Abstract
PURPOSE
We aimed to explore the value of localizing small ground-glass nodules (GGNs; <10 mm) or multiple GGNs within the same lobe in re-aerated lung specimens using CT-guided fine-needle localization.
METHODS
Thirty-five lung specimens containing single small GGNs (<10 mm) and eight specimens containing two or more GGNs in the same lobe were re-aerated with an inflatable aerator. All lesions were localized via CT-guided fine-needle localization following re-aeration. The specimens were then sent for pathologic sampling and qualitative diagnosis.
RESULTS
All 69 nodules from 43 cases were successfully localized using CT-guided fine-needle localization following re-aeration.
CONCLUSIONS
CT-guided fine-needle localization of lesions in surgical specimens under constant, moderate mechanical aeration allows for the rapid and accurate localization of lesions and helps avoid damage from preoperative localization.
In 2011, the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society proposed a new classification for lung adenocarcinomas. The new classification system fully affirmed the role of preoperative computed tomography (CT) examinations in the diagnosis of early-staged lung cancer; the data provided by studies using the new classification system indicate that the lymph node metastasis rates for adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) are extremely low and that patients are expected to have 100% or nearly 100% disease-specific survival following complete resections (1). With the rapid development of multidetector spiral CT (MDCT) and video-assisted thoracoscopic surgery (VATS), not only can small pulmonary nodules be detected clearly but also more and more patients with small nodules that are highly suspicious of lung cancer can be cured with minimally invasive surgery. Atypical adenomatous hyperplasia (AAH), AIS, and MIA often present as pure ground-glass nodules (pGGNs) or mixed ground-glass nodules (mGGNs) containing small amounts of solid elements that are detected on CT images; the diameter of such nodules is often less than 10 mm, and early intervention can improve the prognoses of patients with these lesions. However, it is widely known that these lesions, which are small in volume and low in density, can be extremely difficult to accurately position during surgery and in surgically resected specimens, leading to problems for both thoracic surgeons and pathologists. In addition, it is much more difficult for pathologists to localize all lesions without CT-guided fine-needle localization when two or more GGNs are present in the same lobe. In such cases, the localization of all the GGNs is crucial because the pathologic diagnosis (including gene mutations) of each nodule could help to guide subsequent treatments (1, 2). The aim of this study was to analyze the value of CT-guided fine-needle positioning of lesions that presented as GGNs on CT images under constant, moderate mechanical aeration of the surgical specimens. To the best of our knowledge, this is the first study describing needle localization of small lung nodules in postoperative specimens.
Methods
Patients and specimens
Forty-three consecutive patients were involved in this study (From February 2012 to May 2014), including 16 men (37%) and 27 women (63%), with a mean age of 53.37±11.31 years (range, 27–75 years). All patients were informed and accepted the procedure, and the procedure was approved by the institutional ethics committee. All patients were clinically asymptomatic, and their pulmonary GGNs were identified by CT screening. There were one or more than one small nodules (with a diameter of 10 mm or smaller) in all 43 cases. Of 43 cases, eight had two or more nodules in their resected specimens (Tables 1 and 2). There were 69 nodules in total. Nodules were in the upper lobe of the right lung in 20 cases (47%), in the right middle lobe in six cases (13.9%), in the right lower lobe in five cases (11.6%), in the left upper lobe in six cases (13.9%), and in the left lower lobe in six cases (13.9%). All lesions were considered highly suspicious for early-stage lung adenocarcinoma during the preoperative CT examinations. All patients were operated using VATS; 21 patients (48.8%) underwent pulmonary lobectomy, six patients (13.9%) wedge resection, 15 patients (34.8%) segmentectomy, and one patient sublobar resection. None of the lesions detected by the CT scans were visible or could be palpated on the surface of the surgically resected specimens.
Table 1.
Patient characteristics (35 cases with one small nodule in the specimen)
| Patient no. | Age (y)/sex | Size (mm) | CT finding | Treatment | Pathologic finding | Stage/T category |
|---|---|---|---|---|---|---|
| 1 | 37/F | 6 | pGGN | Segmentectomy | AIS | 0/Tis |
| 2 | 27/F | 8 | pGGN | Segmentectomy | AIS | 0/Tis |
| 3 | 45/M | 7 | pGGN | Segmentectomy | AIS | 0/Tis |
| 4 | 55/M | 8 | pGGN | Segmentectomy | AIS | 0/Tis |
| 5 | 66/F | 9 | pGGN | Segmentectomy | AIS | 0/Tis |
| 6 | 54/M | 6 | pGGN | Wedge resection | AIS | 0/Tis |
| 7 | 70/M | 10 | mGGN | Lobectomy | MIA | 0/T1a |
| 8 | 75/M | 5 | pGGN | Wedge resection | AIS | 0/Tis |
| 9 | 38/F | 6 | pGGN | Wedge resection | AIS | 0/Tis |
| 10 | 50/M | 7 | pGGN | Lobectomy | AIS | 0/Tis |
| 11 | 52/F | 7 | pGGN | Segmentectomy | AIS | 0/Tis |
| 12 | 61/F | 7 | pGGN | Segmentectomy | AIS | 0/Tis |
| 13 | 63/M | 8 | pGGN | Lobectomy | AIS | 0/Tis |
| 14 | 66/F | 8 | mGGN | Lobectomy | MIA | 0/T1a |
| 15 | 34/F | 6 | pGGN | Wedge resection | AIS | 0/Tis |
| 16 | 67/M | 8 | pGGN | Lobectomy | AIS | 0/Tis |
| 17 | 33/F | 9 | pGGN | Lobectomy | AIS | 0/Tis |
| 18 | 71/F | 10 | pGGN | Lobectomy | AIS | 0/Tis |
| 19 | 56/M | 6 | pGGN | Wedge resection | AIS | 0/Tis |
| 20 | 58/F | 7 | pGGN | Segmentectomy | AIS | 0/Tis |
| 21 | 56/F | 8 | pGGN | Lobectomy | AIS | 0/Tis |
| 22 | 53/M | 8 | pGGN | Lobectomy | AIS | 0/Tis |
| 23 | 45/F | 7 | pGGN | Segmentectomy | AIS | 0/Tis |
| 24 | 40/F | 6 | pGGN | Segmentectomy | AIS | 0/Tis |
| 25 | 45/M | 9 | mGGN | Segmentectomy | MIA | 0/T1a |
| 26 | 54/M | 8 | pGGN | Segmentectomy | AIS | 0/Tis |
| 27 | 57/F | 7 | pGGN | Wedge resection | AIS | 0/Tis |
| 28 | 60/F | 7 | mGGN | Segmentectomy | AIS | 0/T1a |
| 29 | 59/F | 9 | pGGN | Lobectomy | AIS | 0/Tis |
| 30 | 49/F | 8 | mGGN | Lobectomy | MIA | 0/T1a |
| 31 | 46/F | 9 | pGGN | Segmentectomy | AIS | 0/Tis |
| 32 | 54/F | 7 | pGGN | Segmentectomy | AIS | 0/Tis |
| 33 | 60/M | 9 | mGGN | Lobectomy | MIA | 0/T1a |
| 34 | 61/F | 10 | pGGN | Lobectomy | AIS | 0/Tis |
| 35 | 66/F | 8 | pGGN | Lobectomy | AIS | 0/Tis |
CT, computed tomography; F, female; pGGN, pure ground-glass nodule; AIS, adenocarcinoma in situ; M, male; mGGN, mixed ground-glass nodule; MIA, minimally invasive adenocarcinoma.
Table 2.
Patient characteristics (two or more small nodules in the specimen)
| Patient no. | Age (y)/sex | No. of lesions | Size (mm) | CT finding | Treatment | Pathologic finding | Stage/T category |
|---|---|---|---|---|---|---|---|
| 1 | 32/M | 1 | 6 | pGGN | Lobectomy | AAH | 0/Tis |
| 2 | 7 | pGGN | AIS | ||||
|
| |||||||
| 2 | 45/F | 1 | 7 | pGGN | Lobectomy | AIS | 0/Tis |
| 2 | 7 | pGGN | AAH | ||||
|
| |||||||
| 3 | 57/F | 1 | 22 | mGGN | Lobectomy | Adenocarcinoma | IA/T1 |
| 2 | 6 | pGGN | AIS | ||||
|
| |||||||
| 4 | 55/F | 1 | 5 | Solid nodule | Lobectomy | Adenocarcinoma | IA/T1a |
| 2 | 6 | pGGN | AAH | ||||
| 3 | 6 | pGGN | AAH | ||||
|
| |||||||
| 5 | 60/M | 1 | 12 | mGGN | Lobectomy | Adenocarcinoma | IA/T1a |
| 2 | 5 | pGGN | AAH | ||||
|
| |||||||
| 3 | 6 | pGGN | AAH | ||||
| 4 | 6 | pGGN | AAH | ||||
| 6 | 66/F | 1 | 6 | pGGN | Lobectomy | AAH | 0/Tis |
| 2 | 8 | pGGN | AIS | ||||
|
| |||||||
| 7 | 46/M | 1 | 8 | pGGN | Lobectomy | AIS | 0/Tis |
| 2 | 6 | pGGN | AAH | ||||
|
| |||||||
| 8 | 51/F | 1 | 6 | pGGN | Sublobar resection | AAH | 0/T0 |
| 2 | 6 | pGGN | AAH | ||||
CT, computed tomography; M, male; pGGN, pure ground-glass nodule; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; F, female; mGGN, mixed ground-glass nodule.
Fine-needle localization and CT examination
Surgical specimens were sent to the CT examination room immediately after resection, without washing, fixation, or incision. An inflatable aerator was used to inflate the lung specimens. The air out-let of an electric pump was connected to a rubber tube, and the end of the rubber tube was connected to a fine needle of the type that is commonly used for clinical infusions. The needle was then inserted into the bronchi of a resected specimen, and the specimen was aerated after ensuring that no leak was present. There were metal clips for sealing the notch in specimens resected by VATS. The lung specimens were well re-aerated although there might have been a small amount of gas leakage because of continuous aeration. The positions we placed the specimens were consistent with the in vivo positions on the examination couch according to the metal clips and marks by thoracic surgeons (up and down, ventral and dorsal). We performed the CT scans when the specimens were considered to be well re-aerated. We reviewed the images and found the location of nodules (CT image-guided), and localized these nodules by fine needles according to the position lines. We used an inflatable aerator that is made for home aquariums, so the pressure was not high enough to destroy the lung structures. When the CT images indicated poor aeration, the specimens were scanned again after inflation with more air; this process was repeated until the lesions were clearly displayed (Fig. 1). After an accurate location of the lesion was obtained in the specimen, in combination with the preoperative CT images for the patient, the fine needle was inserted for positioning the lesion (Fig. 2). The specimens were then sent to the pathology department with the fine needle left in place (Fig. 3).
Figure 1.
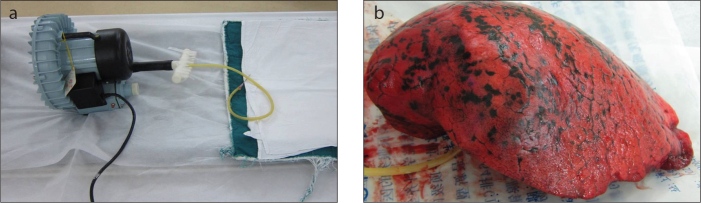
a, b. Inflatable aerator on the CT examination couch is shown in panel (a). Fine-needle localization of the lung nodule on the re-aerated lung specimen is shown in panel (b).
Figure 2.
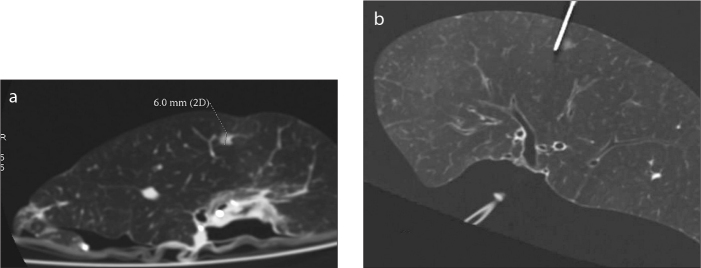
a, b. Reformatted sagittal images after re-aeration show a 6 mm nodule (a, b), and the fine needle positioned near the nodule (b).
Figure 3.
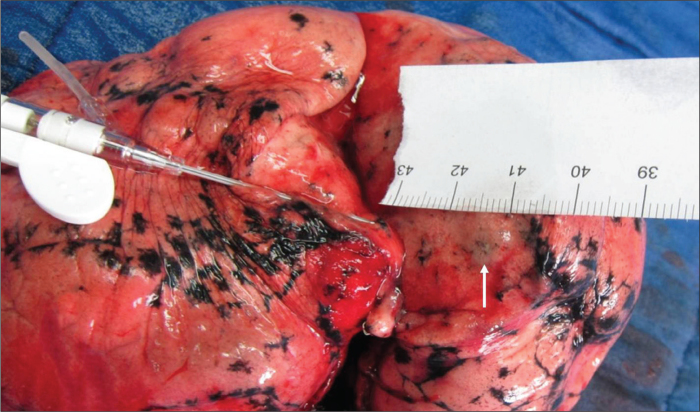
The incision surface (cut along the path of the needle) shows the nodule (white arrow) localized by fine needle.
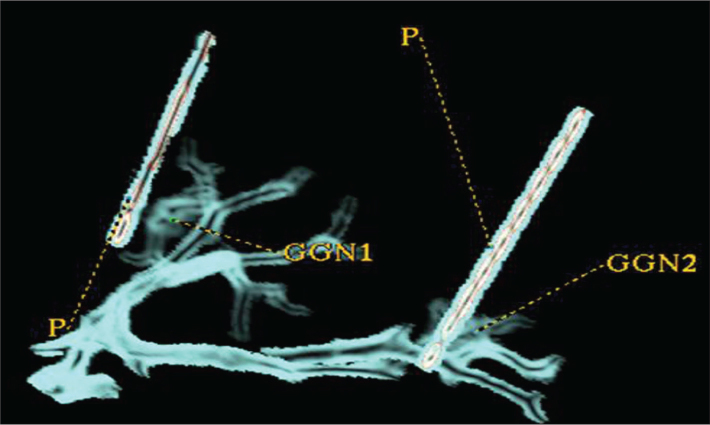
A 64-detector row CT scanner (GE Light speed VCT or GE Discovery CT750 HD, GE Healthcare) was used for both preoperative CT examinations and postoperative specimen scans with the following protocol: 0.625 mm section width with a 0.625 mm reconstruction interval and a pitch of 0.984. All images were reviewed with a high-resolution 20.8-inch (52.8 cm) 2048×1560 pixel gray-scale monitor with standard lung (window width, 1500 HU; window level, −500 HU) and mediastinal (window width, 350 HU; window level, 50 HU) window settings. Multiplanar reconstruction, volume rendering and curved planar reformation were used for further nodule assessment (Fig. 4).
Figure 4.
Volume rendering images showing two GGNs localized with two fine needles (P, fine needle; GGN, ground-glass nodule).
Pathologic diagnosis
The pathologic diagnoses and categorization of AAH, AIS, MIA, and IAC were carried out based on the most recent pulmonary adenocarcinoma classifications (2011 edition) (1). GGNs were resected using video-assisted thoracoscopy or thoracotomy surgery. The specimens were fixed in 10% formalin and embedded in paraffin. Representative hematoxylin-eosin-stained sections were reviewed. In cases of equivocal pathologic classification using light microscopy, immunohistochemical analyses were performed for clarification. All histologic preparations and analyses were performed by two senior pathologists. In cases of disagreement, a mutual consensus was reached after discussion and/or consultation with a third pathologist.
Results
All 43 cases were positioned successfully without damage and with the needle at the edge of the lesion; all samples were obtained successfully. Pathologic diagnoses were established for all 69 nodules (Tables 1 and 2).
Discussion
With the help of CT-guided fine-needle localization, we were able to take pathologic samples successfully from all 43 patients. With high quality samples, definitive pathologic diagnoses were obtained and used as guidelines for subsequent treatments of these patients. Thus, in our daily clinical practice, our multidisciplinary team can operate on more asymptomatic lung nodule patients without worrying about the difficulty of pathologic sampling.
Following the popularization and application of MDCT, an increasing number of ground-glass nodules (GGNs) have been found in otherwise healthy patients during routine checkups. The percentage of malignant lesions found in GGNs is higher than that found in solid pulmonary nodules (3). The majority of malignant lesions in GGNs are adenocarcinomas (1, 3). Both AIS and AAH lesions are classified as preinvasive adenocarcinomas under the new classification system, and the data provided by studies utilizing the new classifications indicate that patients with AIS or MIA have very high five-year survival rate following surgical treatment (1). AIS and MIA lesions are generally very small and often appear as ground-glass opacities on CT images, conventional chest X-rays, and thick-layer CT scans, possibly leading to misdiagnosis. With the development of MDCT technologies and a deeper understanding of the characteristics of early-staged lung cancer by radiologists, the accurate preoperative diagnosis rate for these small lung lesions is continually improving. Furthermore, with the growing popularity of VATS, the risk of surgical resection has been greatly reduced (4). It is often difficult to differentiate neoplastic GGNs from non-neoplastic lesions, such as inflammatory or fibrotic lesions (5).
Following the guidelines of the Fleischner society as well as the ACCP guidelines a resection of such small lesions is rarely indicated (6). However, there are some nodules regarded as highly suspicious of malignancy by radiologists. According to research on CT diagnosis of lung adenocarcinomas, small size of GGNs could also be AIS or MIA (7). Approximately half of these tumors bear a mutation in the epidermal growth factor receptor (EGFR). These tumors have a tendency to occur in the Asian population, particularly in Asian women, and for this reason much of the original work describing their pathologic and clinical characteristics has come from groups in Japan (6). Based on the lung cancer CT screening experience accumulated over the past several years, thousands of subcentimeter nodules were proved to be cancers at the time of resection (6). In cases of suspected invasive adenocarcinoma, some patients underwent lobectomy, which was confirmed as AIS or MIA by pathology. We often encounter patients with multiple primary lung cancers with ground-glass opacity. However, there are no established guidelines regarding the optimal extent of resection for multifocal lung adenocarcinoma (8). Moreover, GGNs are often localized to one lobe of the lung, and although the lobe can be resected, these GGNs are generally too small to be found pathologically if not localized prior to the operation, which can be problematic for both surgeons and pathologists. In other words, all lesions may not be identified or accurately diagnosed by the pathologist if all of the small GGNs within a lobe are not identified prior to surgery. Many lesions cannot be identified by surgeons because the lesions are similar to normal lung tissue in density. Therefore, the identification, localization, and removal of such lesions from specimens is very difficult for thoracic surgeons and pathologists due to the small size and density of these lesions. At worst, the inability to locate lesions can negatively impact histopathologic diagnoses and subsequent treatments (9). Indeed, the pathologic localization of all GGNs less than 10 mm in size can be difficult, or even impossible, in real-world practice, particularly when multiple GGNs are found in the same lobe. This is particularly important in cases with two or more GGNs in the same lobe, in which two or more GGNs are diagnosed as adenocarcinomas. This fact is crucial because it is very important to pathologically diagnose all GGNs for evaluation of prognosis (2). Multiple nodules in the same lobe could be of different gene mutational types such as EGFR gene or K-ras gene mutations (10). Ultimately, the gene mutation types will guide subsequent treatment strategies. Therefore, it is crucial to accurately define the nature of all lesions. In this study, we were able to localize all lesions in the specimens using CT-guided fine-needle localization following re-aeration of the specimens, even in cases where the scapula or hilum was nearby.
When lung nodules suspicious of malignancy are particularly small in size (e.g., less than 10 mm) or low in density, it can be extremely difficult for pathologists to accurately identify the location of the lesions in postoperative specimens, which may require sectioning and microscopic examination of the entire portion of the sample where the lesions may exist. The latter scenario would require significant time and effort to carry out, particularly the intraoperative examination of frozen sections. Indeed, the rapid and accurate localization of lesions can dramatically shorten the anesthesia time, and the accurate localization of lesions can lead to a more accurate histopathologic diagnosis, which can greatly affect the scope of a given operation by indicating an expanded resection or cleaning of local lymph nodes.
There are several techniques used to localize pulmonary nodules: 1) Preoperative or intraoperative injection of methylene blue dye at the site of a pulmonary nodule; 2) Intraoperative ultrasound; 3) Radio-guided detection; 4) CT-guided positioning of a metal wire. Each of these techniques has advantages and drawbacks. Due to overinfusion of the methylene blue dye or errors in the nodule localization, the failure rate for methylene blue injection is approximately 13% (11). Intraoperative ultrasound localization of pulmonary nodules also has limitations; namely, this process is operator dependent and requires a specialized flexible probe and complete collapse of the lung being assessed (12, 13). In contrast, the localization of lesions via preoperative CT scans allows the surgeon to determine the focal positions of lesions quickly and accurately during the operation procedure, reducing intraoperative injuries and aiding in accurate pathologic diagnoses. Preoperative CT-guided hook-wire positioning is one of these techniques, and this technology allows for much greater accuracy in positioning the pulmonary nodules and greatly decreases rates of conversion to thoracotomy. (14). However, this technology also has the following disadvantages: 1) Primary preoperative CT-guided percutaneous fine-needle localization positioning can be traumatic and may lead to complications, including hemorrhage, pneumothorax, hemothorax, and tumor spreading or implantation, among others (15, 16); 2) Between the time the metal hooks are embedded in the tissue and when the lesions are removed during surgery, the positions of the hooks can change (17), affecting the resection of the lesions and sampling of the specimens following the operation; 3) Many lesions are not suitable for hook-wire localization if they are located close to the scapula or hilum.
CT-guided positioning of lesions that present as GGNs on CT images using fine needles under constant, moderate mechanical aeration of the specimen (i.e., maintaining the charging state of the lung tissue) can increase the density of lesions relative to the surrounding lung tissue, allowing for better access and higher quality CT images compared with the preoperative CT examination. This highly accurate method of lesion positioning allows for effective fine-needle localization of surgical specimens and avoids excessive damage to the patient. In addition, this method is suitable for specimens resected during thoracotomy and thoracoscopic surgeries that involve either lobectomy or wedge resection. However, the success of this approach is highly dependent on the airway integrity of the surgical specimens, requiring thoracic surgeons to avoid damaging the specimens as much as possible. In case of wedge resections, the operative incision should be stitched carefully; if leaks are observed during the inflation process, these must be immediately repaired to avoid inflation failure. However, if the surgical specimens being CT-scanned are kept under constant, moderate mechanical aeration, local air leakage may be avoided. If the surgical specimens have not been seriously damaged, then the lesions should be clearly visible as long as the pulmonary segments surrounding the lesions are well aerated.
We do note some limitations of this study, including the small sample size and relatively few specimens with two or more nodules in the same lobe.
In conclusion, based on the cases analyzed here, CT-guided positioning of lesions in surgical specimens using fine needles under constant, moderate mechanical aeration allows for the rapid and accurate localization of lesions and helps avoid preoperative damage from fine-needle localization, thereby effectively decreasing the occurrence of complications in patients.
Main points.
Increasing number of small pulmonary nodules are detected by CT screening, and many of them are resected with minimally invasive surgery for being highly suspicious of malignancy.
After surgery, it is difficult to accurately localize small pulmonary nodules on resected specimens for pathologic sampling.
CT-guided fine-needle localization on re-aerated lung specimens can accurately position small nodules with a high rate of success.
Acknowledgements
Funding: This work has been financially supported by the National Natural Science Foundation of China (Grant No. 81472794), Shanghai Municipal Commission of Health (20134360), Shanghai Municipal Commission of Science and Technology (24119a0400), Key talents training program of Huadong Hospital (HDGG2014011).
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. http://dx.doi.org/10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. http://dx.doi.org/10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 3.Henschke CI, Yankelevitz DF, Mirtcheva R, Mc-Guinness G, McCauley D, Miettinen OS. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002;178:1053–1057. doi: 10.2214/ajr.178.5.1781053. http://dx.doi.org/10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 4.Ciriaco P, Negri G, Puglisi A, Nicoletti R, Del MA, Zannini P. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg. 2004;25:429–433. doi: 10.1016/j.ejcts.2003.11.036. http://dx.doi.org/10.1016/j.ejcts.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui S, Ashizawa K, Minami K, et al. Multiple focal pure ground-glass opacities on high-resolution CT images: clinical significance in patients with lung cancer. AJR Am J Roentgenol. 2010;195:W131–W138. doi: 10.2214/AJR.09.3828. http://dx.doi.org/10.2214/AJR.09.3828. [DOI] [PubMed] [Google Scholar]
- 6.Shrager JB. Approach to the patient with multiple lung nodules. Thorac Surg Clin. 2013;23:257–266. doi: 10.1016/j.thorsurg.2013.01.004. http://dx.doi.org/10.1016/j.thorsurg.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Gu B, Burt BM, Merritt RE, et al. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced disease. Ann Thorac Surg. 2013;96:411–418. doi: 10.1016/j.athoracsur.2013.04.048. http://dx.doi.org/10.1016/j.athoracsur.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda M, Nagashima A, Haro A, Saitoh G. How should synchronous multiple primary adenocarcinomas of the lung be resected? Ann Thorac Surg. 2014;97:e151–e153. doi: 10.1016/j.athoracsur.2014.02.057. http://dx.doi.org/10.1016/j.athoracsur.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Chen L, Qiang G, Chen Z, Jing J, Xiong S. Using an image-guided navigation system for localization of small pulmonary nodules before thoracoscopic surgery: a feasibility study. Surg Endosc. 2007;21:1883–1886. doi: 10.1007/s00464-007-9198-8. http://dx.doi.org/10.1007/s00464-007-9198-8. [DOI] [PubMed] [Google Scholar]
- 10.Chung JH, Choe G, Jheon S, et al. Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol. 2009;4:1490–1495. doi: 10.1097/JTO.0b013e3181bc9731. http://dx.doi.org/10.1097/JTO.0b013e3181bc9731. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999;115:563–568. doi: 10.1378/chest.115.2.563. http://dx.doi.org/10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 12.Nakano N, Miyauchi K, Imagawa H, Kawachi K. Immediate localization using ultrasound-guided hookwire marking of peripheral lung tumors in the operating room. Interact Cardiovasc Thorac Surg. 2004;3:104–106. doi: 10.1016/S1569-9293(03)00222-6. http://dx.doi.org/10.1016/S1569-9293(03)00222-6. [DOI] [PubMed] [Google Scholar]
- 13.Santambrogio R, Montorsi M, Bianchi P, Mantovani A, Ghelma F, Mezzetti M. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg. 1999;68:218–222. doi: 10.1016/s0003-4975(99)00459-2. http://dx.doi.org/10.1016/S0003-4975(99)00459-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc. 2011;25:1723–1729. doi: 10.1007/s00464-010-1502-3. http://dx.doi.org/10.1007/s00464-010-1502-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol. 2012;13:694–701. doi: 10.3348/kjr.2012.13.6.694. http://dx.doi.org/10.3348/kjr.2012.13.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Wang Y, He X, et al. Combination of CT-guided hookwire localization and video-assisted thoracoscopic surgery for pulmonary nodular lesions: Analysis of 103 patients. Oncol Lett. 2012;4:824–828. doi: 10.3892/ol.2012.800. http://dx.doi.org/10.3892/ol.2012.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang DY, Kim HK, Kim YK, Yong HS, Kang EY, Choi YH. Needlescopy-assisted resection of pulmonary nodule after dual localisation. Eur Respir J. 2011;37:13–17. doi: 10.1183/09031936.00021410. http://dx.doi.org/10.1183/09031936.00021410. [DOI] [PubMed] [Google Scholar]