Abstract
PURPOSE
We aimed to evaluate the apparent diffusion coefficient (ADC) values of metastatic lymph nodes in patients with squamous cell carcinoma (SCC) of the head and neck.
METHODS
Patients with metastatic lymph nodes underwent 1.5 Tesla diffusion-weighted magnetic resonance imaging (MRI). The ADC values of the histologically proven metastases were evaluated retrospectively and mean ADC values were compared using one-way analysis of variance test. Receiver operating characteristic analysis was performed to identify ADC threshold values.
RESULTS
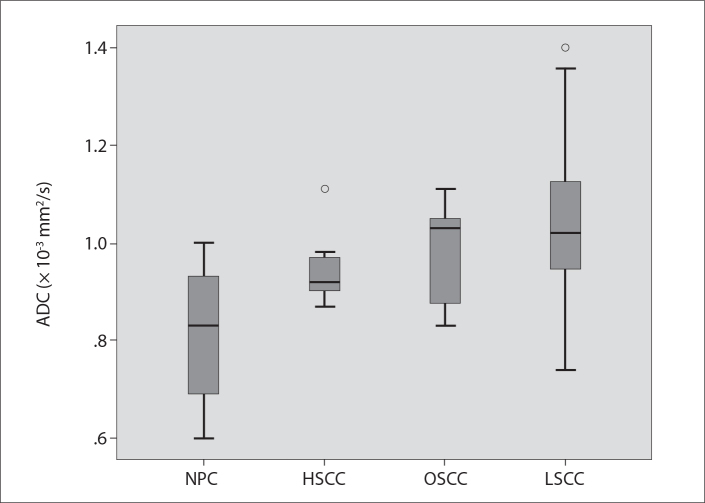
We included 33 patients (27 males, 6 females; mean age, 60.7 years) with 53 metastatic lymph nodes in the study. Mean ADC values for nodal metastases of nasopharyngeal carcinoma (NPC) (n=7), oropharyngeal (n=12), laryngeal (n=27), and hypopharyngeal (n=7) carcinoma were (0.810±0.158)×10−3 mm2/s, (0.985±0.099)×10−3 mm2/s, (1.037±0.150)×10−3 mm2/s, and (0.948±0.081)×10−3 mm2/s, respectively. The mean ADC values of nodal metastases of NPC were significantly lower than ADC values of laryngeal carcinoma (LSCC) (P = 0.002). An ADC value less than 0.890×10−3 mm2/s was found to facilitate differentiation of NPC from LSCC with a sensitivity of 71% and specificity of 85% (area under the curve, 0.852).
CONCLUSION
The mean ADC values showed significant differences between nodal metastases of NPC and LSCC. Considering SCCs as a single group may affect the accuracy of ADC-based differentiation. Location of the primary tumor should be taken into account and cutoff values should be determined separately for each anatomical location.
Diffusion-weighted imaging (DWI) has the potential to characterize and differentiate various head and neck carcinomas (1–4). Differentiating nodal metastases of SCC from other less common tumors of the head and neck is important for treatment planning. Previous studies have shown that apparent diffusion coefficient (ADC) values may be used to differentiate metastatic lymph nodes due to SCC from lymphoma (5). However, the results of some studies indicated that ADC values of SCCs and their nodal metastases (e.g., poorly differentiated SCC and nasopharyngeal carcinoma) may sometimes overlap with the ADC values of lymphoma (5–7). Thus, the efficacy of using DWI for differentiation depends largely on the histologic characteristics of the lymph node.
Approaching all pharyngeal space SCCs as a single homogeneous group may affect the accuracy of ADC-based discrimination of metastatic lymph nodes due to SCC from other tumors. Therefore, we aimed to retrospectively evaluate and compare the ADC values of metastatic lymph nodes from carcinoma of the nasopharynx, oropharynx, larynx, and hypopharynx.
Methods
Patients
This study was approved by the regional ethics committee. From January 2012 to April 2014, conventional magnetic resonance imaging (MRI) and DWI studies of 33 patients with histologically proven metastatic lymph nodes due to SCC were included in the study. Of these, seven had nasopharyngeal carcinoma (NPC; all had non-keratinizing SCC: differentiated, n=5; undifferentiated, n=2), six had hypopharyngeal carcinoma (HPCC; differentiated, n=3; moderately-differentiated n=3), eight had oropharyngeal carcinoma (OPCC; differentiated, n=5; moderately differentiated, n=2; poorly differentiated, n=1) and 12 had laryngeal carcinoma (LPCC; differentiated, n=4; moderately differentiated, n=8). In each patient, all of the nodal metastases size criteria for malignancy (minimum 1 cm short-axis diameter) were selected and mean ADC values were measured retrospectively. Not all patients were treated surgically, but all lymph nodes met the size criteria of metastases and were biopsy-proven SCC metastases.
Of a total of 42 patients, nine were excluded due to susceptibility and/or motion artifacts in DWI that prevented ADC measurements (nodal metastases of the nasopharyngeal carcinoma, n=3; hypopharyngeal carcinoma, n=3; laryngeal carcinoma, n=2; oropharyngeal carcinoma, n=1).
MRI technique
All studies were obtained using a 1.5 Tesla system (Achieva; Philips Medical Systems) using a sensitivity-encoding head-neck coil. All patients underwent conventional MRI of the entire neck. Axial fat-suppressed T2-weighted turbo spin-echo MRI (2500/100 ms repetition time/echo time, 4 mm slice thickness and two signals acquired), axial T1-weighted spin-echo MRI (477/12 ms repetition time/echo time, other parameters were the same as for T2 weighted imaging), and contrast-enhanced T1-weighted MRI were performed.
DWI was performed before contrast-enhanced T1-weighted imaging. Images were obtained in the axial orientation with an echo-planar imaging sequence. The following parameters were used: repetition time, 2000 ms; echo time, 75 ms; b values, 0 and 1000 s/mm2; field of view, 220 mm; matrix size, 256×128; slice thickness, 4 mm; interslice gap, 1 mm; number of signals, 4; acquisition time, 1–2 min. Parallel imaging techniques (SENSE) with a reduction factor of 1 were used. ADC maps were generated automatically on the operating console from concurrent images.
Image analysis
Measurements were performed by a single radiologist on a workstation (Easy Vision, Philips Medical Systems) in the axial plane. Tumors were first evaluated on conventional images. The most solid and/or homogeneous portions of the lesion, according to T2-weighted fat-suppressed, gadolinium-enhanced T1-weighted fat-suppressed, and DWI sequences, were selected for region of interest (ROI) measurement and copied to ADC maps. ROI positions were adjusted to avoid contamination from adjacent tissues and exclude geometric distortions. When necrosis in the signal intensity was observed, at least three small, uniform, round or oval ROIs were placed on solid areas of the ADC map and mean ADC measurements was selected for statistical analyses.
Statistical analysis
For statistical analyses (SPSS 15.0 statistical software for Windows, SPSS Inc.), nodal metastases of head and neck SCCs were categorized as LSCC, OSCC, HSCC, or NPC. A power analysis was performed. The total sample of 53 nodal metastases from 33 patients achieved 82% power to detect differences.
Because of normally distributed data, mean ADC values of nodal metastases were compared with one-way analysis of variance (ANOVA) test. Post hoc pairwise comparisons were then performed using the Bonferroni’s test. In these analyses, P < 0.05 was considered as statistical significant. Then, receiver operating characteristic (ROC) analyses were employed to find ADC threshold values among nodal metastases of SCC. The mean ADC value that corresponded to the nearest point of the ROC curve to the top left corner was chosen as the optimal ADC threshold value for optimizing both sensitivity and specificity in equal weighting.
Results
Final patient population included 33 patients (27 males, six females; mean age, 60.7 years) with 53 nodes (Figs. 1–3). The groups were similar in terms of age and gender. Mean ADC values for nodal metastases of each group are outlined in the Table and displayed in Fig. 4. Upon pairwise comparisons, the mean ADC values of NPC were significantly lower than the mean ADC values of LSCC (P = 0.002). The mean ADC values did not show significant differences between NPC and OSCC (P = 0.062), between NPC and HSCC (P = 0.399), between HSCC and LSCC (P= 0.805), between HSCC and OSCC (P = 1.000) and between LSCC and OSCC (P = 1.000).
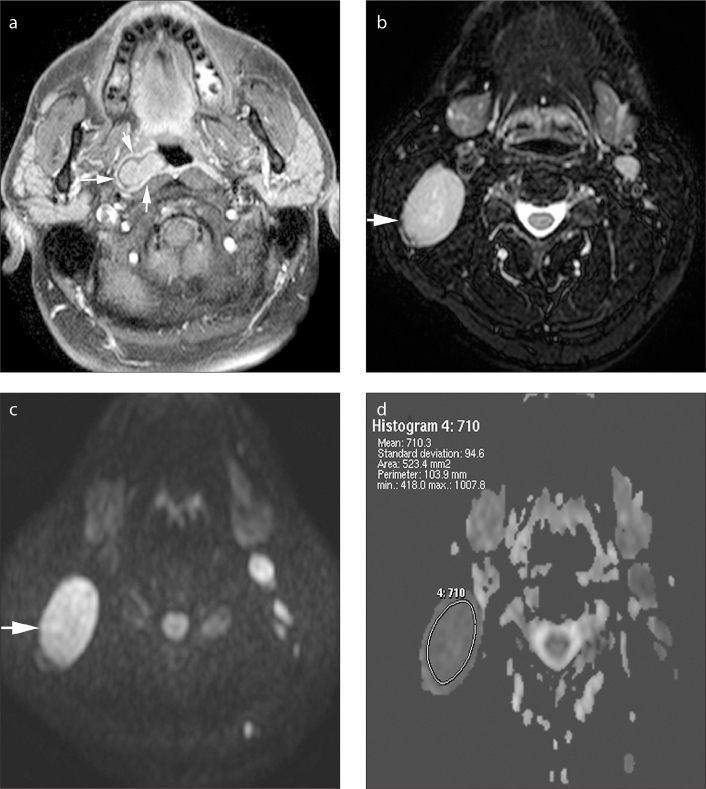
Figure 1.
a–d. Axial gadolinium-enhanced fat-suppressed T1-weighted image (a) in a 43-year-old male patient shows carcinoma of the nasopharynx on the right (arrows). Axial fat-suppressed T2-weighted image (b) demonstrates an enlarged, homogeneous metastatic lymph node at level 2, on the right (arrow). The lymph node is hyperintense on b=1000 s/mm2 DWI (c), and it appears as a hypointense lesion on the ADC map (d). The ADC value is 0.71×10−3 mm2/s.
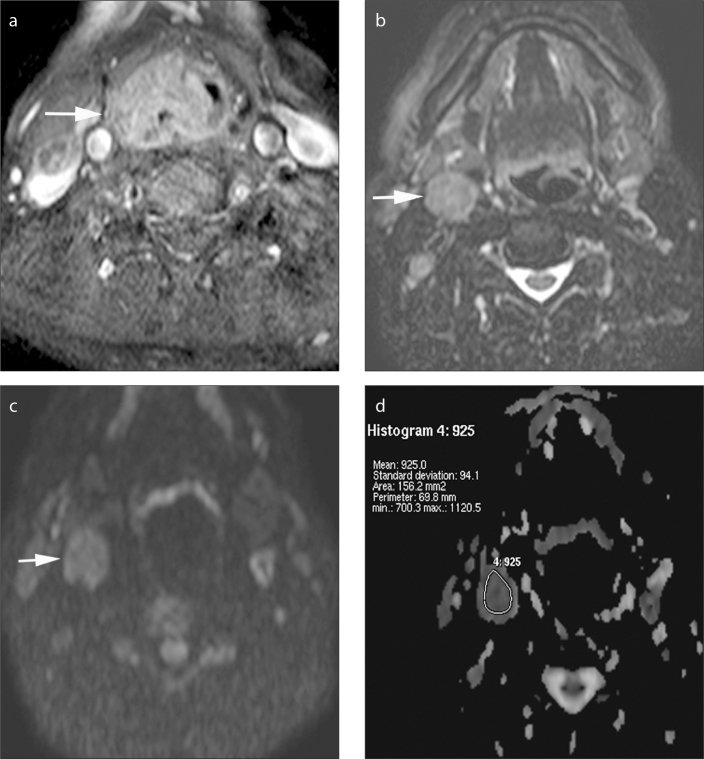
Figure 2.
a–d. Axial gadolinium-enhanced fat-suppressed T1-weighted image (a) in a 65-year-old male patient shows a tumor on the right piriform sinus extending posterior to the pharyngeal wall and larynx (arrow). Axial fat-suppressed T2-weighted image (b) demonstrates an enlarged lymph node at level 2, on the right (arrow). The lymph node is hyperintense on b=1000 s/mm2 DWI (c), and the ADC value is 0.93×10−3 mm2/s on the ADC map (d).
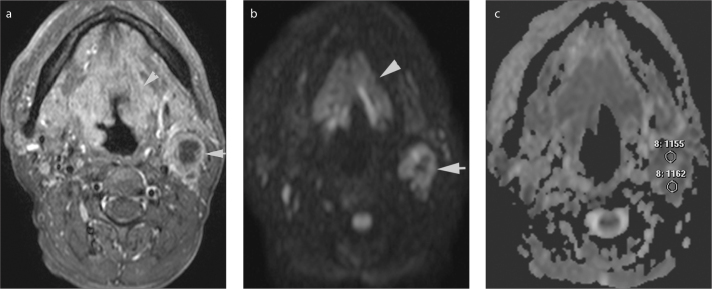
Figure 3.
a–c. Axial gadolinium-enhanced fat-suppressed T1-weighted image (a) in a 53-year-old male shows a squamous cell carcinoma of the supraglottic larynx extending cranially to the base of the tounge (arrowhead). There is a necrotic metastatic lypmh node at level 2, on the left (arrow). On b=1000 s/mm2 DWI (b) the lymph node was hyperintense with a central hypointense region that represents necrosis. On the ADC map (c) multiple small, uniform, round region of interests were placed on solid areas (two of them shown) and mean ADC was selected for statistical analyses.
Table.
The mean apparent diffusion coefficient values of nodal metastases
| Nodal metastases | No. of nodes | ADC (×10−3 mm2/s) | |
|---|---|---|---|
| Mean±SD | Range | ||
| NPC | 7 | 0.810±0.158 | 0.600–1.0 |
| HSCC | 7 | 0.948±0.081 | 0.870–1.11 |
| OSCC | 12 | 0.985±0.099 | 0.830–1.11 |
| LSCC | 27 | 1.037±0.150 | 0.740–1.40 |
ADC, apparent diffusion coefficient; NPC, nasopharyngeal carcinoma; HSCC, hypopharyngeal carcinoma; OSCC, oropharyngeal carcinoma; LSCC, laryngeal carcinoma.
Figure 4.
The mean ADC values of the nodal metastases due to nasopharyngeal carcinoma (NPC), hypopharyngeal carcinoma (HSCC), oropharyngeal carcinoma (OSCC), and laryngeal carcinoma (LSCC). The horizontal bars in the boxes represent mean ADC values. O, outliers.
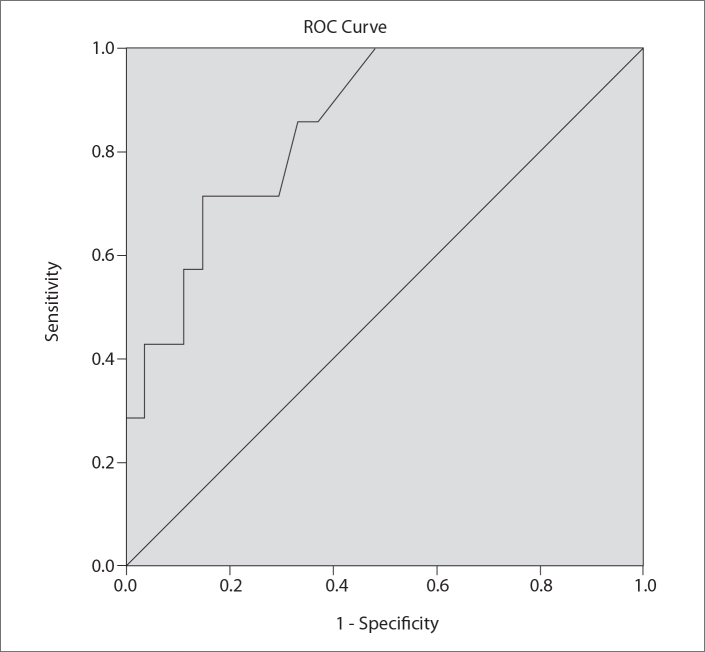
ROC analysis indicated that an ADC threshold value lower than 0.89×10−3 mm2/s may differentiate metastasis of the NPC from LSCC (sensitivity: 71%, specificity: 85%, area under the curve: 0.852) (Fig. 5).
Figure 5.
ROC curve for the ADC values for discrimination of metastatic lymph nodes due to nasopharyngeal carcinoma (NPC) from laryngeal carcinoma (LSCC). ADC threshold value, 0.89×10−3 mm2/s; area under the curve, 0.852; sensitivity, 71%; specificity, 85%; P = 0.005.
Discussion
We evaluated ADC-based differentiation among nodal metastases of SCCs in the head and neck region and found that the mean ADC values of NPC were significantly lower than the mean ADC values of LSCC. Although some ADC threshold values have been determined to distinguish metastases of head and neck SCC from other benign and malignant lymph nodes in this region; according to our study, considering SCCs as a single group may affect the accuracy of ADC-based differentiation (5, 6). Location of the primary tumor is very important and cutoff values could be more accurate when determined separately for each anatomical location.
NPC is very likely to have lower mean ADC values than other SCCs due to its histologic characteristics. NPC has histologic similarities with lymphoma in terms of high cellular density, absence of keratinization, and lower levels of necrotic tissue than other carcinomas (5, 7). Although lymphoma tends to have lower ADC values, comparisons of lymphoma and NPC in the nasopharynx and their nodal metastases have been reported to yield similar ADC values (5, 7). Thus, using DWI to distinguish NPC from lymphoma is challenging and may cause misdiagnosis. According to our study, it is also challenging and may give incorrect results, when ADC values are used to differentiate nodal metastases of NPC from those of OPCC and HPCC.
DWI is increasingly used for nodal staging and has been shown to be especially useful in the demonstration of small nodal metastases (8, 9). Differentiation of benign from malignant lymph nodes was suggested to be possible using ADC values with cutoff values of 1.0×10−3 mm2/s and 0.94×10−3 mm2/s (1, 8). The ADC value has also potential to characterize metastatic lymph nodes (10–13). The histologic characteristics of a tumor directly influence its ADC values, and the different ADC values among metastatic lymph nodes originating from nasopharyx and larynx found in our study may be attributable to variations in histologic composition and content. Therefore, for DWI to be used for characterization and differentiation of lymph nodes, location of the tumor should be considered and comparison of the mean ADC values should be done for each anatomical location individually.
Keratinization is a component of the SCC and reflects desmoplastic stroma infiltrated by varying numbers of cells. It is considered to impair water movement and cause a decrease in the ADC value (7). Nonkeratinizing carcinomas, the most common type of NPC, have solid sheets of cells mixed with lymphocytes and plasma cells, and restrict diffusion due to cell density (7, 8). This restriction of water movement is greater than keratinizing SCC and it is considered the main reason for the lower ADC values in metastatic lymph nodes due to NPC. All patients in our study had nonkeratinizing NPC.
Another important factor that may affect the ADC values is the histologic grade of the head and neck SCC. This has been investigated in few studies, and the relation of histologic grade and the ADC value is still unclear (10, 13, 14). Some investigators suggested that there were no significant differences among different histologic grades (10). While some others suggested that the ADC values are significantly greater in highly and moderately-differentiated SCCs when compared to poorly-differentiated SCCs (13). Recently, a study in a larger patient population with more homogeneous tumor groups showed that DWI with high b values (i.e., b=2000 s/mm2) could differentiate histologic grades of the head and neck SCC (14). In the present study, only one patient had poorly-differentiated SCC (one patient with oropharynx SCC; mean ADC value of nodal metastases, 1.04×10−3 mm2/s). The rest of the oropharyngeal and all laryngeal and hypopharyngeal SCC nodal metastases were either well or moderately differentiated. Although the histologic grades of the SCCs were homogeneous (either well or moderately differentiated) in our study group, the results could be different with a patient group that also includes poorly differerentiated SCC nodal metastases. Therefore, besides location of the primary tumor, histologic grade of the tumor should also be considered on ADC-based differentiation.
Necrosis also contributes to the calculated ADC value, which increases with increasing amount of necrosis (15). We excluded the visible necrotic areas for ADC measurement and considered solid components for comparisons, as in most previous studies. However, the resolution of MRI is insufficient to detect the presence of micronecrosis, which has been histologically demonstrated in SCC and lymphoma, and so could not be excluded (5, 15). In the present study, the proportion of micronecrosis may have made some contribution to evaluation of the differences in ADC values between nodal metastases of SCC.
Perfusion also has an influence on ADC values (5, 16). However, by increasing the diffusion gradient strength (at b values >100 s/mm2) contribution of the perfusion effect is reduced considerably. We used high b values (b=1000 s/mm2), and although the effects of the perfusion factor may not have been eliminated completely, we feel that it had little influence on our results.
Positioning ROIs in head-and-neck imaging requires experience and familiarity with DWI. All ADC values in our study were obtained from the most solid and/or homogeneous part of the lesion, which was determined by a radiologist using the corresponding fat-suppressed T2-weighted, contrast-enhanced MRI and DWI. This is the most commonly accepted method of measurement of ADC values in head and neck tumors, with acceptable intra- and interobserver agreement (17). Diffusion-weighted evaluations should thus be correlated with morphologic images and endoscopy findings until the imaging technique and analyses are standardized.
The major limitation of this study was the relatively small number of patients in each group. Although power analysis indicated that the patient number in each group was adequate to identify statistical significance, larger cohorts are required to obtain definitive threshold values among metastatic cervical lymph nodes due to SCC. The results may not be appropriate for different centers; thus at present each center should determine their own cutoff values. Another limitation was that not all patients in our study were treated surgically. Some were treated with radiotherapy and/or chemotherapy. Therefore, the diagnosis of some lymph nodes were based on biopsy. All patients with NPC and other SCC patients that are not candidate for surgery are now treated with radiotherapy and/or chemotherapy. We evaluated the patients retrospectively and included the lymph nodes that had size criteria for malignancy. Our main purpose was to determine whether there was a difference in ADC values among them and we did not include the subcentimeter lymph nodes since mean ADC value calculation were difficult with the method we used for DWI. A further prospective study that includes subcentimeter metastatic lymph nodes may be valuable.
In conclusion, the mean ADC values showed significant differences between nodal metastases of NPC and LSCC. Considering SCCs as a single group may affect the accuracy of ADC-based differentiation. Location of the primary tumor should be taken into account and cutoff values should be determined separately for each anatomical location.
Main points.
Although apparent diffusion coefficient (ADC) values have been used to differentiate metastatic lymph nodes of squamous cell carcinoma (SCC) from lymphoma, the efficacy of using DWI for differentiation depends largely on the histological characteristics of the lymph node.
We compared the ADC values of metastatic lymph nodes due to SCC of the nasopharynx, oropharynx, larynx, and hypopharynx and found significant differences between nodal metastases of nasopharynx and larynx.
Location of the primary tumor should be taken into account and cutoff ADC values should be determined separately for each anatomical location.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.de Bondt RB, Hoeberigs MC, Nelemans PJ, et al. Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology. 2009;51:183–192. doi: 10.1007/s00234-008-0487-2. http://dx.doi.org/10.1007/s00234-008-0487-2. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich KM, Matzek W, Gentzsch S, Sulzbacher I, Czerny C, Herneth AM. Diffusion-weighted magnetic resonance imaging of head and neck squamous cell carcinomas. Eur J Radiol. 2008;68:493–498. doi: 10.1016/j.ejrad.2007.10.011. http://dx.doi.org/10.1016/j.ejrad.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Hauser T, Essig M, Jensen A, et al. Characterization and therapy monitoring of head and neck carcinomas using diffusion-imaging-based intravoxel incoherent motion parameters-preliminary results. Neuroradiology. 2013;55:527–536. doi: 10.1007/s00234-013-1154-9. http://dx.doi.org/10.1007/s00234-013-1154-9. [DOI] [PubMed] [Google Scholar]
- 4.Vandecaveye V, De Keyzer F, Dirix P, Lambrecht M, Nuyts S, Hermans R. Applications of diffusion-weighted magnetic resonance imaging in head and neck squamous cell carcinoma. Neuroradiology. 2010;52:773–784. doi: 10.1007/s00234-010-0743-0. http://dx.doi.org/10.1007/s00234-010-0743-0. [DOI] [PubMed] [Google Scholar]
- 5.King AD, Ahuja AT, Yeung DK, et al. Malignant cervical lymphadenopathy: diagnostic accuracy of diffusion-weighted MR imaging. Radiology. 2007;245:806–813. doi: 10.1148/radiol.2451061804. http://dx.doi.org/10.1148/radiol.2451061804. [DOI] [PubMed] [Google Scholar]
- 6.Sumi M, Ichikawa Y, Nakamura T. Diagnostic ability of apparent diffusion coefficients for lymphomas and carcinomas in the pharynx. Eur Radiol. 2007;17:2631–2637. doi: 10.1007/s00330-007-0588-z. http://dx.doi.org/10.1007/s00330-007-0588-z. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa Y, Sumi M, Sasaki M, Sumi T, Nakamura T. Efficacy of diffusion-weighted imaging for the differentiation between lymphomas and carcinomas of the nasopharynx and oropharynx: correlations of apparent diffusion coefficients and histologic features. AJNR Am J Neuroradiol. 2012;33:761–766. doi: 10.3174/ajnr.A2834. http://dx.doi.org/10.3174/ajnr.A2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandecaveye V, De Keyzer F, Vander Poorten V, et al. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology. 2009;251:134–146. doi: 10.1148/radiol.2511080128. http://dx.doi.org/10.1148/radiol.2511080128. [DOI] [PubMed] [Google Scholar]
- 9.Varoquaux A, Rager O, Lovblad KO, et al. Functional imaging of head and neck squamous cell carcinoma with diffusion-weighted MRI and FDG PET/CT: quantitative analysis of ADC and SUV. Eur J Nucl Med Mol Imaging. 2013;40:842–852. doi: 10.1007/s00259-013-2351-9. http://dx.doi.org/10.1007/s00259-013-2351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda M, Kato H, Sakuma H, Maier SE, Takeda K. Usefulness of the apparent diffusion coefficient in line scan diffusion-weighted imaging for distinguishing between squamous cell carcinomas and malignant lymphomas of the head and neck. AJNR Am J Neuroradiol. 2005;26:1186–1192. [PMC free article] [PubMed] [Google Scholar]
- 11.Perrone A, Guerrisi P, Izzo L, et al. Diffusion-weighted MRI in cervical lymph nodes: differentiation between benign and malignant lesions. Eur J Radiol. 2011;77:281–286. doi: 10.1016/j.ejrad.2009.07.039. http://dx.doi.org/10.1016/j.ejrad.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Kanematsu M, Kato Z, et al. Necrotic cervical nodes: usefulness of diffusion-weighted MR imaging in the differentiation of suppurative lymphadenitis from malignancy. Eur J Radiol. 2013;82:e28–35. doi: 10.1016/j.ejrad.2012.08.014. http://dx.doi.org/10.1016/j.ejrad.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Sumi M, Sakihama N, Sumi T, et al. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003;24:1627–1634. [PMC free article] [PubMed] [Google Scholar]
- 14.Yun TJ, Kim JH, Kim KH, Sohn CH, Park SW. Head and neck squamous cell carcinoma: differentiation of histologic grade with standard- and high-b-value diffusion-weighted MRI. Head Neck. 2013;35:626–631. doi: 10.1002/hed.23008. http://dx.doi.org/10.1002/hed.23008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Chen J, Shen J, Zhong J, Ye R, Liang B. Apparent diffusion coefficient values of necrotic and solid portion of lymph nodes: differential diagnostic value in cervical lymphadenopathy. Clin Radiol. 2013;68:224–231. doi: 10.1016/j.crad.2011.04.002. http://dx.doi.org/10.1016/j.crad.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Thoeny HC, De Keyzer F, King AD. Diffusion-weighted MR imaging in the head and neck. Radiology. 2012;263:19–32. doi: 10.1148/radiol.11101821. http://dx.doi.org/10.1148/radiol.11101821. [DOI] [PubMed] [Google Scholar]
- 17.Abdel Razek AA, Gaballa G, Elhawarey G, Megahed AS, Hafez M, Nada N. Characterization of pediatric head and neck masses with diffusion-weighted MR imaging. Eur Radiol. 2009;19:201–208. doi: 10.1007/s00330-008-1123-6. http://dx.doi.org/10.1007/s00330-008-1123-6. [DOI] [PubMed] [Google Scholar]