Abstract
We investigated changes in circulating T helper type 17 (Th17) cells following anti-tumour necrosis factor (TNF) in rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) patients. Peripheral blood mononuclear cells (PBMC) were isolated from 25 RA, 15 AS and eight PsA patients at baseline 4 and 12 weeks after treatment, and Th17 cell frequencies were analysed using interleukin (IL)-17 enzyme-linked immunospot (ELISPOT) and flow cytometry. A significant increase in IL-17-producing cells was observed by ELISPOT in RA and AS patients at 12 weeks. Flow cytometry confirmed significant increases in CD4+IL-17+ cells at 12 weeks in RA and AS and 4 weeks in PsA patients. Anti-TNF treatment increases circulating Th17 cells in three different diseases.
Keywords: ankylosing spondylitis, anti-TNF, psoriatic arthritis, rheumatoid arthritis, T cells
Introduction
T helper type 17 (Th17) cells, a proinflammatory subset of CD4+ T helper cells whose signature cytokine is interleukin (IL)-17, have been implicated in the pathogenesis of different inflammatory arthritides [1]. Increased frequencies of Th17 cells have been observed in the peripheral blood of patients with rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) compared to healthy controls [2,3] and have been shown to be further enriched in synovial fluid from these patients, as well as in synovial tissue from RA and PsA patients, where their levels correlate with systemic inflammation [4–6].
Anti-tumour necrosis factor (TNF) agents have revolutionized the treatment of RA, AS and PsA, with more than two-thirds of patients demonstrating therapeutic responses. The kinetics of change in circulating Th17 cells during anti-TNF treatment in patients with inflammatory arthritis are not well characterized. Data from the collagen-induced arthritis (CIA) mouse model of RA indicate that amelioration of arthritis after anti-TNF therapy is accompanied by decreasing Th17 cell numbers in arthritic joints while, paradoxically, increased Th17 cell numbers are observed in draining lymph nodes [7]. Small studies involving RA patients have suggested that anti-TNF treatment may increase circulating Th17 cells. In a cross-sectional study, IL-17 production by cultured peripheral blood mononuclear cells (PBMC) in vitro was higher 12 weeks after anti-TNF initiation compared to pretreatment levels [8]. In another study in RA patients, our group reported increased frequency of circulating Th17 cells up to 12 weeks after anti-TNF initiation [9]. These preliminary observations suggest that anti-TNF may have similar effects in human disease as in the CIA model. However, longitudinal investigations of the dynamics of Th17 cell numbers and function at predefined time-points during therapy are needed to elucidate more clearly the effects of anti-TNF treatment on these cells, to determine whether these post-treatment changes are unique to RA, or a more general occurrence in different types of inflammatory arthritis treated with anti-TNF, and whether the changes are specific to the pharmacology of particular anti-TNF agents.
The aim of this study was to characterize changes in the frequency of circulating Th17 cells in three different types of inflammatory arthritis (RA, AS and PsA) at predefined time-points during the initial 12 weeks of treatment with anti-TNF. We assessed changes in circulating Th17 cells using two different but complementary techniques for evaluating cellular immune responses, enzyme-linked immunospot (ELISPOT) and flow cytometry.
Materials and methods
Study population
Forty-eight patients with a confirmed diagnosis of RA (n = 25), AS (n = 15) or PsA (n = 8) were recruited and followed at four predetermined protocol visits prior to treatment initiation with anti-TNF agents and at 1, 4 and 12 weeks following treatment initiation (Table 1). Inclusion criteria were as follows: patients with RA had active disease with a Disease Activity Score of 28 joints (DAS28) >5·1 on two occasions at least 1 month apart and had failed therapy with at least two disease-modifying agents (DMARDs), including methotrexate; patients with AS had active disease as defined by the Bath Disease Activity Index (BASDAI) >4 on two occasions, at least 3 months apart, and an inadequate response to two non-steroidal anti-inflammatories (NSAIDs); patients with PsA had evidence of active skin disease and ≥3 swollen or tender joints at baseline. Patients were treated by subcutaneous injection with etanercept 50 mg weekly or adalimumab 40 mg fortnightly. Peripheral blood was collected and disease activity assessed at each visit using DAS28 in RA, BASDAI in AS and Psoriatic Arthritis Response Criteria (PsARC) in PsA. Patients were excluded if they had received previous biological agents, had intercurrent active infection, dose change in DMARDs in the 4 weeks preceding study entry or had received oral, intramuscular or intra-articular steroids in the preceding 4 weeks.
Table 1.
Patient characteristics and disease activity indices
| Rheumatoid arthritis (n = 25) | Ankylosing spondylitis (n = 15) | Psoriatic arthritis (n = 8) | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 57·4 ± 11·7 | 36·4 ± 11·8 | 50·9 ± 8·4 |
| Sex | 18 female, 7 male | 3 female, 12 male | 5 female, 3 male |
| Disease duration (years) | 10·6 ± 9·2 | 10·9 ± 10·7 | 7·8 ± 7·3 |
| DMARDs, n (%) | 21 (84) | 4 (27) | 5 (63) |
| Prednisolone, n (%) | 7 (28) | – | 3 (38) |
| Disease activity indices | |||
| DAS28 score (baseline) | 5·7 ± 0·8 | – | – |
| DAS28 score (12 weeks) | 3·9 ± 1·1*** | – | – |
| BASDAI (baseline) | – | 5·3 ± 2·0 | – |
| BASDAI (12 weeks) | – | 2·2 ± 1·9**** | – |
| BASFI (baseline) | – | 4·2 ± 1·9 | – |
| BASFI (12 weeks) | – | 2·5 ± 1·7* | – |
| Swollen joint count (76 joints) baseline | – | – | 8·1 ± 4·8 |
| Swollen joint count (76 joints) 12 weeks | – | – | 1·2 ± 0·8* |
| Tender joint count (78 joints) baseline | – | – | 33·5 ± 19·3 |
| Tender joint count (78 joints) 12 weeks | – | – | 9·7 ± 6·8** |
| CRP (baseline) | 15·7 ± 18·2 | 7·0 ± 7·7 | 16·0 ± 16·3 |
| CRP (12 weeks) | 8·7 ± 16·4*** | 1·4 ± 1·9*** | 2·6 ± 3·4* |
| ESR (baseline) | 27·2 ± 22·6 | 22·3 ± 18·3 | 22·2 ± 17.1 |
| ESR (12 weeks) | 19·9 ± 14·8** | 9·7 ± 9·4** | 13·8 ± 15·0 |
DMARDs = disease-modifying anti-rheumatic drugs; DAS28 = Disease Activity Score of 28 joints; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BASFI = Bath Ankylosing Spondylitis Functional Index; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate. Numbers are expressed as mean ± standard error of the mean. *P < 0·05; **P < 0·001; ***P < 0·0005; ****P < 0·0001.
The study was conducted in compliance with the Helsinki declaration with ethical approval from the West London 1 Research Ethics Committee (formerly known as Hammersmith REC), reference number 09/H0707/80. All subjects gave written informed consent; study registration (NCT01060098).
Peripheral blood mononuclear cell isolation
Peripheral blood was collected in tubes containing sodium heparin and PBMC were isolated by density gradient centrifugation. In order for all time-points to be analysed simultaneously, PBMC were cryopreserved at a density of 5–10/106/ml in heat-inactivated fetal bovine serum (Gibco, Paisley, UK) containing 10% dimethyl sulphoxide (Sigma, Gillingham, UK). Aliquots were placed overnight at −80 °C in a cryogenic vessel containing isopentane and transferred subsequently to liquid nitrogen.
IL-17 ELISPOT
PBMC from each time-point were thawed, washed and resuspended at 2 × 106/ml in RPMI containing 10% human AB serum (Sigma). Cell viability by trypan blue exclusion was consistently >95%; 2 × 105 cells were cultured in triplicate in RPMI/10%AB serum containing 1 μg/ml anti-CD3 (eBiosciences, Hatfield, UK) for 20 h. Phytohaemagglutinin (PHA) (1 μg/ml) or medium alone were used as assay controls. Sterile multi-screen 96-well plates (Millipore, Bedford, MA, USA) were coated with IL-17 capture antibody (R&D Systems, Abingdon, UK) and incubated at 4°C for 16 h. The stimulated cells were transferred to the coated plates for a further 24 h. The plates were washed and biotinylated anti-IL-17 antibody was added for 2 h. Streptavidin-alkaline phosphatase (R&D Systems) was added to the plates for 2 h. 5-Bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) solution (R&D Systems) was added for 30 min. The spots were counted using an automated ELISPOT reader (AID, Strassberg, Germany). The number of spots in the control wells was very low, mean 0·39 ± 0·9, median 0. The number of specific spot-forming cells was determined as the mean number of spots in the presence of stimulation agent minus mean number of spots in wells containing medium only.
Flow cytometry
Thawed PBMC were cultured at 15 × 106/ml in RPMI containing 50 ng/ml phorbol myristate acetate (PMA) and 500 ng/ml ionomycin for 5 h in the presence of 10 μg/ml brefeldin A. Cells were stained with anti-CD4-fluorescein isothiocyanate (FITC) and anti-CD8-peridinin chlorophyll/cyanin 5·5 (PerCP/Cy5·5) (BD Biosciences, San Jose, CA, USA) for 30 min at 4°C before fixation with Cytofix (BD Biosciences). Cells were permeabilized with phosphate-buffered saline containing 1% bovine serum albumin and 0·05% saponin and stained with anti-IL-17-PE (BD Biosciences) for 30 min at room temperature. Cells were acquired and analysed on a fluorescence activated cell sorter (FACS)Canto II using FACS diva software.
Statistical analysis
Time-points on treatment were compared to baseline using Wilcoxon's signed-rank matched-pairs test. Correlation was obtained using Spearman's rank test. P-values less than 0·05 were considered significant. Data were analysed using Prism version 5 (Graphpad Software Inc, La Jolla, CA, USA).
Results
There was significant improvement in clinical disease activity scores and inflammatory markers 12 weeks after anti-TNF initiation in all three disease groups (Table 1).
Anti-TNF treatment increases frequency of circulating IL-17-producing cells (by ELISPOT)
In RA patients, there was a significant increase in the frequency of circulating IL-17-producing cells 1 week (466·4 ± 277·6 versus 776·5 ± 533 spSFC/106, P = 0·02) and 12 weeks (466·4 ± 277·6 versus 759·8 ± 510 spSFC/106, P = 0·003) after anti-TNF initiation compared to baseline. Similarly, a significant increase was also observed 12 weeks after anti-TNF initiation in AS patients (432 ± 474 versus 651 ± 532 spSFC/106, P = 0·04) (Fig. 1). There was a trend towards an increase in frequency of IL-17-producing cells in PsA patients at week 4 (450 ± 276 versus 744 ± 444 spSFC/106, P = 0·16) and week 12 (450 ± 276 versus 609 ± 373 spSFC/106, P = 0·48), but these did not reach statistical significance.
Fig. 1.
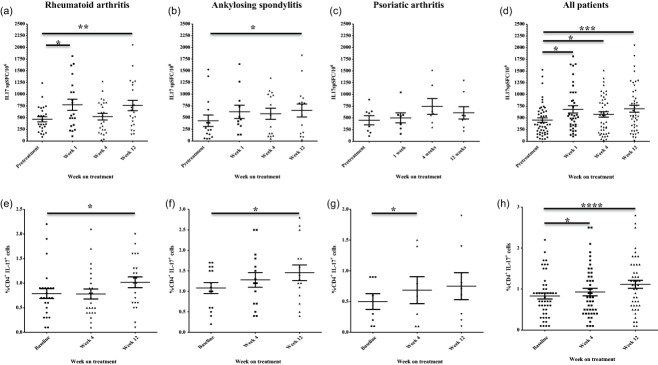
Changes in frequency of T helper type 17 (Th17) cells in peripheral blood during 12 weeks of anti-tumour necrosis factor (TNF) treatment. Changes in numbers of interleukin (IL)-17-producing peripheral blood mononuclear cells (PMBC) during anti-TNF treatment as determined by IL-17 enzyme-linked immunospot (ELISPOT) assay are shown for rheumatoid arthritis (RA) patients (n = 25) (a), ankylosing spondylitis (AS) patients (n = 15) (b), psoriatic arthritis (PsA) patients (n = 8) (c) and the whole study cohort (n = 48 patients); (d) 200 000 PBMC were seeded in triplicate in each experiment and stimulated with 1 μg/ml anti-CD3 antibody for 20 h and the numbers of cytokine-producing cells were enumerated. Changes in the percentages of circulating CD4+IL-17+ cells in the peripheral blood of RA patients (n = 25) (d), AS patients (n = 15) (e), PsA patients (n = 8) (f) and the whole study cohort (n = 48 patients) (g) as determined by flow cytometry. Bars represent mean ± standard error of the mean (s.e.m.). *P < 0·05, **P < 0·01 versus baseline visit by Wilcoxon's matched-pairs test. spSFC/106 = specific spot-forming cells per million PBMC.
There was a significant increase in the frequency of IL-17-producing cells as early as week 1 (453·2 ± 49·5 versus 681·7 ± 78·07 spSFC/106, P = 0·04) after anti-TNF initiation in all study patients (n = 48) and at weeks 4 (453·2 ± 49·5 versus 575·7 ± 58·9 spSFC/106, P = 0·04) and 12 (453·2 ± 49·5 versus 696·8 ± 73·2, P = 0·0008) (Fig. 1d).
Anti-TNF treatment increases frequency of circulating CD4+IL-17+ cells
Flow cytometry analysis of PBMC confirmed a significant increase in the frequency of circulating CD4+IL-17+ cells 12 weeks after anti-TNF initiation in RA (0·8 ± 0·5 versus 1·1 ± 0·5%, P = 0·01) and AS groups (1·1 ± 0·5 versus 1·6 ± 0·7%, P = 0·01) (Fig. 2). In the PsA group, there was a significant increase in the frequency of circulating CD4+IL-17+ cells 4 weeks after anti-TNF initiation (0·5 ± 0·3 versus 0·7 ± 0·5%, P = 0·03).
Fig. 2.
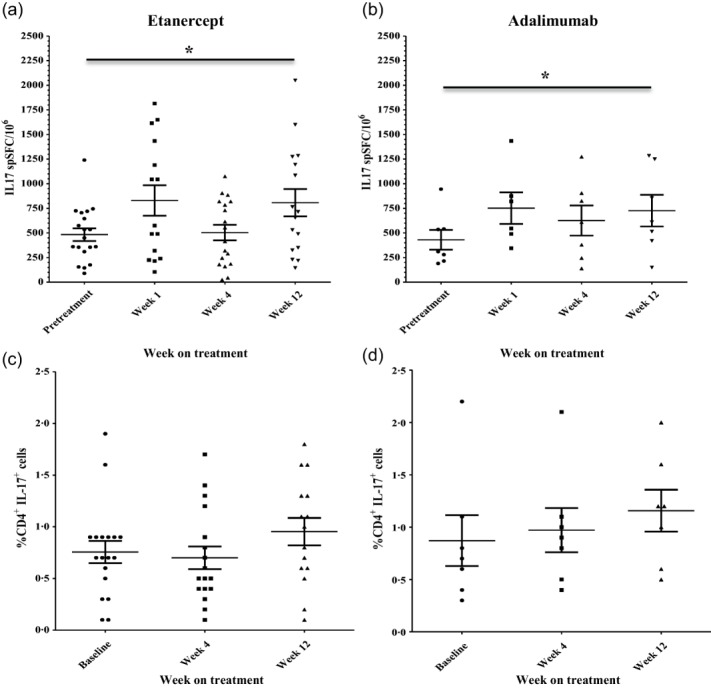
Anti-tumour necrosis factor (TNF) treatment with adalimumab and etanercept leads to an increase in frequency of circulating T helper type 17 (Th17) cells in peripheral blood. The kinetics of change of circulating interleukin (IL)-17-producing cells during anti-TNF treatment were the same in rheumatoid arthritis (RA) patients treated with etanercept (n = 18) (a) and with adalimumab (n = 7) (b), as determined by IL-17 enzyme-linked immunospot (ELISPOT) assay; 200 000 PBMC were seeded in triplicate in each experiment and stimulated with 1 μg/ml anti-CD3 antibody for 20 h and the numbers of cytokine-producing cells were enumerated. The percentages of circulating CD4+IL-17+ cells in the peripheral blood of RA patients increased similarly in patients treated with etanercept (n = 18) (c) and adalimumab (n = 7) (d). Bars represent mean ± standard error of the mean (s.e.m.). *P < 0·05, **P < 0·01 versus baseline visit by Wilcoxon's matched-pairs test. spSFC/106 = specific spot-forming cells per million PBMC.
In the whole study cohort, there were significant increases in the frequency of circulating CD4+IL-17+ cells at week 4 (0·83 ± 0·07 versus 0·93 ± 0·09, P = 0·04) and week 12 (0·83 ± 0·07 versus 1·11 ± 0·09, P < 0·0001) after anti-TNF initiation (Fig. 1h).
The kinetics of change of Th17 cells in RA patients were observed to be the same whether TNF inhibition was mediated by adalimumab, a monoclonal antibody, or etanercept, a TNF-R2 fusion protein (Fig. 1e,f).
In this study we used two different but complementary techniques to assess the changes in the frequency of circulating Th17 cells following anti-TNF initiation. Although these techniques differ in their methodology and numerical assessment of the frequency of Th17 cells, there was a significant correlation between the results obtained using both methods to assess the frequency of Th17 cells at baseline in the whole study cohort (r = 0·4, P = 0·0047).
Discussion
In this longitudinal study, we demonstrate that anti-TNF therapy leads to a similar increase in frequency of circulating Th17 cells in patients with different types of inflammatory arthritis, suggesting that this may be a common feature of inflammation suppression by TNF inhibition rather than a disease-specific phenomenon. Previous studies investigating effects of anti-TNF on circulating Th17 cells have been small, cross-sectional and focused on RA [8,9]. The kinetics of change in circulating Th17 cells were observed to be the same following treatment with etanercept or adalimumab, suggesting this may be a class effect of TNF blockade.
This increase in circulating Th17 cells during anti-TNF is an intriguing finding, as Th17 cells are considered to be a highly inflammatory cell type. The mechanism of increase in Th17 cells is unclear, but may be due to redistribution of inflammatory cells from inflamed joints following altered chemokine gradients or synovial expression of adhesion molecules, consistent with known effects of anti-TNF [10,11]. In the CIA model, increases in Th17 cells during anti-TNF are attributed to an increase in IL-12/23p40 subunit shared between IL-12 and IL-23, the key cytokines involved in differentiation of Th1 and Th17 cells, respectively [7]. Thus, anti-TNF may influence Th17 cell margination or production. These two hypotheses are not mutually exclusive, and require further investigation.
A novel approach in this study was to employ two complementary techniques for analysing cellular immune responses, ELISPOT and flow cytometry, to quantify effects of anti-TNF on the frequency of circulating Th17 cells. Consistency of results using both techniques adds strength to the findings. ELISPOT is particularly suited to studying low-frequency immune responses such as Th17 cells and is a highly sensitive technique providing objective analysis of cellular function [12].
In conclusion, we have shown that TNF inhibition is associated with a rise in circulating Th17 cells in three different disease phenotypes. Further investigation of the relationship between this increase and treatment response is needed.
Acknowledgments
The authors would like to thank Ms Catherine McClinton for nursing support throughout this study. This study was sponsored by Imperial College London, and was supported in part by the Medical Research Council (G802513). P. T. thanks Arthritis Research UK (ARUK) for their funding of Arthritis Research UK Early Arthritis Treatment Centre at the University of Oxford and the National Institute of Health Research (NIHR) for their funding of The NIHR Biomedical Research Centre in Musculoskeletal Disease at Oxford University Hospitals NHS Trust and the University of Oxford. S. A. thanks the National Institute of Health Research for funding the research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare National Health Service (NHS) Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of ARUK, the NHS the NIHR or the Department of Health. Registration (NCT01060098).
Author contributions
The authors made the following substantial contributions to the work and preparation of the manuscript: conception or design of the work (S. A., P. T., R. W.), acquisition (D. H., R. W., E. P., S. Al., S. A., P. T.), analysis (D. H., P. T., R. W.) and interpretation of data for the work (D. H., R. W., E. P., S. Al., S. A., P. T.); all authors (D. H., R. W., E. P., S. Al., S. A., P. T.) contributed to drafting the work or revising it critically for important intellectual content and gave approval of the final version to be published; they agree to be accountable for all aspects.
Disclosure
S. A. received consultancy funding to the institution from Roche, grant funding from Pfizer and UCB Pharma, payment for lectures from Abbvie and Pfizer and payment for development of educational presentations from Abbvie and Pfizer. P. T. has received grant funding to institution from UCB Pharma, consultancy fees or honoraria from UCB, Pfizer and Merck. D. H., R. W., S. Al. and E. P. have no declared conflict of interests.
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Jandus C, Bioley G, Rivals J-P, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–17. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 3.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 4.Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–85. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 5.Gullick NJ, Evans HG, Church LD, et al. Linking power doppler ultrasound to the presence of Th17 cells in the rheumatoid arthritis joint. PLOS ONE. 2010;5:e12516. doi: 10.1371/journal.pone.0012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendling D, Cedoz J-P, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–5. doi: 10.1016/j.jbspin.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Notley CA, Inglis JJ, Alzabin S, McCann FE, McNamee KE, Williams RO. Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J Exp Med. 2008;205:2491–7. doi: 10.1084/jem.20072707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aerts NE, De Knop KJ, Leysen J, et al. Increased IL-17 production by peripheral T helper cells after tumour necrosis factor blockade in rheumatoid arthritis is accompanied by inhibition of migration-associated chemokine receptor expression. Rheumatology. 2010;49:2264–72. doi: 10.1093/rheumatology/keq224. [DOI] [PubMed] [Google Scholar]
- 9.Alzabin S, Abraham SM, Taher TE, et al. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann Rheum Dis. 2012;71:1741–8. doi: 10.1136/annrheumdis-2011-201024. [DOI] [PubMed] [Google Scholar]
- 10.Tak PP, Taylor PC, Breedveld FC, et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor α monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1077–81. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PC, Peters AM, Paleolog E, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson AC, Martin JN, Younger SR, et al. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods. 2003;283:141–53. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]


