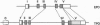Abstract
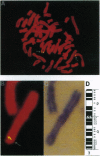
Thrombopoietin (TPO), a lineage-specific cytokine affecting the proliferation and maturation of megakaryocytes from committed progenitor cells, is believed to be the major physiological regulator of circulating platelet levels. Recently we have isolated a cDNA encoding a ligand for the murine c-mpl protooncogene and shown it to be TPO. By employing a murine cDNA probe, we have isolated a gene encoding human TPO from a human genomic library. The TPO locus spans over 6 kb and has a structure similar to that of the erythropoietin gene (EPO). Southern blot analysis of human genomic DNA reveals a hybridization pattern consistent with a single gene locus. The locus was mapped by in situ hybridization of metaphase chromosome preparations to chromosome 3q26-27, a site where a number of chromosomal abnormalities associated with thrombocythemia in cases of acute myeloid leukemia have been mapped. A human TPO cDNA was isolated by PCR from kidney mRNA. The cDNA encodes a protein with 80% identity to previously described murine TPO and is capable of initiating a proliferative signal to murine interleukin 3-dependent BaF3 cells expressing the murine or human TPO receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew C., Ihle J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991 Apr;11(4):1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R., Bagg A., Pinto M., Lewis D., Mendelow B. Chromosome 3q21 abnormalities associated with hyperactive thrombopoiesis in acute blastic transformation of chronic myeloid leukemia. Blood. 1986 Sep;68(3):652–657. [PubMed] [Google Scholar]
- Carbonell F., Hoelzer D., Thiel E., Bartl R. Ph1 -positive CML associated with megakaryocytic hyperplasia and thrombocythemia and an abnormality of chromosome no. 3. Cancer Genet Cytogenet. 1982 Jun;6(2):153–161. doi: 10.1016/0165-4608(82)90080-2. [DOI] [PubMed] [Google Scholar]
- Carrington P. A., Hill R. J., Stenberg P. E., Levin J., Corash L., Schreurs J., Baker G., Levin F. C. Multiple in vivo effects of interleukin-3 and interleukin-6 on murine megakaryocytopoiesis. Blood. 1991 Jan 1;77(1):34–41. [PubMed] [Google Scholar]
- Evatt B. L., Levin J. Measurement of thrombopoiesis in rabbits using 75selenomethionine. J Clin Invest. 1969 Sep;48(9):1615–1626. doi: 10.1172/JCI106127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evatt B. L., Shreiner D. P., Levin J. Thrombopoietic activity of fractions of rabbit plasma: studies in rabbits and mice. J Lab Clin Med. 1974 Mar;83(3):364–371. [PubMed] [Google Scholar]
- Foster D. C., Rudinski M. S., Schach B. G., Berkner K. L., Kumar A. A., Hagen F. S., Sprecher C. A., Insley M. Y., Davie E. W. Propeptide of human protein C is necessary for gamma-carboxylation. Biochemistry. 1987 Nov 3;26(22):7003–7011. doi: 10.1021/bi00396a022. [DOI] [PubMed] [Google Scholar]
- Hoffman R. Regulation of megakaryocytopoiesis. Blood. 1989 Sep;74(4):1196–1212. [PubMed] [Google Scholar]
- Ishibashi T., Kimura H., Shikama Y., Uchida T., Kariyone S., Hirano T., Kishimoto T., Takatsuki F., Akiyama Y. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989 Sep;74(4):1241–1244. [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- KELEMEN E., CSERHATI I., TANOS B. Demonstration and some properties of human thrombopoietin in thrombocythaemic sera. Acta Haematol. 1958 Dec;20(6):350–355. doi: 10.1159/000205503. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., Lok S., Holly R. D., Broudy V. C., Lin N., Bailey M. C., Forstrom J. W., Buddle M. M., Oort P. J., Hagen F. S. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994 Jun 16;369(6481):568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- Kievits T., Dauwerse J. G., Wiegant J., Devilee P., Breuning M. H., Cornelisse C. J., van Ommen G. J., Pearson P. L. Rapid subchromosomal localization of cosmids by nonradioactive in situ hybridization. Cytogenet Cell Genet. 1990;53(2-3):134–136. doi: 10.1159/000132913. [DOI] [PubMed] [Google Scholar]
- Law M. L., Cai G. Y., Lin F. K., Wei Q., Huang S. Z., Hartz J. H., Morse H., Lin C. H., Jones C., Kao F. T. Chromosomal assignment of the human erythropoietin gene and its DNA polymorphism. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6920–6924. doi: 10.1073/pnas.83.18.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Kaushansky K., Holly R. D., Kuijper J. L., Lofton-Day C. E., Oort P. J., Grant F. J., Heipel M. D., Burkhead S. K., Kramer J. M. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994 Jun 16;369(6481):565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- Manavalan P., Swope D. L., Withy R. M. Sequence and structural relationships in the cytokine family. J Protein Chem. 1992 Jun;11(3):321–331. doi: 10.1007/BF01024870. [DOI] [PubMed] [Google Scholar]
- McDonald J. D., Lin F. K., Goldwasser E. Cloning, sequencing, and evolutionary analysis of the mouse erythropoietin gene. Mol Cell Biol. 1986 Mar;6(3):842–848. doi: 10.1128/mcb.6.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. P. A comparison of platelet size, latelet count, and platelet 35S incorporation as assays for thrombopoietin. Br J Haematol. 1976 Oct;34(2):257–267. doi: 10.1111/j.1365-2141.1976.tb00196.x. [DOI] [PubMed] [Google Scholar]
- McDonald T. P. Bioassay for thrombopoietin utilizing mice in rebound thrombocytosis. Proc Soc Exp Biol Med. 1973 Dec;144(3):1006–1012. doi: 10.3181/00379727-144-37730. [DOI] [PubMed] [Google Scholar]
- McDonald T. P., Clift R., Lange R. D., Nolan C., Tribby I. I., Barlow G. H. Thrombopoietin production by human embryonic kidney cells in culture. J Lab Clin Med. 1975 Jan;85(1):59–66. [PubMed] [Google Scholar]
- McDonald T. P., Cottrell M., Clift R., Khouri J. A., Long M. D. Studies on the purification of thrombopoietin from kidney cell culture medium. J Lab Clin Med. 1985 Aug;106(2):162–174. [PubMed] [Google Scholar]
- McDonald T. P. Thrombopoietin. Its biology, clinical aspects, and possibilities. Am J Pediatr Hematol Oncol. 1992 Spring;14(1):8–21. doi: 10.1097/00043426-199221000-00002. [DOI] [PubMed] [Google Scholar]
- McDonald T. P. Thrombopoietin: its biology, purification, and characterization. Exp Hematol. 1988 Mar;16(3):201–205. [PubMed] [Google Scholar]
- Methia N., Louache F., Vainchenker W., Wendling F. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood. 1993 Sep 1;82(5):1395–1401. [PubMed] [Google Scholar]
- Mitani K., Ogawa S., Tanaka T., Miyoshi H., Kurokawa M., Mano H., Yazaki Y., Ohki M., Hirai H. Generation of the AML1-EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic crisis in chronic myelocytic leukemia. EMBO J. 1994 Feb 1;13(3):504–510. doi: 10.1002/j.1460-2075.1994.tb06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parganas E., William C. L., Whittaker M. H., Drabkin H., Oval J., Taetle R., Valentine M. B., Ihle J. N. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300-400 kilobases on chromosome band 3q26. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3937–3941. doi: 10.1073/pnas.89.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parker D. S., Mucenski M. L., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell lines. Cell. 1988 Sep 9;54(6):831–840. doi: 10.1016/s0092-8674(88)91175-0. [DOI] [PubMed] [Google Scholar]
- Neben T. Y., Loebelenz J., Hayes L., McCarthy K., Stoudemire J., Schaub R., Goldman S. J. Recombinant human interleukin-11 stimulates megakaryocytopoiesis and increases peripheral platelets in normal and splenectomized mice. Blood. 1993 Feb 15;81(4):901–908. [PubMed] [Google Scholar]
- Nucifora G., Begy C. R., Erickson P., Drabkin H. A., Rowley J. D. The 3;21 translocation in myelodysplasia results in a fusion transcript between the AML1 gene and the gene for EAP, a highly conserved protein associated with the Epstein-Barr virus small RNA EBER 1. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7784–7788. doi: 10.1073/pnas.90.16.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Begy C. R., Kobayashi H., Roulston D., Claxton D., Pedersen-Bjergaard J., Parganas E., Ihle J. N., Rowley J. D. Consistent intergenic splicing and production of multiple transcripts between AML1 at 21q22 and unrelated genes at 3q26 in (3;21)(q26;q22) translocations. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4004–4008. doi: 10.1073/pnas.91.9.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington D. G. Isotope bioassay for "thrombopoietin". Br Med J. 1970 Mar 7;1(5696):606–608. doi: 10.1136/bmj.1.5696.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M. R., King M. A., Goss G. D., Bezwoda W. R., Fernandes-Costa F., Mendelow B., McDonald T. P., Dowdle E., Bernstein R. Acute megakaryoblastic leukaemia with 3q inversion and elevated thrombopoietin (TSF): an autocrine role for TSF? Br J Haematol. 1985 Dec;61(4):687–694. doi: 10.1111/j.1365-2141.1985.tb02883.x. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Shoemaker C. B., Mitsock L. D. Murine erythropoietin gene: cloning, expression, and human gene homology. Mol Cell Biol. 1986 Mar;6(3):849–858. doi: 10.1128/mcb.6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoda R. C., Seldin D. C., Chiang M. K., Peichel C. L., Vogt T. F., Leder P. Murine c-mpl: a member of the hematopoietic growth factor receptor superfamily that transduces a proliferative signal. EMBO J. 1993 Jul;12(7):2645–2653. doi: 10.1002/j.1460-2075.1993.tb05925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souyri M., Vigon I., Penciolelli J. F., Heard J. M., Tambourin P., Wendling F. A putative truncated cytokine receptor gene transduced by the myeloproliferative leukemia virus immortalizes hematopoietic progenitors. Cell. 1990 Dec 21;63(6):1137–1147. doi: 10.1016/0092-8674(90)90410-g. [DOI] [PubMed] [Google Scholar]
- Tayrien G., Rosenberg R. D. Purification and properties of a megakaryocyte stimulatory factor present both in the serum-free conditioned medium of human embryonic kidney cells and in thrombocytopenic plasma. J Biol Chem. 1987 Mar 5;262(7):3262–3268. [PubMed] [Google Scholar]
- Vigon I., Florindo C., Fichelson S., Guenet J. L., Mattei M. G., Souyri M., Cosman D., Gisselbrecht S. Characterization of the murine Mpl proto-oncogene, a member of the hematopoietic cytokine receptor family: molecular cloning, chromosomal location and evidence for a function in cell growth. Oncogene. 1993 Oct;8(10):2607–2615. [PubMed] [Google Scholar]
- Vigon I., Mornon J. P., Cocault L., Mitjavila M. T., Tambourin P., Gisselbrecht S., Souyri M. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N., Eger R. R., Jackson H. M., Nelson D. J. Two-factor requirement for murine megakaryocyte colony formation. J Cell Physiol. 1982 Jan;110(1):101–104. doi: 10.1002/jcp.1041100116. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Hass P. E., Spencer S. D., Malloy B. E., Gurney A. L., Spencer S. A., Darbonne W. C., Henzel W. J., Wong S. C., Kuang W. J. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994 Jun 16;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]