos of Ginkgo biloba. Plant Cell Tissue Organ Cult. 1996;44:19–24. [Google Scholar]
- 104.Kang SM, Min JY, Kim YD, Park DJ, Jung HN, Karigar CS, et al. Effect of supplementing terpenoid biosynthetic precursors on the accumulation of bilobalide and Ginkgolides in Ginkgo biloba cell cultures. J Biotechnol. 2006;123:85–92. doi: 10.1016/j.jbiotec.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 105.Kang S, Min J, Kim Y, Kang Y, Park D, Jung H, et al. Effects of methyl jasmonate and salicylic acid on the production of bilobalide and Ginkgolides in cell cultures of Ginkgo biloba. In Vitro Cell Dev Biol Plant. 2006;42:44–9. [Google Scholar]
- 106.Kang SM, Min JY, Kim YD, Karigar CS, Kim SW, Goo GH, et al. Effect of biotic elicitors on the accumulation of bilobalide and Ginkgolides in Ginkgo biloba cell cultures. J Biotechnol. 2009;139:84–8. doi: 10.1016/j.jbiotec.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Martin C, Iridono JM, Benito-Gonzales E, Perez C. The use of tissue culture techniques in the conservation of plant biodiversity. Agro-Food-Ind Hi-Tech. 1998;9:37–40. [Google Scholar]
- 108.Popova EV, Lee E, Wu C, Hahn E, Paek K. A simple method for cryopreservation of Ginkgo biloba callus. Plant Cell Tissue Organ Cult. 2009;97:337–43. [Google Scholar]
- 109.Lu ZW, Popova EV, Wu CH, Lee EJ, Hahn EJ, Paek KY. Cryopreservation of Ginkgo biloba cell culture: Effect of pretreatment with sucrose and ABA. Cryo Letters. 2009;30:232–43. [PubMed] [Google Scholar]


