Abstract
Lymphangiography and percutaneous embolization of injured lymphatics are minimally invasive and effective techniques for the diagnosis and treatment of thoracic and retroperitoneal lymphatic leaks. We present a 58-year-old man who had abdominal chylous collection developed after multiple abdominal surgeries. Retroperitoneal lymphatic duct leakage was detected by ultrasound-guided intranodal lymphangiography and treated successfully using computed tomography (CT)-guided transabdominal embolization with percutaneous N-butyl cyanoacrylate (NBCA) glue and percutaneous NBCA glue and coil embolization by directly catheterizing the leaking lymphatic channel through the chylous collection. To the best of our knowledge, this is the first report of a lymphatic leakage case treated by percutaneous direct catheterization and embolization of leaking lymphatic channels through the chylous fluid collection.
Abdominal lymphatic leakage is an unusual complication after abdominal surgery and has been reported after vascular, gastrointestinal, gynecologic, urologic surgeries, and liver and small bowel transplantations. It may cause loss of essential proteins, lipids, immunoglobulin, vitamins, electrolyte, and water, and lead to increased mortality in postoperative setting due to malnutrition, cachexia, immunosuppression, and sepsis (1, 2). The management of chylous leakage is a challenging condition and extends from conservative treatment (dietary management, medical and percutaneous drainage) to radiological intervention or surgical treatment (1–3). Bipedal or intranodal lymphangiography is the traditional imaging method for localizing the site of lymphatic leakage before surgical and endovascular treatment (3–6). In this report, we present successful treatment of abdominal collection developed after multiple abdominal surgeries using computed tomography (CT)-guided transabdominal embolization and percutaneous embolization through the leakage collection. To the best of our knowledge, this is the first report of a lymphatic leakage case treated by percutaneous embolization by catheterizing the leaking lymphatic channel through the chylous collection.
Technique
A 58-year-old male patient underwent three abdominal operations due to gastric cancer and its recurrences within the last four years. A solid peripancreatic mass measuring 42×30 mm and invading the pancreatic tail and spleen was detected on follow-up CT. The peripancreatic mass was excised along with pancreatic tail and spleen along with extensive lymph node dissection in the fourth operation. Ten days after the last operation, the patient developed progressive abdominal pain and distension. Ultrasonography and CT examinations showed large fluid collection in the left abdomen (Fig. 1). Percutaneous catheter drainage was performed and it revealed milky fluid consistent with chylous leakage 1500 mL per day. Conservative treatment including oral feeding with medium chain triglycerides or cessation of oral feeding and total parenteral nutrition was initiated in order to treat the lymphatic leak. Although the lymphatic leak drainage initially diminished to 800 mL per day, it gradually increased back to former levels. On postoperative day 32, intranodal lymphangiography with possible lymphatic leakage embolization was suggested.
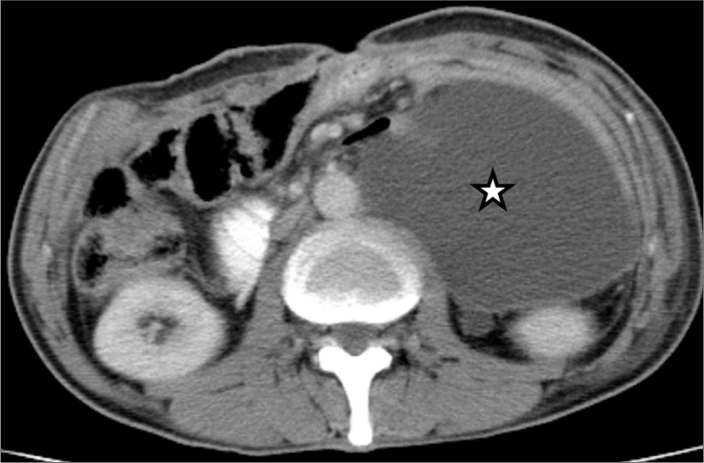
Figure 1.
Axial contrast-enhanced abdominal CT image shows encapsulated large fluid collection (star) on the left side of the abdominal cavity.
Intranodal lymphangiography procedure was carried out according to the previously described standard technique (5, 6). Bilateral inguinal lymph nodes were punctured by 22 gauge (G) needle under ultrasonography guidance and ethiodized contrast (Lipiodol, Laboratorie Guerbet) was slowly injected at a rate of 1–2 mL/5 min under intermittent pulsed fluoroscopy (Neurostar, Siemens Medical Solutions). A total volume of 15 mL lipiodol was injected manually, followed by injection of normal saline solution at 1 mL/5 s to facilitate propagation of lipiodol to improve and expedite opacification of lymphatic ducts (5). Approximately 35 minutes after initial injection of lipiodol, abdominal lymphatic channels were visualized along with a focus of extravasation in the paraaortic region at L3 vertebral level (Fig. 2). In addition to pseudoaneurysm-like leakage, the fluoroscopic spot magnified image showed contrast accumulation within the chylous collection and the drainage catheter (Fig. 2b).
Figure 2.

a, b. Fluoroscopic images show bilateral intranodal lymphangiography. The inguinal lymph node is punctured bilaterally by a 22G needle and lipiodol is slowly injected at a rate of 1–2 mL/5 min. Approximately 35 min after injection of lipiodol the standard (a) and magnified (b) fluoroscopic spot images show the pseudoaneurysm-like contrast accumulation (white arrow), contrast extravasation within the fluid collection (arrowheads, b) and contrast passage within the drainage catheter (black arrows).
On the same day following lymphangiography, CT-guided percutaneous intervention was planned to embolize the leakage site (Fig. 3). Before the intervention, an unenhanced abdominal CT examination was performed which showed lipiodol accumulation within the lymphatic channels and the leakage site (Fig. 3a). Using anterior abdominal approach a 22G needle was percutaneously placed within the opacified retroperitoneal lymphocele-like lesion (Fig. 3b). Embolization of this pouch and the channel was performed using 1.5 mL total volume of a 2:1 mixture of lipiodol:NBCA glue (Histoacryl, B. Braun). At the end of the procedure, the glue cast accumulated within the lymphocele-like lesion and within the channel (Fig. 3c), and in the medial wall of the collection (Fig. 3d). The catheter drainage reduced from 1500 mL to 850 mL daily, but it did not stop completely.
Figure 3.
a–d. Axial contrast-enhanced abdominal CT images show CT-guided percutaneous embolization of the leakage site one hour following lymphangiography. Axial CT scan (a) shows lipiodol accumulation within the lymphatic channels (arrowhead) and pseudoaneurysm-like leakage (black arrow). A 22G needle was inserted within the leak site (arrow, b), and 1.5 mL NBCA glue and lipiodol was injected within the pseudoaneurysm-like leakage site and tract (arrows, c). After embolization, the glue cast (arrows) also accumulated in the medial wall of the collection (d).
Twenty days after the first intervention, we decided to perform another embolization through the collection pouch. After removing the drainage catheter from the collection site, we placed a 6F introducer sheath Arrow-Flex 6F (Arrow International Inc.) within the collection. Then, a 5F Simmons 2 diagnostic catheter was used to find out the leakage site through the collection (Fig. 4). Following many attempts, we were able to opacify the injured lymphatic channel from the collection (Fig. 4a). Using a microguidewire X-Pedion-10 (ev3 Micro Therapeutics Inc.), an Excelsior SL-10 microcatheter (Stryker Neoruvascular) was navigated to leaking lymphatic channel through the diagnostic catheter. First, coil embolization was performed using three bare platinum Micrus coils (Micrus Endovascular) (Fig. 4b). Following coil embolization, a 2 mL total volume of a 2:1 mixture of lipiodol: NBCA glue injection was performed to strengthen the embolization site (Fig. 4c). Control image revealed complete embolization of the injured channel with the same microcatheter (Fig. 4). Following the second embolization, the catheter drainage reduced to approximately 500 mL per day.
Figure 4.
a–d. Fluoroscopic images 20 days after the first intervention show that a 6F guiding catheter placed within the collection and a 5F Simmons catheter were used to find out leakage site through the collection. Contrast injection through the diagnostic catheter opacified the injured lymphatic channel (arrows, a). Using microcatheter, coil embolization (arrow) was performed to occlude the leakage (b). Then, NBCA glue injection was performed to strengthen the embolization site (arrows, c). Control image revealed complete embolization of injured channel (arrow, d).
Catheter drainage continued for two months after the second embolization, and we planned another embolization session through the collection as we did before. Two months later, the third embolization procedure was performed using a total 2.5 mL of a 2:1 mixture of lipiodol:NBCA glue injected through microcatheter around the coils (Fig. 5a). After percutaneous embolization through the collection, the catheter drainage was completely resolved on the same day. However, the patient suddenly died three months after the last embolization because of chemotherapy-related complications. During this period, the patient had no recurrence of lymphatic collection (Fig. 5b).
Figure 5.

a, b. Fluoroscopic (a) and axial unenhanced abdominal CT images (b) two months after the second embolization. Since catheter drainage continued, a third embolization through the collection was performed two months after the second embolization. A total of 2.5 mL NBCA glue and lipiodol mixture was injected around the coils and medial wall of the collection (arrows, a). CT scan (b) obtained one month after the last embolization revealed the NBCA glue cast (arrow) and complete resolution of the collection.
Discussion
Chylous leakage may occur as a complication of extensive abdominal surgical procedures for cancer treatment with an incidence of 5% after gastric surgery (1, 2). In patients with malignant tumors, the high flow chylous fluid has a high rate of mortality reaching up to 50% because of loss of lymphocyte-rich fluid and protein which leads to hypoalbuminemia and susceptibility to infection (1, 3). The diagnosis of the chylous leakage was mainly made based on the typical appearance of milky drainage fluid. Majority of patients with cyhlous leaks heal with diet with medium chain triglycerides, or total parenteral nutrition, and drainage (1, 2). However, conservative treatment fails in 25% to 50% of patients, as we experienced in our patient (1, 3). Surgical treatment of chylous leakage consists of ligature of leaking lymphatic vessels or retroperitoneal shunting with a success rate of 37% (1, 2).
Lymphangiography has been accepted as the gold standard of imaging to delineate the anatomy of the lymphatic system and to identify the site of lymphatic leakage including chylous ascites, chylothorax, and lymphatic fistulae (4–6). Previous studies reported that lymphangiography can detect leakage site in 78% of cases (3, 7, 8). Diagnostic lymphangiography can also play a therapeutic role as it facilitates the healing of lymphatic leak after the procedure (3, 7, 8). Kawasaki et al. (3) reported that successful healing was achieved in 64.2% of patients following diagnostic lymphangiography.
Lymphangiography not only provides visualization of the lymphatic system and leakage site, but also allows for percutaneous embolization for leakage site through the opacified lymphatic channels (3, 4, 7, 8). Percutaneous thoracic duct and pelvic-retroperitoneal lymphatic duct embolizations for the treatment of chylothorax and cyhlous ascites are minimally invasive and effective treatment options alternative to surgery (4, 9, 10). Embolization procedure was reported to be successful between 75% and 100% of the patients (9). The procedure involves lymphangiography followed by transabdominal needle puncture of retroperitoneal lymph ducts or thoracic duct with cannulation and embolization of leakage point (4, 9). Percutaneous glue injection for lymphatic leaks has been reported in few patients (9–12). Ching et al. (11) treated a 68-year-old patient who underwent an aortomesenteric bypass surgery complicated by a high-output chylothorax and chylous ascites after surgery. CT-guided successful percutaneous lymphatic duct embolization was performed using NBCA glue and lipiodol injection. Itou et al. (12) treated a case of chylous ascites developed after nephrectomy using NBCA glue-lipiodol mixture and metallic coils to obliterate lymphocele-like extravasation of the retroperitoneal lymph duct. In a patient with chylothorax, Gaba et al. (10) performed direct glue injection within the lymphatic duct which was injured during thoracic duct embolization performed two weeks ago. In our patient, we performed two different percutaneous embolization techniques: similar to the previous studies we carried out a CT-guided percutaneous injection of lipiodol-NBCA glue mixture to occlude the leakage site detected in lymphangiography in the first intervention. Because the leakage continued, we further performed successful embolization by catheterizing the leaking lymphatic ducts through the collection pouch in the second and third interventions. To the best of our knowledge, the latter embolization technique has not been reported previously. Although percutaneous catheterization and embolization technique is the standard method in the treatment of injured lymphatic ducts, if it fails, transcollection embolization technique can be used as an alternative treatment method in persistent lymphatic leaks.
In conclusion, chylous leakage is an unusual complication after extensive abdominal surgery with a challenging treatment. This case report demonstrates the use of intranodal lymphangiography to find out the lymphatic leakage site. We used two different approaches in the treatment of injured lymphatic duct. In addition to previously described CT-guided percutaneous lipiodol-NBCA glue injection, we also performed direct catheterization and embolization of leaking lymphatic channels through the collection pouch with successful outcome. This novel technique may be an alternative treatment method in persistent lymphatic leaks.
Main points.
Chylous leakage may occur as a complication of extensive abdominal surgical procedures with an incidence of 1%–5%.
Intranodal lymphangiography has been accepted as the gold standard imaging study to delineate the anatomy of the lymphatic system and identify the site of lymphatic leakage.
Intranodal lymphangiography allows for percutaneous embolization of the leakage site through the opacified lymphatic channels.
In addition to previously described CT-guided percutaneous lymphatic duct embolization, transcollectional direct catheterization and embolization of leaking lymphatic channels is a promising minimally invasive treatment option for the treatment of chylothorax and cyhlous ascites.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Cardenas A, Chopra S. Chylous ascites. Am J Gastroenterol. 2002;97:1896–1900. doi: 10.1111/j.1572-0241.2002.05911.x. http://dx.doi.org/10.1111/j.1572-0241.2002.05911.x. [DOI] [PubMed] [Google Scholar]
- 2.Ksas R, Rustman LD, Zoetmulder FA. Chylous ascites after oncological abdominal surgery: incidence and treatment. Eur J Surg Oncol. 2001;27:187–189. doi: 10.1053/ejso.2000.1088. http://dx.doi.org/10.1053/ejso.2000.1088. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki R, Sugimoto K, Fujii M, et al. Therapeutic effectiveness of diagnostic lymphangiography for refractory postoperative chylothorax and chylous ascites: correlation with radiologic findings and preceding medical treatment. AJR Am J Roentgenol. 2013;201:659–666. doi: 10.2214/AJR.12.10008. http://dx.doi.org/10.2214/AJR.12.10008. [DOI] [PubMed] [Google Scholar]
- 4.Lee EW, Shin JH, Ko HK, Park J, Kim SH, Sung KB. Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J Radiol. 2014;15:724–732. doi: 10.3348/kjr.2014.15.6.724. http://dx.doi.org/10.3348/kjr.2014.15.6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadolski GJ, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23:613–616. doi: 10.1016/j.jvir.2012.01.078. http://dx.doi.org/10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Kariya S, Komemushi A, Nakatani M, Yoshida R, Kono Y, Tanigawa N. Intranodal lymphangiogram: technical aspects and findings. Cardiovasc Intervent Radiol. 2014;37:1606–1610. doi: 10.1007/s00270-014-0888-z. http://dx.doi.org/10.1007/s00270-014-0888-z. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto T, Yamagami T, Kato T, Hirota T, Yoshimatsu R, Masunami T, Nishimura T. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. 2009;82:286–290. doi: 10.1259/bjr/64849421. http://dx.doi.org/10.1259/bjr/64849421. [DOI] [PubMed] [Google Scholar]
- 8.Kos S, Haueisen H, Lachmund U, Roeren T. Lymphangiography: forgotten tool or rising star in the diagnosis and therapy of postoperative lymphatic vessel leakage. Cardiovasc Intervent Radiol. 2007;30:968–973. doi: 10.1007/s00270-007-9026-5. http://dx.doi.org/10.1007/s00270-007-9026-5. [DOI] [PubMed] [Google Scholar]
- 9.Cope C, Kaiser LR. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13:1139–1148. doi: 10.1016/s1051-0443(07)61956-3. http://dx.doi.org/10.1016/S1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 10.Gaba RC, Owens CA, Bui JT, Carrillo TC, Knuttinen MG. Chylous ascites: a rare complication of thoracic duct embolization for chylothorax. Cardiovasc Intervent Radiol. 2011;34( Suppl 2):S245–249. doi: 10.1007/s00270-010-9900-4. http://dx.doi.org/10.1007/s00270-010-9900-4. [DOI] [PubMed] [Google Scholar]
- 11.Ching KC, Santos E, McCluskey K, Jeyabalan G. CT-guided injection of N-butyl cyanoacrylate glue for treatment of chylous leak after aor-to-mesenteric bypass. Cardiovasc Intervent Radiol. 2014;37:1103–1106. doi: 10.1007/s00270-013-0811-z. http://dx.doi.org/10.1007/s00270-013-0811-z. [DOI] [PubMed] [Google Scholar]
- 12.Itou C, Koizumi J, Myojin K, Yamashita T, Mori N, Imai Y. A case of refractory chylous ascites after nephrectomy successfully treated with percutaneous obliteration using adhesive glue. Jpn J Radiol. 2013;31:71–74. doi: 10.1007/s11604-012-0146-8. http://dx.doi.org/10.1007/s11604-012-0146-8. [DOI] [PubMed] [Google Scholar]