Prostate cancer continues to be the most common cancer type in men and the second leading cause of cancer death in American men (1). Currently, digital rectal exam and serum prostate specific antigen (PSA) are the standard screening methods for prostate cancer in men. Although this screening approach has led to increased detection of prostate cancer, there are also concerns that screening with PSA has not resulted in convincing survival benefits especially in view of the significant side effects associated with treatment (2). Multiparametric magnetic resonance imaging (mpMRI) has been reported to be a useful adjunct to screening and in many studies it is reported to be useful in localizing and staging prostate cancer (3, 4). However, the success rate of mpMRI has been difficult to ascertain across the world due to a lack of uniformity in the acquisition and interpretation standards. In 2012 the European Society of Urogenital Radiology (ESUR) published the first Prostate Imaging Reporting and Data System (PIRADS 1.0) guidelines in order to address this challenge (5). PIRADS 1.0 included assessment of T2-weighted MRI, diffusion-weighted MRI, dynamic contrast-enhanced (DCE) MRI, and, as an option, magnetic resonance spectroscopy (MRS) separately for each lesion based on a 5-point scale. The total PIRADS 1.0 score was a summation of each of these scores resulting in a score range of 3–15 without MRS and 4–20 with MRS. PIRADS 1.0 has been validated in terms of accuracy by several groups with promising accuracy values for diagnosis of clinically significant prostate cancer. A recent meta-analysis of 14 studies (1785 patients) by Hamoen et al. (6) reported the pooled sensitivity and specificity for PIRADS 1.0 as 0.78 (95% CI, 0.70–0.84) and 0.79 (95% CI, 0.68–0.86), respectively. On the other hand, the interobserver agreement has been reported to be only moderate to good (7–9). Additionally, DCE MRI with curve type analysis, which is included in PIRADS 1.0, was found to be of little value for prostate cancer detection. Moreover, MRS was only rarely incorporated in the studies (10–12). Based on the results of these studies and clinical experience, an effort emerged to revise PIRADS 1.0. The PIRADS steering committee of the American College of Radiology (ACR) and the ESUR prostate MRI working group have diligently developed a revised version called PIRADS 2.0 which was made public in early 2015. While PIRADS 2.0 provides extensive information on how to acquire, interpret, and report mpMRI of the prostate, the highlights of the changes compared to PIRADS 1.0 are:
Introduction of the concept of a “dominant sequence” depending on the location of a lesion; in the peripheral zone, the dominant sequence is diffusion-weighted MRI, whereas in the transition zone, the dominant sequence is T2-weighted MRI (Tables 1 and 2).
The role of DCE MRI is secondary to T2-weighted and diffusion-weighted MRI. Instead of being assessed on a 5-point scale, DCE MRI will be reported as negative or positive. In PIRADS 2.0 curve type no longer plays a role. Positive enhancement on DCE MRI is defined as “focal, and; earlier than or contemporaneously with enhancement of adjacent normal prostatic tissues, and; corresponds to suspicious finding on T2-weighted and/or diffusion-weighted MRI.” The role of DCE MRI is restricted to PI-RADS score 3 lesions in the peripheral zone wherein a positive DCE MRI could increase the score from 3 to 4 for that lesion.
Instead of a 15- or 20-point top score, now an overall 5-point (from 1 to 5) PIRADS assessment score is employed (PIRADS 1, very low probability of clinically significant cancer; PIRADS 2, low probability of clinically significant cancer; PIRADS 3, intermediate probability of clinically significant cancer; PIRADS 4, high probability of clinically significant cancer; PIRADS 5, very high probability of clinically significant cancer).
MRS is no longer included in PIRADS 2.0 since it is considered a research pulse sequence.
Table 1.
PIRADS 2.0 scoring for peripheral zone
| DW MRI | T2W MRI | DCE MRI | Overall PIRADS |
|---|---|---|---|
| 1 | Any* | Any | 1 |
|
| |||
| 2 | Any | Any | 2 |
|
| |||
| 3 | Any | − | 3 |
| + | 4 | ||
|
| |||
| 4 | Any | Any | 4 |
|
| |||
| 5 | Any | Any | 5 |
Any indicates a score of 1–5.
DW MRI, diffusion-weighted magnetic resonance imaging; T2W MRI, T2-weighted magnetic resonance imaging; DCE MRI, dynamic contrast-enhanced magnetic resonance imaging; PIRADS, prostate imaging reporting and data system.
Table 2.
PIRADS 2.0 scoring for transition zone lesions
| T2W MRI | DW MRI | DCE MRI | Overall PIRADS |
|---|---|---|---|
| 1 | Any* | Any | 1 |
|
| |||
| 2 | Any | Any | 2 |
|
| |||
| 3 | ≤4 | Any | 3 |
| 5 | Any | 4 | |
|
| |||
| 4 | Any | Any | 4 |
|
| |||
| 5 | Any | Any | 5 |
Any indicates a score of 1–5.
T2W MRI, T2-weighted magnetic resonance imaging; DW MRI, diffusion-weighted magnetic resonance imaging; DCE MRI, dynamic contrast-enhanced magnetic resonance imaging; PIRADS, prostate imaging reporting and data system.
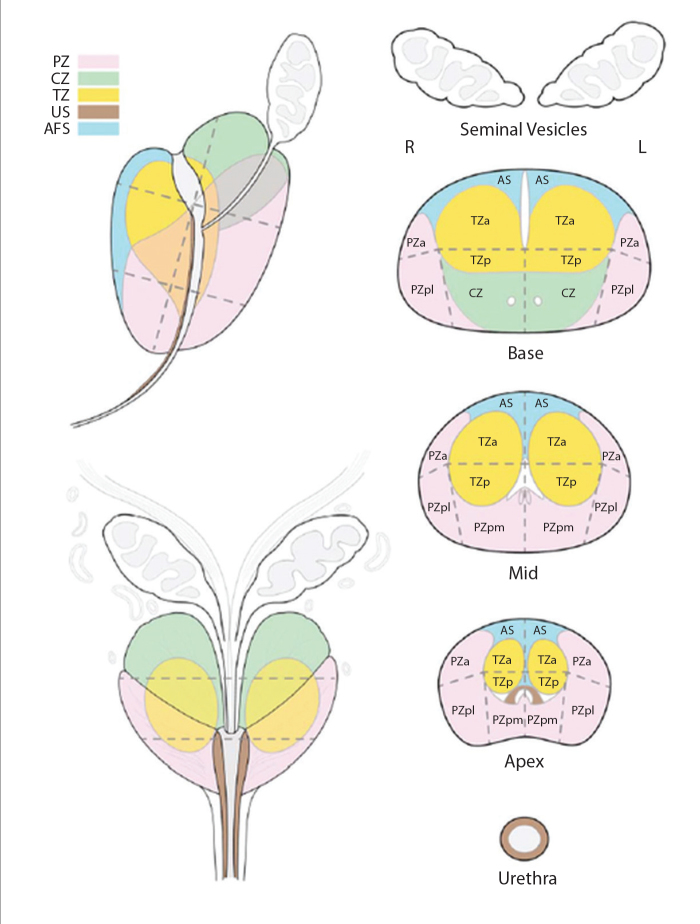
In addition to these changes in the PIRADS 2.0 text, the expert committee provided an updated sector map of the prostate for better communicating the results to referring clinicians and established a lexicon for standardization of terminology for mpMRI (Figs. 1 and 2). The committee is still working on providing report templates and an atlas for practicing radiologists. The most up-to-date versions of PIRADS 2.0 can be found at http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PI-RADS%20V2.pdf (accessed on 2/26/2015).
Figure 1.
Sector map of PIRADS 2.0 from http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf (accessed on 2/26/2015).
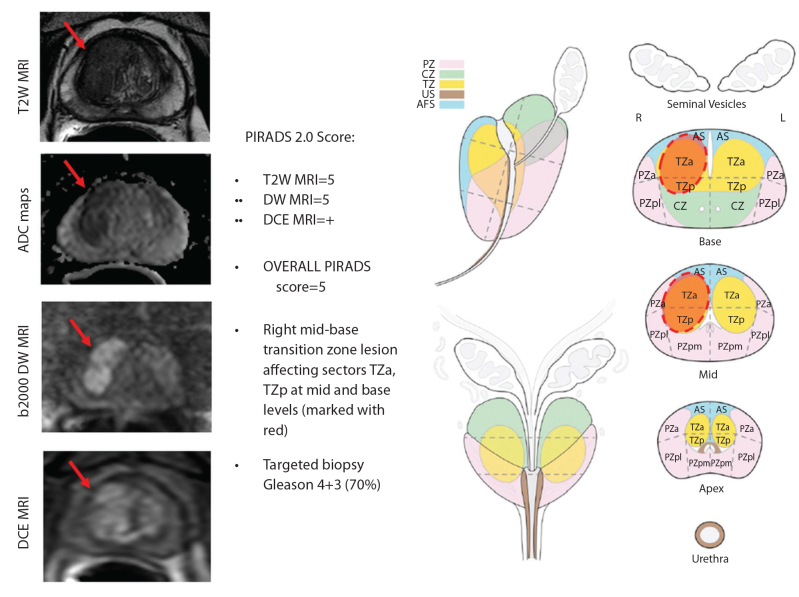
Figure 2.
Sample multiparametric MRI PIRADS evaluation of a 62-year old male with a serum PSA of 17 ng/mL (two negative transrectal ultrasound-guided biopsies prior to MRI).
In conclusion, the aims of developing PIRADS 2.0 were to improve detection, localization, characterization, and risk stratification in patients with suspected cancer in treatment naïve prostate glands and to improve outcomes for patients via establishing minimum standards for mpMRI acquisition, interpretation, and reporting and enhancing the communication between practicing radiologists and clinicians. While these are noble goals to which all can agree, PIRADS 2.0 remains to be tested in multi-center validation trials. PIRADS 2.0 clearly represents a step forward in simplifying the initial efforts of standardization made in PI-RADS 1.0 but will no doubt require its own modification as experience grows and technology evolves.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.American Cancer Society. Cancer facts & figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Miller AB. New data on prostate cancer mortality after PSA screening. N Engl J Med. 2012;366:1047–1048. doi: 10.1056/NEJMe1200185. http://dx.doi.org/10.1056/NEJMe1200185. [DOI] [PubMed] [Google Scholar]
- 3.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. http://dx.doi.org/10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soylu FN, Eggener S, Oto A. Local staging of prostate cancer with MRI. Diagn Interv Radiol. 2012;18:365–373. doi: 10.4261/1305-3825.DIR.4970-11.2. [DOI] [PubMed] [Google Scholar]
- 5.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. http://dx.doi.org/10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamoen EH, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol. 2015;67:1112–1121. doi: 10.1016/j.eururo.2014.10.033. http://dx.doi.org/10.1016/j.eururo.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Schimmöller L, Quentin M, Arsov C, et al. Inter-reader agreement of the ESUR score for prostate MRI using in-bore MRI-guided biopsies as the reference standard. Eur Radiol. 2013;23:3185–3190. doi: 10.1007/s00330-013-2922-y. http://dx.doi.org/10.1007/s00330-013-2922-y. [DOI] [PubMed] [Google Scholar]
- 8.Vaché T, Bratan F, Mège-Lechevallier F, Roche S, Rabilloud M, Rouvière O. Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology. 2014;272:446–455. doi: 10.1148/radiol.14131584. http://dx.doi.org/10.1148/radiol.14131584. [DOI] [PubMed] [Google Scholar]
- 9.Renard-Penna R, Mozer P, Cornud F, et al. Prostate imaging reporting and data system and likert scoring system: multiparametric MR imaging validation study to screen patients for initial biopsy. Radiology. 2015;275:458–468. doi: 10.1148/radiol.14140184. http://dx.doi.org/10.1148/radiol.14140184. [DOI] [PubMed] [Google Scholar]
- 10.Baur AD, Maxeiner A, Franiel T, et al. Evaluation of the prostate imaging reporting and data system for the detection of prostate cancer by the results of targeted biopsy of the prostate. Invest Radiol. 2014;49:411–420. doi: 10.1097/RLI.0000000000000030. http://dx.doi.org/10.1097/RLI.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 11.Junker D, Quentin M, Nagele U, et al. Evaluation of the PI-RADS scoring system for mpMRI of the prostate: a whole-mount step-section analysis. World J Urol. 2015;33:1023–1030. doi: 10.1007/s00345-014-1370-x. [DOI] [PubMed] [Google Scholar]
- 12.Hansford BG, Peng Y, Jiang Y, et al. Dynamic contrast-enhanced MR imaging curve-type analysis: is it helpful in the differentiation of prostate cancer from healthy peripheral zone? Radiology. 2015;275:448–457. doi: 10.1148/radiol.14140847. http://dx.doi.org/10.1148/radiol.14140847. [DOI] [PubMed] [Google Scholar]