Abstract
Background & objectives:
Bone marrow is a rich source of adult stem cells that can differentiate into various cell types. Administration of mesenchymal stem cells (MSCs) in irradiated diabetic rat model has transiently shown to decrease blood glucose level. This study examines the effect of high dose and multiple injections of MSCs on glycemic profile, their localization and regeneration of islet in diabetic Wistar rat.
Methods:
The study was carried out in male Wistar rats categorized into three groups (n=6, in each group): Group 1 as control, group 2 streptozotocin (STZ) (50 mg/kg) induced diabetic group and group 3 experimental group; 5-bromo-2-deoxyuridine (BrdU) labelled allogenic MSCs were injected in the non-irradiated diabetic rat of the experimental group through tail vein. The blood glucose profile was subsequently monitored at regular intervals. Rats were sacrificed on day 45 and pancreas was examined for localization of BrdU labelled stem cells by immunofluorescence and islet-neogenesis by immunohistochemistry.
Results:
There was a significant reduction in blood glucose level after administration of MSCs in the experimental group (P<0.001). The presence of BrdU labelled MSCs in islet suggested their localization in the pancreas. Co-expression of anti-BrdU and anti-insulin antibody indicated trans-differentiation/fusion into insulin producing cells evidenced by significant increase in total number of islet (P=0.004) and insulin positive cells (P<0.0001) in experimental group.
Interpretation & conclusions:
Our results showed that the MSCs administration in non-irradiated diabetic Wistar rat reduced hyperglycaemia and was accompanied by increased islet-neogengesis, possibly through trans- differentiation/fusion.
Keywords: β-cells, diabetes, glucose level, mesenchymal stem cells, Wistar rat
The prevalence of diabetes is rapidly increasing all over the world possibly due to population growth, ageing (increased life expectancy), increasing prevalence of obesity and sedentary lifestyle and as per an estimate by the International Diabetes Federation at least 382 million people are affected by diabetes and this number is likely to grow to 592 million by 20351. India houses 62.4 million people with diabetes and 77.2 million with prediabetes contributing almost one-fifth of the world diabetic population2. The development of both type 1 and type 2 diabetes is attributed to the destruction or dysfunction of β-cell, respectively. Type 1 diabetes is characterized by abnormal activation of T-cells resulting in immune-inflammatory destruction of the β-cell consequently leading to absolute insulin deficiency. Type 2 diabetes is characterized by two defects, namely insulin resistance and insulin deficiency. Insulin resistance remains fairly constant after the onset of hyperglycaemia, while decline in β-cell function is progressive and inexorable. It is attributed to glucotoxicity, oxidative stress, free radical- mediated injury and possibly enhanced programmed β- cell death3.
Irrespective of the type of diabetes, the current focus is to target β- cell dysfunction and/or mass to achieve the cure of diabetes. Islet cell transplantation is a viable option and has been successfully performed with insulin independence in initial years in a majority of diabetic patients4. However, limited availability of donors, progressive decline in insulin independence and graft rejection are major limitations of this therapy5. Stem cells, which have the ability to differentiate into insulin producing cells either in vitro6 or in vivo7, would provide a potentially unlimited source of islet cell for the treatment and alleviation of the major limitations of availability and rejection of allogenic pancreatic islet8.
Bone marrow is an important source of adult stem cells enriched with haematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). MSCs are multipotent and can differentiate into bone, cartilage, fat and connective tissue and also have anti-inflammatory and immunomodulatory properties9. It is proposed that MSCs may be responsible for pancreatic endocrine differentiation and help in proliferation and vascularisation but these donot differentiate into β- cell10,11. Moreover, the MSCs are hypoimmunogenic as compared to HSCs, which lowers the probability of allogenic rejection, thereby providing an opportunity to administer these cells even without prior irradiation.
The present study is designed to demonstrate the effect of allogenic mesenchymal stem cells transplantation in non-irradiated streptozotocin (STZ) induced diabetic Wistar rat to demonstrate its effects on glycaemic profile and their localization into pancreatic islet and effects in islet neogenesis.
Material & Methods
Male Wistar rats weighing between 180-200 g were procured from the animal house of Postgraduate Institute of Medial Education and Research (PGIMER), Chandigarh, India. The study was approved by the institute′s animal ethics committee and was carried out at the department of Endocrinology and Translational and Regenerative Medicine at PGIMER, Chandigarh, from September 2011 to October 2012. The rats were kept individually in polypropylene cages in an environmentally controlled room maintained at 25 ± 2° C with a 12 h dark and light cycle and 40-70 per cent humidity. The animals had free access to food and water. Rats were fed with rat chow diet according to the standard protocol. Experiments were carried out after a week of acclimatization.
Experimental groups: Male Wistar rats were randomly categorized into three groups with six animals in each group: including the control group (group 1), streptozotocin (STZ) induced diabetic group (group 2) and experimental group (group 3) which received MSCs after STZ induced diabetes. The rats were injected freshly prepared STZ (Sigma -Aldrich, USA) prepared in chilled sodium citrate buffer (pH 4.5) at a dose of 50 mg/kg body weight, intraperitoneally at baseline. The animals exhibiting blood glucose level of more than 200 mg/dl on multiple occasions after administration of STZ were considered as diabetic12.
The MSCs were administered in a non-irradiated diabetic rat of the experimental group through tail vein on days 17 and 24 after the establishment of diabetes on several occasions between baseline and day 10. Subsequently, blood glucose was regularly monitored till the end of the study. After six weeks of MSCs transplantation, an intraperitoneal glucose tolerance test (IPGTT) was performed. Rats were sacrificed and the pancreas was isolated and examined for histology, islet neogenesis by immunohistochemistry (IHC), immunofluorescence (IF) and electron microscopy.
Blood glucose level: Blood was drawn from the tail vein and blood glucose was measured between 0800 and 1100 h at baseline, days 3,5,7,10, 17, 24, 31 and 38 by a glucometer using the glucose oxidase method (Optium Xceed, Abbott Diabetes Care Inc, USA) which was regularly calibrated with reference standard solutions.
Intraperitoneal glucose tolerance test (IPGTT): At day 45 after an overnight fast, the rats were challenged by loading a glucose solution administered intraperitoneally at a dose of 2 g/kg. Blood samples were drawn for blood glucose at baseline (0 min) and at 30, 60, 90 and 120 min after glucose load.
Isolation and culture of bone marrow mesenchymal stem cells: The rats were sacrificed after administration of sodium pentobarbital intraperitoneally at a dose of 30 mg/kg. The femurs and tibiae were carefully cleaned of adherent soft tissues. The tip of each bone was cut with a bone cutter. Bone marrow cells were flushed from the medullary cavities of the femurs and tibiae and disaggregated into a single-cell suspension by sequential passage through a 23-gauge needle. This single cell suspension was cultured in 15 ml of MSC specific medium; minimum essential medium (MEM) supplemented with 10 per cent foetal bovine serum (FBS) and penicillin/streptomycin) in T-75 culture flasks at 37° C in a humidified atmosphere of 95 per cent air and 5 per cent carbon dioxide incubator. Three to four days later, non-adherent cells were removed by changing the medium. After 10 days in culture, adherent cells formed homogenous fibroblast-like colonies. When MSCs became confluent (80-90%), adherent cells were passaged with trypsin (0.25%) by incubating for 10 min. Three such passages were done to obtain optimal number of MSCs before transplantation. The de-adhered cells after trypsin treatment were collected in a 15ml falcon tube and centrifuged at 480 g for 5 min and these cells were counted and tested for viability by trypan blue.
Flow cytometery analysis of cell surface markers and labelling of the MSCs: Rat MSCs specific cell surface antigens (CD29 and CD54) were characterized for purity of cultured population and negative markers for haematopoietic lineage (CD 34 and CD 45). The cells were incubated at room temperature for 30 min with monoclonal antibodies CD29, CD54, CD34 and CD 45 (BD Bioscience, USA) labelled with fluorescein isothiocyanate (FITC) and phycoerythrin (PE), respectively, and acquired onto 4-colour flow cytometer (FACS Calibur, BD Bioscience USA).
For localization, 80-90 per cent of confluent MSCs were labelled with 10 μM 5-bromo-2’-deoxyuridine (BrdU, Sigma-Aldrich, USA) containing medium and incubated for 48 h. After incubation, the cells were trypsinized. A part of labelled MSCs was kept for flow cytometer analysis and the remaining cell population was processed for transplantation. The cells for flow cytometric analysis were incubated with mouse anti-BrdU antibody (Sigma -Aldrich, USA) at room temperature for two hours followed by incubation with a secondary antibody, i.e. goat anti-mouse IgG- FITC (Sigma -Aldrich, USA) for two hours and acquired onto 4-colour flow cytometer.
MSCs transplantation: BrdU labelled MSCs 4.8 x 106 (range - 4.5-5.5 X 106) in 0.5ml of phosphate buffer saline, pH 7.4 were administered intravenously through tail vein on days 17 and 24 in the non-irradiated diabetic rat of the experimental group (group 3). The same volume of saline was injected into the STZ induced diabetic group (group 2).
Histological and morphological analysis: Rats were sacrificed at day 45 by administering sodium pentobarbital at a dose of 30mg/kg. The pancreas was dissected out and the tissue was embedded in an optimal cutting temperature (OCT) component and stored at -20 °C. The tissue was cut at a thickness of 4μm in cryostat (Leica CM1850 UV, Thermo Fisher scientific, USA). A portion of the tissue was fixed in 10 per cent buffered formalin and processed for haematoxylin and eosin (H & E) stain for pancreatic histology in all the groups.
Immunohistochemistry: For immunohistochemistry, tissue sections were deparaffinised in xylene, immersed in 3 per cent hydrogen peroxide to quench endogenous peroxidase activity and microwaved in sodium citrate solution (pH= 6.9) for 15 min for antigen retrieval. The tissue sections were incubated with mouse anti-insulin antibody (1:300 v/v, Sigma -Aldrich, USA) at room temperature for two hours and the slides were then incubated with peroxidase labelled secondary antibody (goat secondary antibody against rabbit/mouse with peroxidase labelled dextran, DakoREAL™ Envision™/HRP, Denmark) for 40 min. The chromogen used for obtaining brown colour was 3’3’ diaminobenzidine tetrachloride (DAB) chromogen buffer. Tissue sections were counter-stained with haematoxylin and mounted with DPX and visualized under bright field light microscope. Ten fields were examined in each section to count the number of islet (10x). On higher magnification (40x), insulin positive cells in islets were counted randomly on immunohistochemistry.
Immunofluorescence: Tissue sections were incubated with blocking solution (10% bovine serum albumin) for 30 min at room temperature. For the staining of insulin, the pancreatic tissue sections were incubated with a mouse anti-insulin antibody (primary antibody, 1:300 v/v, Sigma -Aldrich, USA ) at room temperature for two hours and further incubated with 1: 200 dilution of secondary antibody i.e. goat anti- mouse IgG- allophycocyanin (APC, Santa Cruz, USA ) for one hour. For BrdU staining, sections were pre-treated with 1N HCl for one hour at 30 °C, then incubated with anti-mouse anti-BrdU antibody (primary antibody, 1:1000 v/v, Sigma -Aldrich, USA) at room temperature for two hours followed by incubation with secondary antibody i.e. goat anti-mouse IgG- FITC (1:100, Sigma-Aldrich, USA) for one hour. Further, the sections were double stained with insulin and BrdU and were mounted with phosphate buffer glycerine visualized under a fluorescent microscope.
Electron microscopy: Tissue was taken out from paraffin block and deparaffinised in xylene. The tissue was treated with descending concentrations of alcohol to remove xylene. It was incubated with Sorensen buffer with sucrose at 4°C for 30 min and 1 per cent osmic acid for two hours at 4°C. Later, it was treated with propylene oxide and epon mixture and embedded in rubber mould and polymerized at 60°C. Then semi-thin sections were cut (0.5 micron) with ultramicrotome (Leica, EMU16). The sections were taken over 200 mesh nickel grade. The grids were stained with uranyl acetate and 1 per cent lead citrate and examined on electron microscope (7500; Hitachi, Japan)
Statistical analysis: Intra-group comparisons were done by repeated measures ANOVA. Inter-group comparisons were made using one-way ANOVA followed by post-hoc Bonferroni's test. The statistical analysis was carried out using the SPSS version 22 software for window (SPSS Inc., Chicago, USA).
Results
Isolation, characterisation and labelling of MSCs: After in vitro culture for 10-12 days cells having fibroblast like morphology adhered to flask surface (Fig. 1A). Flow cytometeric analysis showed a high expression of CD29 and CD54 (Fig. 1B) and negative staining for CD34 and CD45 (Fig. 1C, D) markers indicating that cultured cells were of mesenchymal origin as well as of high purity without haematopoietic lineage cell contamination. Before transplantation, the nuclei of MSCs were labelled with BrdU. Further, the efficacy of labelling was assessed by staining with anti-BrdU antibody and flow cytometery revealed 90 per cent (n=6) of these MSCs were stained positively for BrdU (Fig. 1E) and cell viability (92%) with trypan blue.
Fig. 1.
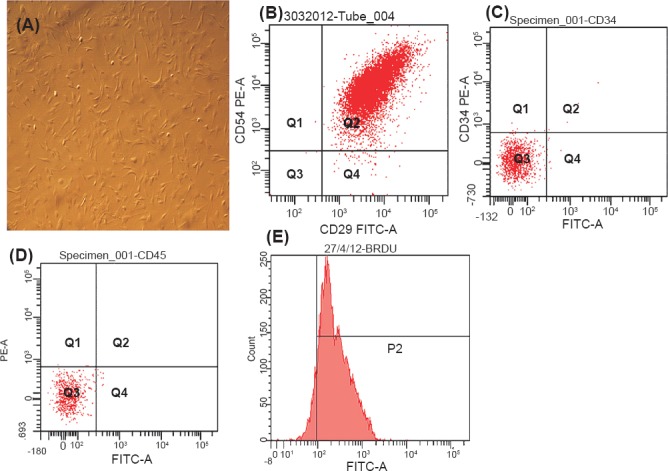
(A). Photomicrographs of cultured mesenchymal stem cells (20X magnification), (B) Flow cytometry analysis of percentage of dual positive (CD29 and CD54) cultured mesenchymal stem cells, (C, D) Flow cytometry analysis of percentage of negative marker (CD 34 and CD 45) for haematopoietic lineage, and (E) Flow cytometry analysis of percentage of labelling of mesenchymal stem cells with anti- BrdU.
Effect of MSCs transplantation in diabetic Wistar rat: All rats fulfilled the criteria of diabetes after streptozotocin as defined by an increase in non-fasting blood glucose level more than 200 mg/dl on multiple occasions. The weight at baseline in all the groups was similar. Administration of STZ resulted in significant decrease in body weight at day 17 by 14 per cent in groups 2 and 3 (P<0.001 for each), while rats fed on normal diet in the control group gained 6 per cent increase in body weight from baseline. After transplantation of MSCs on days 17 and 24, the rate of decline in body weight was slower in MSCs treated group as compared to the diabetic group (-16 v/s -20%), however, it was not significant (Table).
Table.
Weight, blood glucose level, number of islet and number of insulin positive cells in the different study groups
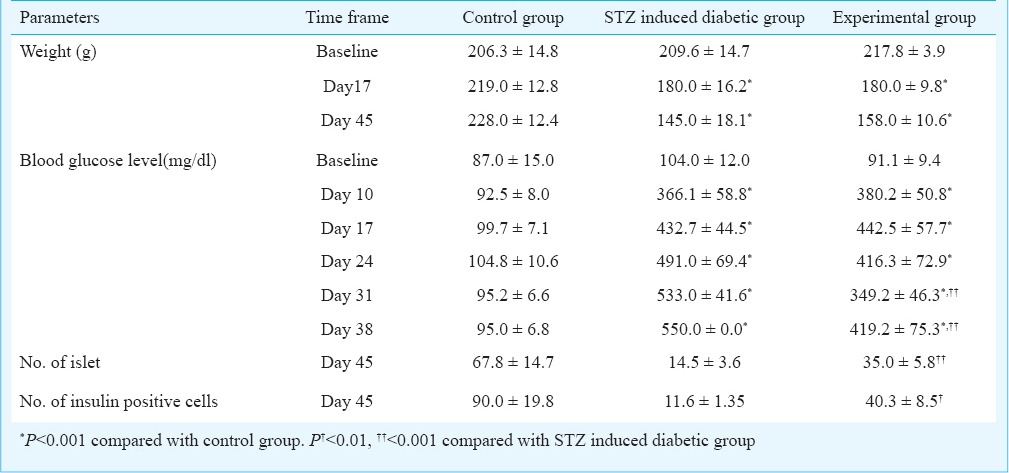
The blood glucose at baseline was similar in all the groups and after administration of STZ, blood glucose level significantly increased from normal to hyperglycaemic level in both group 2 (P<0.001) and group 3 (P<0.001). Administration of MSCs on days 17 and 24 caused a significant decrease in blood glucose level at days 31 and 38 in the experimental group compared with STZ induced diabetic group (P<0.001) (Table).
The assessment of β-cells function in the experimental group was characterized by IPGTT. It was observed that STZ induced diabetic group remained glucose intolerant throughout the study period whereas the experimental group showed a significant improvement in glucose tolerance curves (P<0.001) (Fig. 2).
Fig. 2.
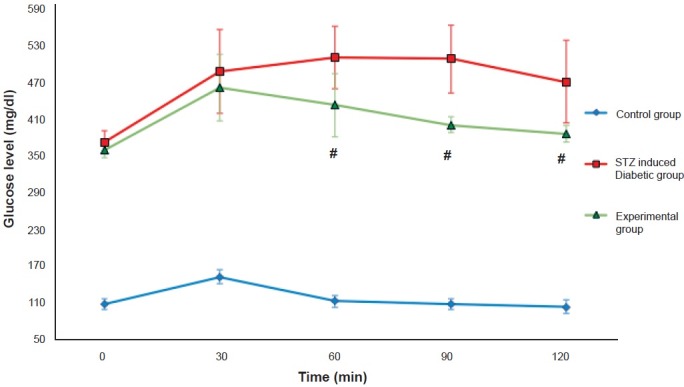
Glucose profile after intraperitoneal glucose tolerance test (IPGTT) in different study groups at the end of the study. Values are mean ± SD (n=6). #P<0.001 compared with STZ induced diabetic group.
Histological assessment of the pancreatic tissue stained with haematoxylin and eosin showed destruction of islet tissue and low insulin reactivity on immunohistochemistry and immunofluorescence in the diabetic group as compared with the control group. Treatment with MSCs in diabetic Wistar rats resulted in reorganization of islet and partial restoration of β-cell as indicated by high insulin reactivity compared with the STZ induced diabetic group as shown in Fig. 3A, B and C. There was a significant increase in number of islet and insulin positive cells the experimental group as compared to the STZ induced diabetic group as shown on immunohistochemistry (P<0.001 and P<0.01) (Table).
Fig. 3.
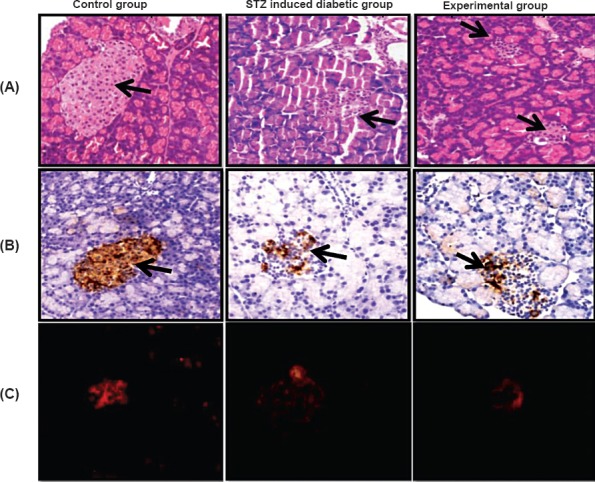
(A). Histology of the rat pancreas from control group, streptozotocin induced diabetic group and experimental group showing pancreatic islets at a magnification of 20X. H&E (arrows), (B) insulin reactivity in pancreatic tissue (arrow) shown by immunohistochemistry, and (C). immunofluorescence.
Electron microscopy showed dense core granules in cytoplasm indicating neuroendocrine cells in MSCs transplanted group (Fig. 4).
Fig. 4.
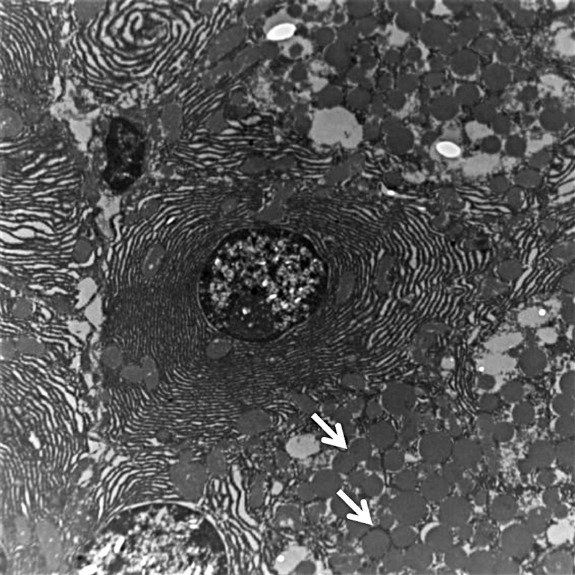
Electron microscopy showing dense core secretory granules (arrow) indicating endocrine differentiation (10000X).
Localization of transplanted MSCs in recipient pancreas: The co-localization of anti-insulin and anti-BrdU antibodies in MSCs treated diabetic group showed that a few of the transplanted MSCs were either trans-differentiated into insulin producing cells or fused with insulin producing cells as compared to diabetic group (Fig. 5).
Fig. 5.
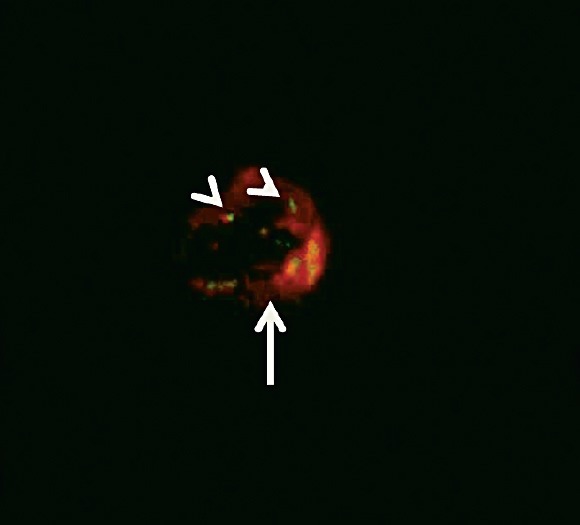
Immunofluorescence on pancreatic islet tissue showing double staining of MSCs with BrdU (green in colour and depicted as arrow head) and insulin (red in colour and depicted as arrow) in the experimental study group (20X).
Discussion
Our study demonstrated that allogenic MSCs transplantation significantly decreased the blood glucose level in streptozotocin induced diabetic Wistar rat. The localization of BrdU labelled mesenchymal stem cells in the islet confirmed their localization in the pancreas and increase in number of islet and insulin positive cells on immunohistochemistry suggest regeneration of β-cells.
Current medical therapies targeting insulin resistance include metformin and pioglitazone, whereas therapy targeting β-cells dysfunction/mass include pioglitazone and glucagon like peptide-1 (GLP-1) agonistic analogue. GLP-1 agonistic analogue have been successfully shown to have islet neogenesis in mice but not in humans13. Therefore, there is a growing interest in cell based therapies which include allogenic or autologous bone marrow derived stem cells transplantation7 or placenta derived mesenchymal stem cells14 in the management of diabetes.
Studies both with haematopoietic stem cells15 and mesenchymal stem cells16 have shown encouraging results in rats. These studies showed that there was an improvement in the glucose profile and development of new islet on histology. Banerjee et al17 administered multiple injections of haematopoietic stem cells in streptozotocin induced diabetes in B alB/c mice and demonstrated a significant reduction in blood glucose level and increased islet neogenesis. Azab et al18 used the haematopoietic stem cells and MSCs derived from human bone marrow and injected separately in alloxan induced diabetic rats. They demonstrated a significant reduction in blood glucose level in these rats and trans-differentiation of MSCs into insulin producing cells in vitro. As expected, the MSCs are likely to have a better outcome than haematopoietic stem cells because of their multipotency, higher proliferative capacity and less restricted lineage to differentiate. However, the overall outcome was more favourable in the haematopoietic stem cells group than in the MSCs in the study, possibly because various growth factors along with haematopoietic stem cells incited residual β-cells. Our study showed significant reduction in blood glucose level after administration of allogenic MSCs into the diabetic rat model.
Transplanted BrdU labelled MSCs in the pancreas of the recipient rats as shown by immunofluorescence, suggested selective localization of these MSCs into the pancreas. The ability of transplanted MSCs to ‘home in’ at the site of tissue injury has been demonstrated in fractured bone, myocardial infarction and ischaemic cerebral injury19,20,21. Sordi et al22 have demonstrated that MSCs are attracted by pancreatic islet both in vitro and in vivo and that the chemokine CXCL12 and its ligand CXCR4 play an important role in ‘homing in’. Therefore, the ability of pancreatic islets to allure MSCs suggests a potential role for these cells in β-cells replacement therapy.
The dose of MSCs is an important determinant of glucose -insulin homeostasis outcome. The doses varying from 2-10 million cell either as single injection18 or as multiple injections17 have been used in animal experiments. The higher dose and the multiple injections have been shown to have a favourable effect on glycaemic profile. In our study two injections with a mean dose of 4.8 million MSCs each, were administered. Despite high doses and two injections of MSCs, euglycaemia could not be achieved, and there was a rise in blood glucose level after two weeks of second injection of MSCs. This ill sustained effect on glucose profile has been attributed to glucotoxity and/or graft rejection23.
The mechanisms implicated in improvement in the β cell mass and/ or function include fusion of MSCs with islet cells or trans-differentiation of MSCs into β-cells. Yanai et al24 showed that co-transplantation of electrofused MSCs and islet cell in rats improved blood glucose profile due to bi-directional reprogramming of both β-cells and MSCs nuclei, thereby allowing the insulin gene expression. Moreover, it also resulted in increased islet cells proliferative capability and decreased apoptosis. The possibility of cell fusion is very likely in our study as substantiated by the presence of both BrdU labelled MSCs and anti-insulin antibody in islet cells. This will translate into increased insulin secretion and improvement in glucose profile without any increase in number of islets. The trans-differentiation of MSCs into β-cells is another mechanism of potentiation of β-cell function. It can be proved only by labelling MSCs with green fluorescent protein (GFP) but this procedure could not be done in our study. However, the surrogate evidences of trans-differentiation which have been demonstrated in our study are increased number of islet and higher number of insulin positive cells in MSCs transplanted group. In addition, increased number of neuroendocrine cells on electron microscopy further supported this finding.
Another potential mechanism of development of new islet is stimulation of resident stem cells in the pancreatic duct to differentiate into islet. The SRY (sex determining region Y) box-9 (Sox9) which is a member of the SRY related transcription factor plays a crucial role in the development of several organs during embryonic period including testis, heart, pancreas, bile duct and central nervous system. The resident stem cells in the pancreatic duct expressing Sox-9 have a capacity to differentiate into endocrine cells but they lose their capability soon after birth. However, the hepatic progenitor cells in the biliary tree have a switch from Sox 9- negative to Sox9- positive and may differentiate into islet. This suggests that adult liver, exocrine pancreas and intestine as a unit provide a source of continuous cell supply of Sox-9 expressing progenitor cells thereby helping in islet differentiation25.
Several studies have shown beneficial effect of MSCs in amelioration of hyperglycaemia in diabetic rats18. However, one study has shown an improvement in insulin sensitivity with MSCs transplantation16. It was attributed to an increase in GLUT-4 expression and enhanced phosphorylation of insulin receptor substrate-1(IRS-1) and Akt (protein kinase B) in skeletal muscle. So administration of MSCs not only improves β-cell function but also decreases insulin resistance16,26.
The strength of the study includes multiple injections of MSCs, demonstration of beneficial effect of allogenic MSCs transplantation in non-irradiated diabetic rats and successful localization of MSCs into islets and β-cells regeneration. The limitations of the study include small sample size, shorter duration of follow up and failure to demonstrate trans-differentiation
In conclusion, mesenchymal stem cells transplantation in streptozotocin induced diabetic Wistar rat resulted in a decrease in blood glucose level associated with increased islet neogenesis. Further studies need to be done to understand the mechanism.
Acknowledgment
This study was financially supported by the Endocrine Society of India, Hyderabad, India and Department of Biotechnology, Ministry of Science of Technology, Government of India.
Footnotes
Conflicts of interest: None.
References
- 1.IDF diabets atlas. 6th ed. Brussels, Belgium: IDF; 2013. international Diabetes Federation (IDF) [PubMed] [Google Scholar]
- 2.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 3.Rother KI. Diabetes treatment--bridging the divide. N Engl J Med. 2007;356:1499–501. doi: 10.1056/NEJMp078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 5.Halban PA, German MS, Kahn SE, Weir GC. Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab. 2010;95:1034–43. doi: 10.1210/jc.2009-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Q, Luo S, Jin H, Cai J, Jia H, Feng L, et al. Insulin-producing cells from human adipose tissue-derived mesenchymal stem cells detected by atomic force microscope. Appl Microbiol Biotechnol. 2012;94:479–86. doi: 10.1007/s00253-012-3904-8. [DOI] [PubMed] [Google Scholar]
- 7.Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N, et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18:1407–16. doi: 10.1089/scd.2009.0164. [DOI] [PubMed] [Google Scholar]
- 8.Zhu D, Chen L, Hong T. Position Statement of the Chinese Diabetes Society regarding stem cell therapy for diabetes. J Diabetes. 2012;4:18–21. doi: 10.1111/j.1753-0407.2011.00166.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathews V, Hanson PT, Ford E, Fujita J, Polonsky KS, Graubert TA. Recruitment of bone marrow-derived endothelial cells to sites of pancreatic beta-cell injury. Diabetes. 2004;53:91–8. doi: 10.2337/diabetes.53.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–70. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 12.Ventura-Sobrevilla J, Boone-Villa VD, Aguilar CN, Roman-Ramos R, Vega-Avila E, Campos-Sepulveda E, et al. Effect of varying dose and administration of streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol Soc. 2011;54:5–9. [PubMed] [Google Scholar]
- 13.Portha B, Tourrel-Cuzin C, Movassat J. Activation of the GLP-1 receptor signalling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res. 2011;2011:376509. doi: 10.1155/2011/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang X, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med. 2011;5:94–100. doi: 10.1007/s11684-011-0116-z. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Song L, Shen K, Wang H, Niu W, Qin X. Transplantation of bone marrow derived cells promotes pancreatic islet repair in diabetic mice. Biochem Biophys Res Commun. 2008;371:132–7. doi: 10.1016/j.bbrc.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, et al. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. 2012;61:1616–25. doi: 10.2337/db11-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee M, Kumar A, Bhonde RR. Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem Biophys Res Commun. 2005;328:318–25. doi: 10.1016/j.bbrc.2004.12.176. [DOI] [PubMed] [Google Scholar]
- 18.Azab N NI, Al Kholy A AF, Salem RF, Gabr H, EI Abd AM. Comparison between bone marrow derived mesenchymal stem cells and hematopoietic stem cells in Â-islet transdifferentiation. Stem Cell. 2011;2:1–10. [Google Scholar]
- 19.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–74. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 20.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 21.Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–27. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 22.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–27. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 23.Eizirik DL, Korbutt GS, Hellerstrom C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992;90:1263–8. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanai G, Hayashi T, Zhi Q, Yang KC, Shirouzu Y, Shimabukuro T, et al. Electrofusion of mesenchymal stem cells and islet cells for diabetes therapy: a rat model. PLoS One. 2013;8:e64499. doi: 10.1371/journal.pone.0064499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 26.Pileggi A. Mesenchymal stem cells for the treatment of diabetes. Diabetes. 2012;61:1355–6. doi: 10.2337/db12-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]


