Abstract
HIV-infected children are less capable of mounting and maintaining protective humoral responses to vaccination against measles compared to HIV-uninfected children. This poses a public health challenge in countries with high HIV burdens. Administration of anti-retroviral therapy (ART) and revaccinating children against measles is one approach to increase measles immunity in HIV-infected children, yet it is not effective in all cases. Immune anergy and activation during HIV infection are factors that could influence responses to measles revaccination. We utilized a flow cytometry-based approach to examine whether T cell anergy and activation were associated with the maintenance of measles-specific immunoglobulin (Ig)G antibodies generated in response to measles revaccination in a cohort of HIV-infected children on ART in Nairobi, Kenya. Children who sustained measles-specific IgG for at least 1 year after revaccination displayed significantly lower programmed cell death 1 (PD-1) surface expression on CD8+ T cells on a per-cell basis and exhibited less activated CD4+ T cells compared to those unable to maintain detectable measles-specific antibodies. Children in both groups were similar in age and sex, CD4+ T cell frequency, duration of ART treatment and HIV viral load at enrolment. These data suggest that aberrant T cell anergy and activation are associated with the impaired ability to sustain an antibody response to measles revaccination in HIV-infected children on ART.
Keywords: ART, HIV, measles, revaccination, T cells
Introduction
Despite increased coverage of measles vaccination programmes, measles is still a threat to many African countries. Kenya documented 2395 measles cases in 2011 and greater than 2169 cases in 2012, despite having a high rate of vaccination against this disease [1,2]. One possible contributing factor to this is high HIV prevalence among adults and children [3]. HIV-infected children are less capable of mounting and maintaining responses to vaccination against measles than uninfected, otherwise healthy children [4–7]. Studies in Kenya have demonstrated that administration of anti-retroviral therapy (ART) to HIV-infected individuals alone is not sufficient to restore measles antibody titres [8,9].
One approach to obviate the detrimental effects HIV exacts on measles vaccination efficacy is to revaccinate individuals after ART-elicited immune system reconstitution. This method has shown promise in the United States, Thailand and Kenya, but the mechanisms underpinning whether individuals respond durably to revaccination have not been investigated [8,10,11]. Underlying immune system dysfunction may influence whether HIV-infected individuals mount durable responses to revaccination. We utilized a flow cytometry-based approach to examine whether T cell anergy and activation associate with the ability to generate a durable measles-specific antibody response after revaccination in a cohort of HIV-infected children on ART in Nairobi, Kenya.
Materials and methods
Study participants and follow-up procedures
This work was nested within a larger study in which HIV-infected children on ART were revaccinated for measles when their CD4+ T cell frequency reached at least 15% of total T cells (Newman 2015, in prep.). During clinic visits at the time of revaccination, 1 month post-revaccination and 1 year post-revaccination, serum was collected to assess levels of measles-specific immunoglobulin (Ig)G by enzyme-linked immunosorbent assay (ELISA) and peripheral blood mononuclear cells (PBMC) were collected.
In order to define immunological parameters that may determine the ability to sustain measles-specific IgG antibody responses upon revaccination, we examined PBMC from a subset of participants who were measles-seronegative (measles-specific IgG antibody titre ≤ 350 mIU/ml [12]) at enrolment and developed detectable measles antibodies 1 month after revaccination. We randomly selected 10 children who met these criteria and maintained measles antibodies 1 year after revaccination (responders) and 10 children who did not exhibit detectable measles antibodies 1 year after revaccination (non-responders).
Flow cytometry
The immunophenotype of T cells in PBMC was determined at baseline, 1 month and 1 year post-revaccination in the 20 nested study participants.
For surface staining, PBMC were thawed in RPMI supplemented with 10% fetal calf serum, 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 1 × penicillin/streptomycin and 5 U/ml benzonase nuclease (Novagen, Darmstadt, Germany); ∼1–4 × 106 cells were then incubated with monoclonal antibodies as indicated below at 4 °C in a final volume of 100 μl in a 96-well tissue culture plate. After 30 min, samples were washed and data were acquired immediately using an LSR II (BD Biosciences, San Jose, CA, USA). Data were analysed using FlowJo software (TreeStar Inc., Ashland, OR, USA). Doublets were excluded before gating on live cells using forward light-scatter and side-scatter.
Immune profiling was performed in two panels. CD4+ and CD8+ T cell activation was assessed using CD3 peridinin chlorophyll cyanin 5·5 (PerCpCy5·5) (clone OKT3), CD4 fluorescein isothiocyanate (FITC) (clone OKT4), CD38 phycoerythrin (PE) (clone HB7) and human leucocyte antigen D-related (HLA)-DR allophycocyanin (APC) (clone LN3). The CD4+ and CD8+ T cell anergy panel consisted of CD3 PerCpCy5.5, CD4 FITC and programmed cell death PD-1 PE (clone J105). Antibodies were purchased from eBioscience (San Diego, CA, USA) and used as per the manufacturer's directions. At each time-point (baseline, 1 month post-revaccination and 1 year post-revaccination) all samples from the responder and non-responder groups were acquired without changing machine settings between samples.
Viral load
HIV viral load was assessed using plasma samples that were collected at enrolment and 12 months post-revaccination, frozen and shipped to Seattle, Washington, USA on dry ice for measurement. HIV-1 RNA levels were measured by the Gen-Probe HIV-1 viral load assay (Gen-Probe, San Diego, CA, USA). This analysis was performed post hoc after group segregation, flow cytometric analysis and measles antibody determination.
Statistical analyses
Statistical significance was determined using a two-tailed Student's t-test for numerical variables or χ2 analysis for categorical variables. Graphs were generated and statistical analyses were performed using Prism (GraphPad, San Diego, CA, USA) or Stata version 11·1 (StatCorp, College Station, TX, USA).
Human subjects approval
This study was approved by the Kenyatta National Hospital/University of Nairobi Ethics Review Committee and the University of Washington Human Subjects Division.
Results
Demographic and clinical characteristics of study participants
The demographic characteristics of the overall cohort have been described previously [9]. Briefly, 232 children attending routine clinic visits at the Comprehensive HIV Care Centre at Kenyatta National Hospital in Nairobi, Kenya were enrolled. They had an average age of 7·5 years [interquartile range (IQR) = 5·5–9·5] and 123 (53%) were male. All children were on ART, had been on treatment for a median time of 3·4 years (IQR = 1·8–4·9) and exhibited a median CD4+ T cell percentage of 32 (IQR = 27–38).
In this ancillary study, we examined 10 children who responded durably to measles revaccination (responders) and 10 who did not (non-responders). One individual in the non-responder group was omitted from analysis due to extremely low CD4+ T cell frequencies (<2·5% of all T cells) at 1 month and 1 year post-revaccination, therefore the analysis of this group was confined to nine individuals.
The age, CD4+ T cell frequency, HIV viral load per ml of blood, time on ART and sex of responders and non-responders at enrolment, 1 month post-revaccination and 1 year post-revaccination can be found in Table 1. There was no statistically significant difference between responders and non-responders in any of these parameters at any time-point assayed (P > 0·05).
Table 1.
Demographic and clinical characteristics of study participants
| Measles-seropositive* at 1 year post- revaccination (n = 10) | Measles-seronegative* at 1 year post- revaccination (n = 9) | ||
|---|---|---|---|
| Enrolment | Mean (range) or n (%) | Mean (range) or n (%) | P-value (χ2 or t-test) |
| Age | 9·7 (3·3–11·9) | 7·7 (4·2–11·5) | 0·0769 |
| Male | 8 (80%) | 6 (67%) | 0·4343 |
| CD4% | 33 (18–60) | 29 (15-50) | 0·8132 |
| HIV viral load† | 41 473 (0–155 430) | 22 753 (0–226 820) | 0·5575 |
| Time on ART (years) | 3·8 (0·2–7·0) | 3·2 (1·3–6·0) | 0·5709 |
| Measles antibody titre (log10 mIU/ml) | Seronegative | Seronegative | n.a. |
| 1 month post-revaccination | |||
| CD4% | 43·7 (35·3–46·0) | 41·3 (26·7–49·9) | 0·6546 |
| Measles antibody titre (log10 mIU/ml) | 3·4 (2·8–3·8) | 3·0 (2·6–3·5) | 0·0057 |
| 1 year post-revaccination | |||
| CD4% | 32 (16–55) | 30 (15–47) | 0·4675 |
| HIV viral load | 1721 (0–15 302) | 16 096 (0–49 509) | 0·0813 |
| HIV virological failure‡ | 1 (10%) | 3 (33%) | 0·2130 |
| Measles antibody titre (log10 mIU/ml) | 3·0 (2·6–4·0) | Seronegative | n.a. |
Measles-seropositive refers to a measles-specific immunoglobulin (Ig)G antibody titre ≥ 350 mIU/ml of serum. †Copies of HIV per ml of blood.
Two consecutive HIV viral load measurements greater than 1000 copies per ml of blood.
ART = anti-retroviral therapy; n.a. = not available.
Individuals in both groups were seronegative for measles-specific IgG at enrolment (Table 1). All participants generated measles-specific IgG antibodies at 1 month post-revaccination, but responders had a significantly higher mean serum titre [3·4 log10 (mean = 2·8–3·8)] compared to non-responders [3·0 log10 (mean = 2·6–3·5)] (P = 0·0057). At 1 year post-revaccination, responders had a mean measles-specific IgG serum antibody titre of 3·0 log10 (mean = 2·6–4·0), whereas non-responders did not have detectable measles antibodies (Table 1).
Responders and non-responders have equivalent percentages of T cells
CD4+ T cells were identified as CD3+CD4+ and CD8+ T cells were identified as CD3+CD4− within the live cell gate (Fig. 1a). We did not observe any difference in the frequency of CD4+ and CD8+ T cells between responders and non-responders at any of the time-points assayed (Fig. 1b).
Fig. 1.
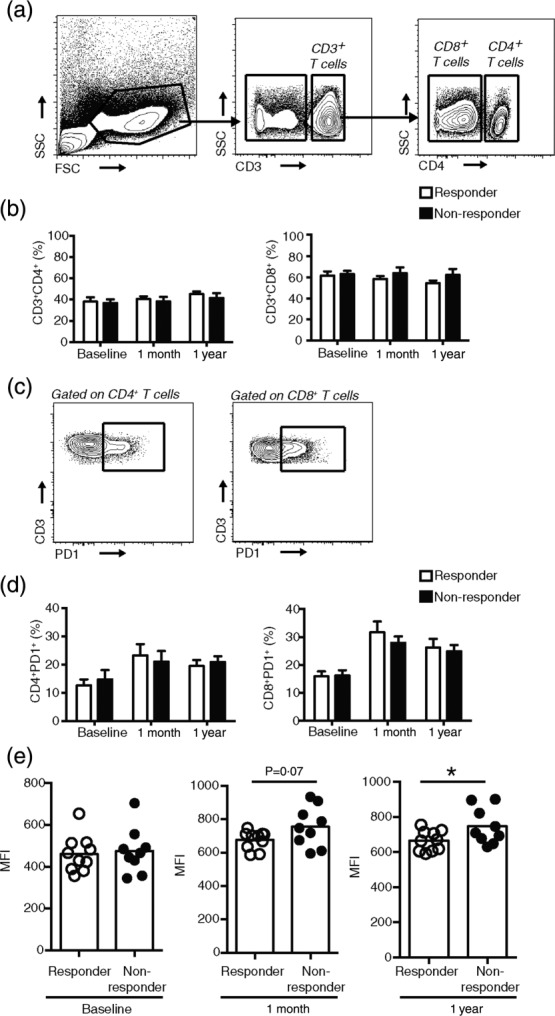
Responders and non-responders to measles revaccination do not exhibit differences in CD4+ or CD8+ T cell frequency but responders have significantly diminished programmed cell death-1 (PD-1) mean fluorescence intensity (MFI) on CD8+PD-1+ CD8+ T cells compared to non-responders at 1 year post-revaccination. (a) Gating strategy and representative flow cytometry plots for identifying CD4+ and CD8+ (identified as CD3+CD4−) T cells in peripheral blood mononuclear cells (PBMC). (b-d) CD8+ T cells were identified as CD3+CD4−. (b) Percentage of CD4+ T cells and CD8+ T cells in responders and non-responders at baseline, 1 month post-revaccination and 1 year post-revaccination. (c) Gating strategy and representative flow cytometry plots for identifying PD-1+CD4+ and PD-1+CD8+ T cells in PBMC. (d) Percentage of PD-1+CD4+ T cells and PD-1+CD8+ T cells at baseline, 1 month and 1 year post-revaccination. (e) MFI of PD-1 expression on CD8+PD-1+ T cells at the indicated time-points. Each dot represents one individual. In bar graphs (b,d) data are presented as mean ± standard error of the mean (s.e.m.). (b,d,e) Ten responders and nine non-responders at each time-point. Statistics were conducted using two-tailed Student's t-test; *P < 0·05.
Responder PD-1+CD8+ T cells exhibit significantly fewer PD-1 molecules on a per-cell basis compared to non-responders 1 year post-revaccination
We used expression of PD-1 as a marker of T cell anergy. We observed no difference in the frequency of CD4+PD-1+ or CD8+PD-1+ T cells between responders and non-responders at any time-point (Fig. 1c,d). However, responders exhibited a trend towards lower PD-1 expression per CD8+PD-1+ T cells, as measured by mean fluorescence intensity (MFI) of PD-1 on CD8+PD-1+ T cells by 1 month post-revaccination compared to non-responders (Fig. 1e). At 1 year post-revaccination, the CD8+PD-1+ T cells of responders displayed significantly less PD-1 on a per-cell basis compared to non-responders (average MFI = 665 responders, 746·3 non-responders, P = 0.0452) (Fig. 1e). We observed no difference in CD4+ T cell PD-1 MFI between responders and non-responders at any time-points (data not shown).
Responders exhibit decreased CD4+ T cell activation compared to non-responders 1 year post-revaccination
T cell activation was determined by tabulating the frequency of CD4+ and CD8+ T cells that co-expressed CD38 and HLA-DR. We found no difference in the frequency of CD38+HLA-DR+ CD8+ T cells between responders and non-responders at any time-points assayed (data not shown). However, responders exhibited a strong trend towards a decrease in the percentage of activated CD4+ T cells at 1 year post-revaccination, but not at other time-points (P = 0·0637) (Fig. 2a,b and data not shown).
Fig. 2.
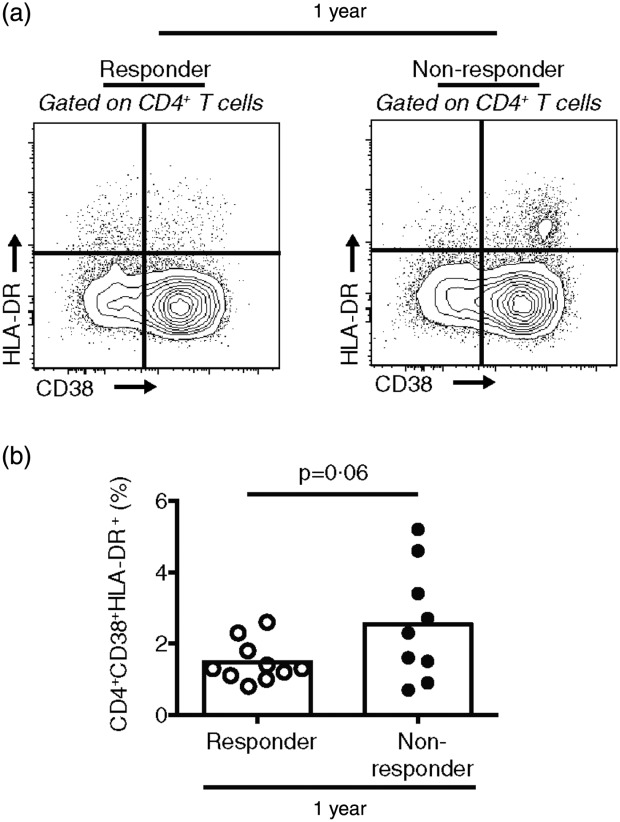
Responders to measles revaccination exhibit a trend towards a diminished frequency of activated CD38+human leucocyte antigen D-related (HLA-DR)+CD4+ T cells compared to non-responders 1 year post-revaccination. (a) Gating strategy and representative flow cytometry plots for identifying CD38+HLA-DR+CD4+ T cells in peripheral blood mononuclear cells (PBMC). (b) Percentage of CD38+HLA-DR+CD4+ at 1 year post-revaccination. Each dot represents one individual. Data are presented as mean of 10 responders and nine non-responders. Statistics were conducted using a two-tailed Student's t-test; *P < 0·05.
Discussion
Here, we report that durable humoral responses to measles revaccination in HIV-infected children on ART in Nairobi, Kenya coincide with restrained CD8+ T cell PD-1 expression and CD4+ T cell activation. Other groups have demonstrated that revaccinating HIV-infected individuals for measles after initiating ART can generate lasting measles-specific IgG in between 65 and 85% of individuals, but the precise immune mechanisms underlying these observations have not been investigated previously [8,10,11]. Thus, this is the first work, to our knowledge, to outline a subset of immunological parameters that may underpin a resilient response to measles revaccination.
Measles vaccination results typically in a protective, durable antibody response that lasts for at least several decades in healthy individuals [13]. However, those with HIV are less capable of generating a durable protective measles-specific antibody titre [4–7]. The dual approach of administering ART to ensure immune system reconstitution and then revaccinating HIV-infected children against measles is one means to diminish measles susceptibility. Garnering an understanding of why some HIV-infected individuals on ART who are revaccinated do not maintain durable measles antibodies is important if this approach is to be utilized further in Kenya and other countries. Here, we examined levels of T cell anergy and activation to evaluate if these parameters were associated with sustained measles antibody levels in response to revaccination in HIV-infected children on ART.
T cells are important for maintaining low HIV viral loads and generating robust antibody responses to vaccinations. We observed no difference in the frequency of CD4+ or CD8+ T cells between responders and non-responders throughout the duration of the study, demonstrating that the inability of non-responders to maintain durable measles antibodies 1 year post-revaccination is not due to a selective diminishment of either T cell subset.
In our cohort, responders exhibited significantly lower PD-1 expression on PD-1+CD8+ T cells compared to non-responders. Surface expression of PD-1 by antigen-specific CD4+ and CD8+ T cells is associated with chronic activation and anergy in some prolonged infections, such as HIV, although the precise role of this molecule is controversial [14,15]. PD-1 cross-linking by its ligands PD-1 or -2 can attenuate T cell receptor/CD28 proximal signal transduction and thereby limit effector functions such as cytokine production and proliferation [16]. Recent work in Uganda has also correlated PD-1 expression on CD4+ and CD8 T cells with suboptimal immune CD4+ T cell reconstitution in HIV-infected individuals on ART [17]. A direct, biological relationship between PD-1 expression on CD8+ T cells and the ability to sustain an antibody response is unlikely. However, at 1 month post-revaccination, the non-responder group had significantly lower measles antibody titres compared to responders and exhibited a trend towards increased CD8+ T cell PD-1 expression, raising the possibility that immune dysregulation in non-responders was evident early after revaccination and is associated with CD8+ T cell anergy. The precise mechanism promoting CD8+ T cell anergy in non-responders is unknown, but could be due to co-infection with other microbes, inadequate nutrition or other variables that were not evaluated here.
Immune activation is crucial for host defence, but can undermine the immune integrity of the host if activation is protracted [18]. Several phenotypical markers can be used to identify activated T cells, but CD38 and HLA-DR are among the most well-characterized in HIV infection [19]. CD4+ and CD8+ T cell activation has been correlated with increased HIV viral load and in Uganda [19]. CD4+ and CD8+ T cell activation was also associated with suboptimal CD4+ T cell reconstitution in HIV+ individuals on ART in Uganda [17]. CD4+ T cells are important in generating productive antibody responses from B cells. We found that responders who are able to maintain measles-specific antibodies 1 year post-revaccination have a trend towards a lower frequency of activated CD4+ T cells compared to non-responders. This suggests that CD4+ T cell hyperactivation may be implicated in impaired B cell function to revaccination. T cell activation can occur via antigen-dependent or independent mechanisms [20]. As there is no meaningful difference in viral load between responders and non-responders, we would speculate that this activation is HIV-antigen-independent and perhaps due to co-infection with other microbes that cause activation of CD4+ T cells. Overall, the link between CD4+ activation and the inability to maintain antibody responses to revaccination is intriguing, and adds to the literature indicating negative consequences of aberrant CD4+ T cell activation during HIV infection.
In conclusion, we identify T cell anergy and activation as factors that may impair a productive humoral response to measles revaccination in HIV-infected children on ART in Nairobi, Kenya. Overall, this study lays a foundation for future work in investigating the interplay between T cell activation and anergy and responses to revaccination in HIV-infected children. Future work will expand the number of individuals assayed, discriminate between T cell subsets and directly examine B cell activation, anergy and function.
Acknowledgments
We thank the study participants and the Measles Vaccination Study team, Dr Julie Overbaugh and Sandy Emery from the Fred Hutchinson Cancer Research Center for their help in determining HIV vial load and Dr Julius Oyugi and the University of Manitoba staff in Nairobi. This work was supported in part by NSF GRFP grant DGE-0718124 (MBB). Research reported in this publication was supported by the Institute of Allergy and Infectious Disease of the National Institutes of Health under award number K24AI087399. LPN received support from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR000422 and the UW Thomas Francis Jr Global Health Fellowship. The publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author contributions
M. B. B. designed and conducted the experiments, analysed the data and wrote the manuscript. L. P. N. designed the experiments, conducted statistical analyses and assisted in writing the manuscript. B. H.C. designed the experiments. A. N. and D. W. designed and implemented the parent study. C. F. designed the experiments and wrote the manuscript. All authors reviewed, edited and approved the manuscript.
Disclosure
The authors do not have commercial or other associations that might pose conflicts of interest.
References
- 1.World Health Organization (WHO) World Health Statistics. Geneva: WHO. Available at: http://www.who.int/gho/publications/world_health_statistics/EN_WHS2013_Full.pdf. Accessed June 17, 2014.
- 2.World Health Organization (WHO) Regional Office for Africa. Kenya launches US$9million Integrated Measles Vaccination Campaign targeting 6 million children. Brazzaville, Congo: WHO Regional Office for Africa, 2012. Available at: http://www.afro.who.int/en/kenya/press-materials/item/5094-kenya-launches-us$9million-integrated-measles-vaccination-campaign-targeting-6-million-children/5094-kenya-launches-us$9million-integrated-measles-vaccination-campaign-targeting-6-million-children.html (accessed 2 September 2014)
- 3.Nilsson A, Chiodi F. Measles outbreak in Africa – is there a link to the HIV-1 epidemic? PLOS Pathog. 2011;7:e1001241. doi: 10.1371/journal.ppat.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudy BJ, Rutstein RM, Pinto-Martin J. Responses to measles immunization in children infected with human immunodeficiency virus. J Pediatr. 1994;125:72–4. doi: 10.1016/s0022-3476(94)70125-3. [DOI] [PubMed] [Google Scholar]
- 5.Tejiokem MC, Gouandjika I, Béniguel L, et al. HIV-infected children living in Central Africa have low persistence of antibodies to vaccines used in the Expanded Program on Immunization. PLOS ONE 2007; 2:e1260. doi: 10.1371/journal.pone.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindgren-Alves CR, Freire LM, Oliveira RC, et al. Search of antimeasles antibodies in HIV-infected children after basic immunization. J Pediatr (Rio J) 2001; 77:496–502. doi: 10.2223/jped.360. [DOI] [PubMed] [Google Scholar]
- 7.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–55. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar C, Wamalwa D, Selig S, et al. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre- and post-highly active antiretroviral therapy and revaccination. Pediatr Infect Dis J 2009; 28:295–9. doi: 10.1097/INF.0b013e3181903ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman LP, Njoroge A, Ben-Youssef L, et al. Measles Seropositivity in HIV-infected Kenyan children on antiretroviral therapy. Pediatr Infect Dis J. 2014;33:843–5. doi: 10.1097/INF.0000000000000332. doi: 10.1097/INF.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Persistence of measles, mumps, and rubella protective antibodies 3 years after revaccination in HIV-infected children receiving antiretroviral therapy. Clin Infect Dis 2010; 50:1415–8. doi: 10.1086/652150. doi: 10.1086/652150. [DOI] [PubMed] [Google Scholar]
- 11.Berkelhamer S, Borock E, Elsen C, Englund J, Johnson D. Effect of highly active antiretroviral therapy on the serological response to additional measles vaccinations in human immunodeficiency virus-infected children. Clin Infect Dis 2001; 32:1090–4. doi: 10.1086/319591. doi: 10.1086/319591. [DOI] [PubMed] [Google Scholar]
- 12.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) Measles vaccines: WHO position paper. Wkly Epidemiol Rec 2009; 84:349–60. [PubMed] [Google Scholar]
- 14.Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory receptor expression depends more dominantly on differentiation and activation than ‘exhaustion’ of human CD8 T cells. Front Immunol 2013; 4:455. doi: 10.3389/fimmu.2013.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLOS ONE 2013; 8:e60186. doi: 10.1371/journal.pone.0060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 17.Nakanjako D, Ssewanyana I, Mayanja-Kizza H, et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis 2011; 11:43. doi: 10.1186/1471-2334-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggena MP, Barugahare B, Okello M, et al. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 20.Haas A, Zimmermann K, Oxenius A. Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J Virol 2011; 85:12102–13. doi: 10.1128/JVI.05607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


