Abstract
Patients with signal transducer and activator of transcription-1 (STAT1)-dependent chronic mucocutaneous candidiasis (CMC) and patients with STAT3-dependent hyper-immunoglobulin (Ig)E syndrome (HIES) display defects in T helper type 17 (Th17) cytokine production capacity. Despite this similar immune defect in Th17 function, they show important differences in the type of infections to which they are susceptible. Recently, our group reported differential regulation of STAT-1 and STAT-3 transcription factors during epigenetic reprogramming of trained immunity, an important host defence mechanism based on innate immune memory. We therefore hypothesized that STAT1 and STAT3 defects have different effects on trained immunity, and this may partly explain the differences between CMC and HIES regarding the susceptibility to infections. Indeed, while trained immunity was normally induced in cells isolated from patients with HIES, the induction of innate training was defective in CMC patients. This defect was specific for training with Candida albicans, the main pathogen encountered in CMC, and it involved a type II interferon-dependent mechanism. These findings describe the role of STAT-1 for the induction of trained immunity, and may contribute to the understanding of the differences in susceptibility to infection between CMC and HIES patients. This study could also provide directions for personalized immunotherapy in patients suffering from these immunodeficiencies.
Keywords: CMC, HIES, trained immunity
Introduction
Chronic mucocutaneous candidiasis (CMC) and hyper-immunoglobulinaemia E syndrome (HIES) are rare primary immunodeficiencies, both characterized by defective T helper type 17 (Th17) cytokine production; this immunological defect is caused mainly by signal transducer and activator of transcription-1 (STAT1) and STAT3 mutations, respectively [1–5]. More rarely, mutations in interleukin (IL)-17F or IL-17RA have also been described in CMC patients [6] and dedicator of cytokinesis 8 gene (DOCK8) mutations in autosomal recessive HIES [7]. Despite the similarities between CMC and HIES concerning genetic and immunological defects, important phenotypical differences distinguish these clinical entities regarding susceptibility to infections. While CMC patients suffer mainly from Candida and dermatophyte infections, and only later during life from respiratory tract infections [8], HIES patients have a strongly increased susceptibility to skin and respiratory tract infections, especially with Staphylococcus aureus and Streptococcus pneumoniae, while their fungal complications are less prominent [4,9–12]. The immunological substrate for these differences in susceptibility is not known. Other striking differences are the skeletal abnormalities in HIES and the autoimmune endocrinopathy in CMC [13,14].
Recently, a novel host defence mechanism has been proposed, consisting of long-term enhanced biological activity of innate immune cells, which was termed ‘trained immunity’ [15,16]. Trained immunity relies on epigenetic reprogramming of monocytes or macrophages, and represents a de-facto memory of the innate immune response [17,18]. The epigenetic and metabolic substrate of trained immunity has been described recently [19,20]. Interestingly, these studies uncovered differential regulation of STAT-1 and STAT-3 during induction of trained immunity and innate immune tolerance [19]. This finding led us to hypothesize that the induction of trained immunity may be regulated differentially or defective in patients suffering from CMC and/or HIES.
In the present study, we explored the trained immunity responses in peripheral blood mononuclear cells isolated from either CMC or HIES patients. We demonstrate that CMC patients display a defective induction of trained immunity by C. albicans, while this was normal in HIES patients. This defect in CMC patients is specific for Candida and is mediated through an interferon (IFN)-γ-dependent pathway. These findings might explain the incapacity of CMC patients to clear fungal infections, and may lead to further understanding of the differences between these two complex immunodeficiency disorders.
Materials and methods
Patients and healthy volunteers
Blood samples were collected from five autosomal dominant CMC patients harbouring mutations in the STAT1 gene, three HIES patients with STAT3 mutations and from age- and gender-matched healthy volunteers who did not suffer from infectious or inflammatory diseases. Blood was collected by venipuncture into 10 ml ethylenediamine tetraacetic acid (EDTA) tubes (367525; Becton Dickinson, San Jose, CA, USA). The study was approved by the Arnhem–Nijmegen Ethical Committee, and the participants gave written informed consent.
Reagents and microorganisms
Culture medium used was RPMI-1640 Dutch modifications (Sigma-Aldrich, St Louis, MO, USA) supplemented with 1% gentamicin, 1% L-glutamine and 1% pyruvate (Life Technologies, Nieuwekerk, the Netherlands). Particulate C. albicans β-1,3-glucan was isolated and purified as described previously [21]. C. albicans American Type Culture Collection (ATCC) MYA-3573 (UC 820) [22] was grown overnight in Sabouraud broth at 29°C; cells were harvested by centrifugation, washed twice and resuspended in phosphate-buffered saline (PBS). Candida conidia were heat-killed for 30 min at 95°C. Other reagents were obtained as follows: lipopolysaccharide (LPS) (Escherichia coli serotype 055:B5; Sigma-Aldrich) with an additional purification step [23] and CD56 MicroBeads, purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Ustekinumab (CNTO 1275) was purchased from Janssen Biotech, Inc. (Horsham, PA, USA). Nanogam [immunoglobulin (Ig)G, human normal immunoglobulin, intravenous Ig (IVIg)] was purchased from Sanquin (Amsterdam, the Netherlands) and IL-18BP was kindly provided by Professor Charles Dinarello (University of Colorado).
Stimulation experiments
Peripheral blood mononuclear cells (PBMCs) were isolated as described previously [24]. PBMCs were counted on a Beckman Coulter Z1 particle counter and adjusted to 5 × 106 cells/ml. The trained immunity protocol was performed as described [17]: in brief, 100 μl of the PBMC suspension were added per well in 96-well round-bottomed plates and preincubated for 24 h with either RPMI, β-glucan (1 μg/ml) or heat-killed Candida conidia 104 colony-forming units (CFU)/ml at 37°C and 5% CO2. After preincubation, cells were washed with warm PBS and left to rest in RPMI supplemented with 10% human pool serum for 5 days (during which media was refreshed on day 3). Thereafter, on day 6, cells were subjected to a second stimulation of cytokine production with various stimuli (RPMI, LPS 10 ng/ml or Candida 105 CFU/ml) in a final volume of 200 μl. After another 24 h, supernatants were collected and stored at −20°C until assayed.
To investigate whether IFN-γ inhibitors affect Candida training, PBMCs were preincubated for 1 h prior to the first stimulation with IgG control, Ustekinumab or IL-18BP in a final concentration of 10 μg/ml. Subsequently, RPMI or Candida were added to the cells with the inhibitors for an additional 24 h. Thereafter, PBMCs were washed with PBS and incubated for a further 5 days in culture medium supplemented with 10% pooled human serum. On day 6, the trained macrophages were subjected to a second stimulation for 24 h with either LPS or Candida. The supernatants were collected and stored at −20°C until assessed.
To assess the role of natural killer (NK) cells in this process, NK cells were isolated via positive selection with CD56 isolation beads from Miltenyi Biotec, according to the manufacturer's instructions. Thereafter, the CD56-positive cells were stimulated for 48 h with heat-killed Candida (106 CFU/ml) or heat-killed S. aureus (107 CFU/ml). PBMCs depleted of NK cells were further included in training experiments, trained with Candida (104 CFU/ml) as described above, and restimulated with either LPS (10 ng/ml) or Candida (105 CFU/ml). After 24 h of cell stimulation, supernatants were collected and stored at −20°C until the amounts of TNF-α and IL-6 were assayed.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of TNF-α (R&D Systems, Abingdon, UK) and IL-6 (Sanquin) were measured 24 h after the second stimulation in cell culture supernatants with ELISA, according to the manufacturer's instructions. The concentration of IFN-γ (Sanquin) was measured in cell culture supernatants at 48 h after stimulation.
Statistical analysis
Results from at least two sets of experiments with a minimum of four volunteers were pooled and analysed using GraphPad. The data are presented as the means ± standard error of the mean (s.e.m.) and Wilcoxon's signed-rank test was used to compare differences between groups. The level of significance was set at *P < 0·05.
Results
PBMCs isolated from patients with CMC, but not HIES, display defective Candida-mediated trained immunity
As shown previously, PBMCs of patients who suffer from CMC due to STAT1 mutations stimulated for 24 h with heat-killed Candida were able to produce normal amounts of TNF-α and IL-6 compared to the healthy controls [2]. We therefore tested whether trained immunity could be triggered by Candida. PBMCs from five CMC patients and nine healthy donors were exposed to β-glucan, Candida and RPMI (negative control) for 24 h. On day 6 after recovery, cells were subjected to a second exposure to LPS, Candida or RPMI (negative control) for an additional 24 h. Interestingly, while PBMCs from CMC patients could be trained by β-glucan (Supporting information, Fig. S1), they could not be trained by C. albicans (Fig. 1).
Fig. 1.
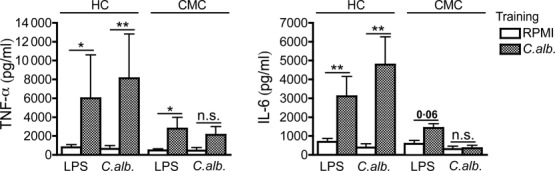
Defective trained immunity in chronic mucocutaneous candidiasis (CMC) patients. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and CMC patients were trained for 24 h by incubation with culture medium or low concentrations of Candida albicans [104 colony-forming units (CFU)/ml]. Thereafter the stimulus was removed and cells were kept in RPMI with 10% human serum. On day 6, a second stimulation with lipopolysaccharide (LPS) (10 ng/ml) or C. albicans (105 CFU/ml) was performed for an additional 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation; n = 9 healthy controls, n = 5 CMC patients. The data are presented as the means ± standard error of the mean (s.e.m.). HC = healthy control.
In contrast, PBMCs isolated from HIES patients showed a normal degree of training with both Candida (Fig. 2) and β-glucan (Supporting information, Fig. S2), as shown by the enhanced production of TNF-α and IL-6 upon restimulation with either LPS or Candida.
Fig. 2.
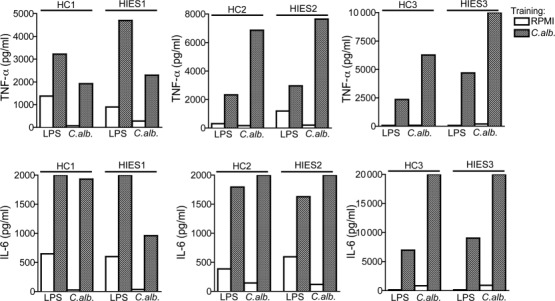
Normal trained immunity in hyper-immunoglobulin (Ig)E syndrome (HIES) patients. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and HIES patients were trained for 24 h with culture medium or to very low concentrations of Candida albicans [104 colony-forming units (CFU)/ml]. At day 6, a second stimulation with lipopolysaccharide (LPS) (10 ng/ml) or C. albicans (105 CFU/ml) was performed for an additional 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation. n = 9 healthy controls; n = 3 HIES patients. The data are presented as the means ± standard error of the mean (s.e.m.). HC = healthy control.
Defective IFN-γ production by NK cells from CMC patients
The defective trained immunity in PBMCs from CMC patients suggests that STAT-1, but not STAT-3, is crucial for Candida-induced trained immunity. IFN-γ is a proinflammatory cytokine produced by NK and T cells that induces activation of both innate and adaptive immunity against candidiasis. Because IFN-γ acts through an interferon-gamma receptor (IFN-GR)- and STAT-1-dependent signalling pathway, and has been reported previously to reverse immune tolerance epigenetically [25], we hypothesized that it may be involved in the defective trained immunity in CMC. Interestingly, while purified NK cells from HIES patients produced similar amounts of IFN-γ to the healthy controls (Fig. 3a), IFN-γ production was diminished significantly in CMC patients (Fig. 3a), suggesting defective NK cell function in patients suffering from CMC only. Moreover, when PBMCs from both groups of patients were depleted of NKs and trained subsequently with Candida, not only the cells from patients with CMC showed a defect in training, but NK-depleted cells from HIES were also not able to respond to a similar extent as PBMCs from healthy controls (Fig. 3b), demonstrating an essential role for the NK cell-derived IFN-γ production for the induction of training in HIES patients.
Fig. 3.
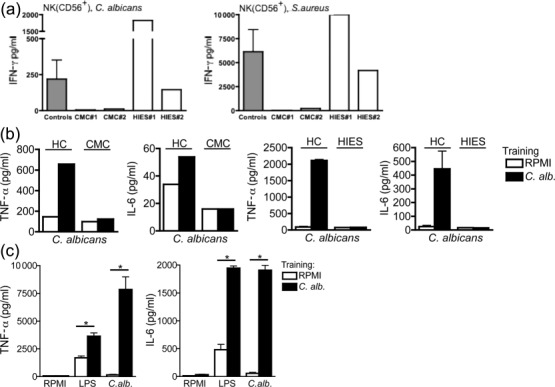
Natural killer (NK)-dependent production of interferon (IFN)-γ mediated trained immunity. (a) Defective NK cells in chronic mucocutaneous candidiasis (CMC) patients. NK cells from two CMC and two hyper-immunoglobulin (Ig)E syndrome (HIES) patients were isolated with CD56-positive beads and stimulated thereafter with either Candida albicans or Staphylococcus aureus. IFN-γ was measured in cell culture supernatants after 48 h of incubation. (b) Defective training of peripheral blood mononuclear cells (PBMCs) depleted of NK cells in CMC and HIES patients. PBMCs from one CMC and two HIES patients were first depleted of NK cells and then trained for 24 h with culture medium or to very low concentrations of C. albicans [104 colony-forming units (CFU)/ml]. At day 6, a second stimulation with C. albicans (105 CFU/ml) was performed for an additional 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation. The data are presented as the means ± standard error of the mean (s.e.m.). HC = healthy control. (c) Training of PBMCs from healthy controls depleted of NK cells. PBMCs from healthy controls were trained for 24 h with culture medium or to very low concentrations of C. albicans (104 CFU/ml), after which they were stimulated as described above; n = 6, three independent experiments. The data are presented as the means ± s.e.m. LPS = lipopolysaccharide.
Of importance, depletion of NK cells in PBMCs from healthy volunteers did not abolish their capacity to mount a trained immunity response (Fig. 3c), suggesting that in healthy volunteers T cell-derived IFN-γ is able to complement for the loss of NK cell function. The defective Th1 cell function in CMC and HIES patients leads, however, to a strong dependency on NK cell-derived IFN-γ.
Inhibiting IFN-γ production by blocking IL-12 and IL-23 with Ustekinumab or by blocking IL-18 with IL-18BP significantly decreased the training by Candida in PBMCs from healthly controls, both for TNF-α and IL-6 ( Fig. 4). Of note, the training was not abolished by IgG control (Fig. 4). Moreover, the inhibition was significant only for Candidia training and Candidia restimulation, and not for LPS restimulation (data not shown).
Fig. 4.
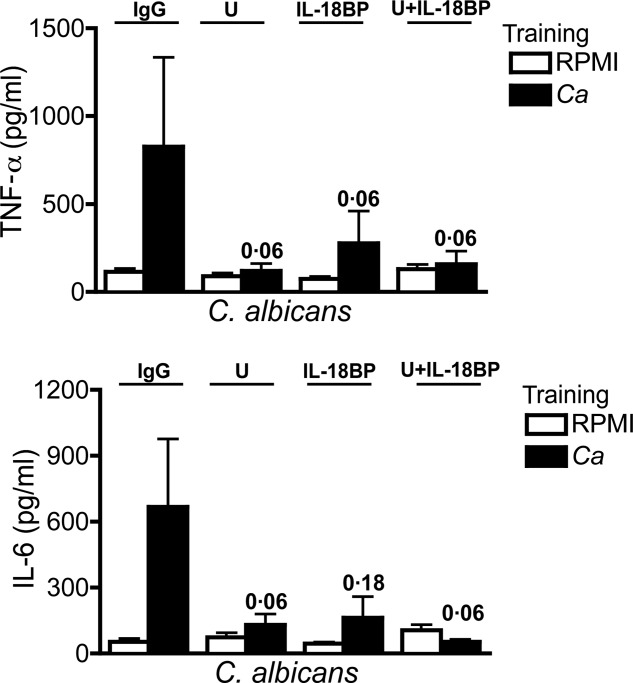
Interferon (IFN)-γ-dependent Candida-induced training. Before training, peripheral blood mononuclear cells (PBMCs) were preincubated for 1 h with immunoglobulin (Ig)G (isotype control) (10 μg/ml), Ustekinumab (10 μg/ml) or interleukin (IL)-18BP (10 μg/ml). Thereafter, 100 µl of culture medium or C. albicans [104 colony-forming units (CFU)/ml] was added to the cell suspension, without removing the inhibitors, and incubated for an additional 23 h. At day 6, macrophages were subjected to a second stimulation with lipopolysaccharide (LPS) (10 ng/ml) or C. albicans (105 CFU/ml) for another 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation. Data are presented as means ± standard error of the mean (s.e.m.); n = 5, three independent experiments; *P < 0·05. Wilcoxon's signed-rank test was used to detect significant differences. U = Ustekinumab.
Discussion
In this study we demonstrated that PBMCs from CMC patients failed to mount Candida-induced trained immunity. This process was caused by STAT1 defects and mediated through NK cell-dependent production of IFN-γ.
The findings of the present study are relevant at several levels. First, they point to an important immunological difference between patients with CMC and HIES. As both these immunodeficiencies are caused by genetic defects in STAT molecules and have a similar immunological defect in the proper activation of Th17 pathway [1,3], why their susceptibility to infection is different remained unexplained. The identification of specific defects in Candida-induced trained immunity in CMC, but not HIES patients, represents a first clue for the severe susceptibility to fungal infections in CMC, compared with the less prominent susceptibility to fungal infections in HIES.
Secondly, the discovery of trained immunity defects in CMC patients, and not in HIES, provides important insights into understanding the communication between important players in host defence. Through in-vitro experiments with NK cells and NK cell depletions, we concluded that T and NK cell-derived IFN-γ plays a central role in potentiating trained immunity in a paracrine and possibly an endocrine manner. Although trained immunity has been described initially to be induced in monocytes, it should be expected that important interactions should take place with other cell types. The first of such interactions is described here, with NK cell-derived IFN-γ playing a central role in potentiating trained immunity in a paracrine and possibly an endocrine manner. We found that while the role of NK cells is redundant in healthy individuals with normal T cell function, the release of IFN-γ by NK cells rescues the training in HIES patients who have defective T cell IFN-γ production. In contrast, CMC patients who display both defective T and NK cell production of IFN-γ explain defective trained immunity. A role for NK cells in the induction of trained immunity has been also described recently after bacillus Calmette–Guérin (BCG) vaccination [26].
The role of IFN-γ and STAT-1 for the induction of trained immunity is in line with previous observations. We have described the differential regulation of STAT-1 and STAT-3 during induction of trained immunity and immune tolerance [19], data that provided the initial impulse to study these mechanisms in CMC and HIES. Furthermore, IFN-γ has been demonstrated to reverse the effects of LPS tolerance, the ‘opposite’ of trained immunity, specifically by activating Brg1 which, in turn, remodels chromatin facilitating the access of transcription factors [25]. IFN-γ exerts its biological activity through activation of intracellular pathways containing Janus kinase 1 (Jak1), Jak2 and STAT-1 [27]. The role of STAT-1 signalling for trained immunity may thus be explained through its role in the type II IFNGR pathway. In line with this, a recent study demonstrated that activation of STAT-1 by IFN-γ correlates with multiple histone modifications, such as H3K4me2, H3K4me3, H3K9me3 and H3K36me3 [28].
It remains unclear why β-glucan is still able to train cells from CMC patients, as it has been proposed that the mechanism through which Candida trains immune cells is through β-glucan/Dectin-1 [17]. However, recent data suggesting that other cell wall Candida structures, such as chitin, are also important for trained immunity induced by the whole fungus (L. Rizzetto, personal communication) may represent one possible explanation. One limitation of the study that should be acknowledged is the limited number of patients. Unfortunately, this was unavoidable due to the rare nature of both CMC and HIES diseases.
Although the in-vitro model of training PBMCs is a simplified system, it provides a direct assessment of the propensity of PBMCs from diverse individuals to be trained. However, to our knowledge, this is the first study reporting defective trained immunity in CMC and normal training effects in HIES patients. Thereby, this study enhances our understanding of specific immunological disturbances in each of these complex immunodeficiencies. Moreover, it provides new openings in the design of novel immunotherapeutic strategies towards a personalized treatment of CMC and HIES.
Acknowledgments
This work was supported by European Union ALLFUN (FP7/2007 2013, HEALTH-2010-260338) (Fungi in the setting of inflammation, allergy and autoimmune diseases: Translating basic science into clinical practices ‘ALLFUN’). M. G. N and J. Q. were also supported by a Vici grant from the Netherlands Organization of Scientific Research (to M. G. N.). F. vd V. was supported by a Veni grant from the Netherlands Organization of Scientific Research.
Author contributions
D. C. I., J. Q., T. S. P., J. W. M. vd M., F. L. vd V. and M. G. N. designed and analysed the experiments. D. C. I. performed the experiments. J. Q. and T. S. P. contributed to some of the experiments. D. C. I., L. A. B. J., F. L. vd V., J. W. M. vd M. and M. G. N. wrote the manuscript, and all authors contributed to the manuscript preparation. M. G. N. supervised the project.
Disclosure
The authors declare no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's Web site:
Fig. S1. β-glucan-induced rained immunity in chronic mucocutaneous candidiasis (CMC) patients. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and CMC patients were trained for 24 h with culture medium or to very low concentrations of β-glucan (1 μg/ml). At day 6, a second stimulation with lipopolysaccharide (LPS) (10 ng/ml) was performed for an additional 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation; n = 9 healthy controls, n = 5 CMC patients. The data are presented as the means ± standard error of the mean (s.e.m.). HC = healthy control.
Fig. 2. β-glucan-induced rained immunity in hyper-immunoglobulin (Ig)E syndrome (HIES) patients. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and HIES patients were trained for 24 h with culture medium or to very low concentrations of β-glucan (1 μg/ml). At day 6, a second stimulation with lipopolysaccharide (LPS) (10 ng/ml) was performed for an additional 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation; n = 9 healthy controls, n = 3 HIES patients. The data are presented as the means ± standard error of the mean (s.e.m.). HC = healthy control.
References
- 1.Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Veerdonk FL, Plantinga TS, Hoischen A, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 3.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452 doi: 10.1038/nature06764. 773–U711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma CS, Chew GYJ, Simpson N, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–7. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–50. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Okada S, Kong XF, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–48. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue L, Yang Y, Wang S. A novel large deletion of the DOCK8 gene in a Chinese family with autosomal-recessive hyper-IgE syndrome. J Eur Acad Dermatol Venereol. 2015;29:599–601. doi: 10.1111/jdv.12394. [DOI] [PubMed] [Google Scholar]
- 8.Soltesz B, Toth B, Shabashova N, et al. New and recurrent gain of-function STAT1 mutations in patients with chronic mucocutaneous candidiasis from Eastern and Central Europe. J Med Genet. 2013;50:567–78. doi: 10.1136/jmedgenet-2013-101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson ML, Freeman AF, Holland SM. Hyper IgE syndrome: an update on clinical aspects and the role of signal transducer and activator of transcription 3. Curr Opin Allergy Clin Immunol. 2008;8:527–33. doi: 10.1097/ACI.0b013e3283184210. [DOI] [PubMed] [Google Scholar]
- 10.Freeman AF, Holland SM. Clinical manifestations, etiology, and pathogenesis of the hyper-IgE syndromes. Pediatr Res. 2009;65:32r–7r. doi: 10.1203/PDR.0b013e31819dc8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 12.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–U1010. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 13.Davis SD, Schaller J, Wedgwood RJ. Job's syndrome. Recurrent, ‘cold’, staphylococcal abscesses. Lancet. 1966;1:1013–5. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- 14.Sowerwine KJ, Holland SM, Freeman AF. Hyper-IgE syndrome update. Ann NY Acad Sci. 2012;1250:25–32. doi: 10.1111/j.1749-6632.2011.06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–61. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Bowdish DM, Loffredo MS, Mukhopadhyay S, Mantovani A, Gordon S. Macrophage receptors implicated in the ‘adaptive’ form of innate immunity. Microbes Infect. 2007;9:1680–7. doi: 10.1016/j.micinf.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Quintin J, Saeed S, Martens JH, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–32. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintin J, Cheng SC, van der Meer JW, Netea MG. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol. 2014;29:1–7. doi: 10.1016/j.coi.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller A, Rice PJ, Ensley HE, et al. Receptor binding and internalization of a water-soluble (1–>3)-beta-D-glucan biologic response modifier in two monocyte/macrophage cell lines. J Immunol. 1996;156:3418–25. [PubMed] [Google Scholar]
- 22.Lehrer RI, Cline MJ. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969;98:996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 24.Endres S, Ghorbani R, Lonnemann G, van der Meer JW, Dinarello CA. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin Immunol Immunopathol. 1988;49:424–38. doi: 10.1016/0090-1229(88)90130-4. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Ivashkiv LB. IFN-gamma abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci USA. 2010;107:19438–43. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. 2014;155:213–9. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- 28.Buro LJ, Chipumuro E, Henriksen MA. Menin and RNF20 recruitment is associated with dynamic histone modifications that regulate signal transducer and activator of transcription 1 (STAT1)-activated transcription of the interferon regulatory factor 1 gene (IRF1) Epigenetics Chromatin. 2010;3:16. doi: 10.1186/1756-8935-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. β-glucan-induced rained immunity in chronic mucocutaneous candidiasis (CMC) patients. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and CMC patients were trained for 24 h with culture medium or to very low concentrations of β-glucan (1 μg/ml). At day 6, a second stimulation with lipopolysaccharide (LPS) (10 ng/ml) was performed for an additional 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation; n = 9 healthy controls, n = 5 CMC patients. The data are presented as the means ± standard error of the mean (s.e.m.). HC = healthy control.
Fig. 2. β-glucan-induced rained immunity in hyper-immunoglobulin (Ig)E syndrome (HIES) patients. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and HIES patients were trained for 24 h with culture medium or to very low concentrations of β-glucan (1 μg/ml). At day 6, a second stimulation with lipopolysaccharide (LPS) (10 ng/ml) was performed for an additional 24 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured in cell culture supernatants after the second stimulation; n = 9 healthy controls, n = 3 HIES patients. The data are presented as the means ± standard error of the mean (s.e.m.). HC = healthy control.


