Abstract
Maternal systemic inflammation is a feature of pre-eclampsia, a condition in pregnancy characterized by hypertension and proteinuria. Pre-eclampsia is caused by the placenta; many placental factors contribute to the syndrome's progression, and proinflammatory cytokines have been identified previously as one such mediator. The interleukin (IL)-1 family of cytokines are key regulators of the inflammatory network, and two naturally occurring regulatory molecules for IL-1 family cytokines, IL-1RA and sST2, have been found previously to be elevated in maternal blood from women with pre-eclampsia. Here we investigate more recently identified IL-1 family cytokines and regulatory molecules, IL-1RAcP, IL-37, IL-18BP, IL-36α/β/γ/Ra and IL-38 in pre-eclampsia. Pregnant women have more circulating IL-18BP and IL-36Ra than non-pregnant women, and sIL-1RAcP is elevated from women with pre-eclampsia compared to normal pregnancies. The placenta expresses all the molecules, and IL-37 and IL-18BP are up-regulated significantly in pre-eclampsia placentas compared to those from normal pregnancies. Together, these changes contribute to the required inhibition of maternal systemic cytotoxic immunity in normal pregnancy; however, in pre-eclampsia the same profile is not seen. Interestingly, the increased circulating levels of sIL-1RAcP and increased placental IL-18BP and IL-37, the latter of which we show to be induced by hypoxic damage to the placenta, are all factors which are anti-inflammatory. While the placenta is often held responsible for the damage and clinical symptoms of pre-eclampsia by the research community, here we show that the pre-eclampsia placenta is also trying to prevent inflammatory damage to the mother.
Keywords: cytokines, IL-1 superfamily, pre-eclampsia, pregnancy
Introduction
Pre-eclampsia is a syndrome that can present from the second half of pregnancy onwards, characterized by new-onset maternal hypertension and proteinuria. It affects 2–8% of all pregnancies [1]. Its pathogenesis is variable, although in early-onset disease (<34 weeks) deficient placentation in the early stages of pregnancy, causing dysfunctional uteroplacental perfusion, hypoxia and oxidative stress is an important component [2]. The subsequent dysfunctional uteroplacental blood supply causes ischaemia/reperfusion injury with associated oxidative, endoplasmic reticulum and inflammatory stress, affecting the placental tissue in direct contact with maternal blood, namely the epithelial syncytiotrophoblast. This has major consequences for both the developing fetus and mother. The fetus may be deprived of nutrients and oxygen leading to growth restriction and because the only effective treatment for pre-eclampsia is the delivery of the placenta, iatrogenic pre-term delivery and its associated complications are common. The maternal features of pre-eclampsia develop as the stressed syncytiotrophoblast releases multiple factors into the mother's blood, which cause systemic vascular inflammation [3,4]. Many of these factors are also present in normal pregnancy, during which mild systemic inflammation increases as pregnancy progresses. Despite this mild inflammation, the immunologically foreign, semi-allogeneic fetus is tolerated by maternal immune cells. Complex maternal immunoregulation skews immunity towards type 2 cytokine-driven humoral responses and away from type 1 cytotoxic responses, and the ratio of peripheral blood regulatory T cell: T helper type 2 (Treg: Th17) cells increases significantly. In pre-eclampsia, however, the inflammation is more pronounced, and proinflammatory immune cells are more responsive to stimuli when compared to those from women with normal pregnancies [5] and the Treg: Th17 skewing does not occur [6].
The placental-derived factors which affect maternal systemic immune function include hormones, enzymes including indoleamine 2,3-dioxygenase (IDO), factors that affect maternal angiogenic balance [7,8] and cytokines [9]. Of particular interest is the IL-1 cytokine family. These cytokines are potent components of the innate immune system, influencing both survival and death of many cell types and bridging innate and adaptive immune responses. They may be pro- or anti-inflammatory, depending on the environment in which they act. Most-studied in pregnancy are the proinflammatory cytokines IL-1α and IL-1β. Both are expressed by the placenta and secreted by placenta explants in vitro in response to hypoxia [10]. IL-1α is not readily detectable in the maternal circulation [11]; in contrast, IL-1β is elevated in the plasma of pre-eclamptic women [12,13]. Most IL-1 family members activate receptors of the Toll/IL-1 receptor/resistance (TIR) family. Ten members are described (R1–10), which activate kinases that typically result in nuclear factor kappa B (NF-κB) activation and proinflammatory effector functions [14]. IL-1α and IL-1β both bind the IL-1 receptor 1 (IL-1R1), which then complexes with the IL-1 receptor accessory protein (IL-1RAcP), triggering signalling. To counter the action of these cytokines, inhibitory IL-1 family members or receptors protect against uncontrolled inflammation. Associations between IL-1 family members and IL-1 receptor proteins, along with regulatory factors, are detailed in Fig. 1, and have been reviewed extensively elsewhere [17,18]. IL-1α and IL-1β functions can be regulated by: (1) an IL-1 receptor antagonist (IL-1Ra), which binds to the IL-1R1, blocking its association with IL-1α/β, (2) cell surface expression of a decoy receptor IL-1R2, which does not initiate signalling owing to its short cytoplasmic domain and (3) expression of a soluble form of the IL-1RAcP (sIL-1RAcP), which enhances IL-1α/β binding to IL-1R2, thereby preventing inflammation [19]. Levels of circulating IL-1Ra and of cellular IL-1R2 therefore determine whether a proinflammatory response will begin, persist or cease. IL-1Ra is reported to be elevated in pre-eclampsia [20–22], and one possible source of this IL-1Ra is the placenta [23].
Fig. 1.

Schematic of interleukin (IL)-1 cytokine family members (F1–10), IL-1 receptors (R1-10) and inhibitory soluble factors (sIL-1RAcP, sST2, IL-18BP, IL-36Ra, IL-38), showing known associations between ligands, receptors and co-receptors. Three receptors, SIGIRR (single immunolglobulin IL-1R-related molecule), TIGIRR-1 and TIGIRR-2, have no known ligand – SIGIRR is the best described, showing an inhibitory regulatory role for signalling through the other receptors and probably does not have an extracellular ligand [15,16].
We have recently studied the expression of another IL-1 family member, IL-33, in normal pregnancy and pre-eclampsia. Similarly to IL-1α/β, IL-33 binds its receptor ST2L forming a heterodimeric complex with the IL-1RAcP. This drives type 2 responses [24], which may be important for immunoregulation in normal pregnancy. A soluble splice variant of ST2L (sST2) is a decoy receptor and inhibits IL-33 functions. IL-33 and sST2 are both expressed by the placenta [25,26], and sST2 is secreted by placental explants in response to proinflammatory cytokines and hypoxia. Elevated plasma levels of sST2 are found in women with pre-eclampsia prior to the onset of the disease [25]. By blocking the action of IL-33, sST2 may contribute to the type 1 cytokine bias associated with this condition [27]. sIL-1RAcP also limits IL-33 functions directly (although only at high concentrations) and enhances the ability of sST2 to inhibit IL-33 [28]. However, circulating sIL-1RAcP or its expression in placental tissue has not been reported either in normal pregnancy or in pre-eclampsia.
Proinflammatory IL-18 triggers interferon (IFN)-γ release from natural killer (NK) and T cells in the presence of IL-12 or IL-15 by binding to IL-18Rα which, in turn, complexes with IL-18Rβ [29,30]. IL-18 expression is elevated in both the serum and placentas of women with pre-eclampsia [31]. However, the levels of IL-18 binding protein (IL-18BP), which is a potent inhibitor of IL-18 activity [32] in normal pregnancy and pre-eclampsia, have not been investigated.
Three more IL-1 family proteins have been discovered recently: IL-37, IL-36Ra and IL-38. IL-37 potently inhibits the production of a number of proinflammatory cytokines [for example IL-1α and -1β, tumour necrosis factor (TNF)-α] [33]. It binds to IL-18Rα, without either agonist or direct IL-18 antagonistic activity [34,35], but enhances IL-18BP function [36]. IL-37 also probably binds to another, as yet unidentified, receptor and a role as an intracellular factor has emerged [18,37].
IL-36Ra and IL-38 are regulatory molecules which antagonize the functions of IL-36α, IL-36β and IL-36γ [38]. IL-36α, IL-36β and IL-36γ have high sequence homology and are expressed highly in epithelial cells. They bind IL-36R which, like several other IL-1 family members, associates with IL-1RAcP leading to NF-κB activation and the production of proinflammatory cytokines [39].
The purpose of this study was to investigate whether the IL-1 family regulatory proteins sIL-1RAcP, IL-18BP, IL-37, IL-36Ra and IL-38 may have a role in normal pregnancy and pre-eclampsia. Their levels were measured in the plasma of normal pregnant and pre-eclamptic women [at the time of writing, an enzyme-linked immunosorbent assay (ELISA) for IL-38 was not available]. We then determined their levels of expression and localization in the placenta and measured their release from placental explants either as soluble molecules or in association with syncytiotrophoblast extracellular vesicles (EVs) (membrane-bound fragments released from the placenta which interact with the mother's immune and endothelial cells [40]). Finally, we investigated whether these factors were secreted in response to hypoxia and proinflammatory stimuli by the trophoblast cell line BeWo as a model for pre-eclampsia.
Methods
Ethical approval
These studies were approved by the South Central Berkshire Research Ethics Committee (07/H6067/74), and written informed consent was obtained from each participant.
Subject recruitment
Placenta samples
All placentas were delivered by caesarean section, without prior labour. Placental tissue samples [Western blotting placental lysates, immunohistochemistry (IHC) sections] were processed and placenta perfusions were established within 10 min of delivery. For placental lysates and sections the decidual layer was removed and a 1-cm3 sample was taken at random from the centre of a cotyledon, with no obvious infarction or calcification. Normal pregnancy placentas were obtained from women undergoing elective caesarean section at term (>38 weeks). Pre-eclampsia was defined as the new onset of a diastolic blood pressure (BP) ≥90 mmHg on at least two occasions within 24 h and new-onset proteinuria ≥300 mg in a 24-h urine collection, >50 mg/mmol protein/creatinine ratio or at least 2+ on dipstick testing on two consecutive measurements. All cases and controls had singleton pregnancies with no known fetal abnormality.
Plasma samples
Plasma samples were collected using ethylenediamine tetraacetic acid (EDTA) anti-coagulation tubes; blood was taken from women with pre-eclampsia, as defined above (n = 13), who were matched for age (±4 years), parity (0, 1–3, 4+) and gestational age (±13 days) to 13 normal pregnant women, and for age and parity to 13 non-pregnant controls. All samples were stored at −80°C until analysis
The mean gestational age of pre-eclamptic women at delivery was 34 ± 3 (weeks ± days). The range of gestational age at diagnosis was 27 ± 0 – 36 ± 2 and at delivery was 27 ± 1 – 36 ± 3. Blood pressure measurements were comparable between normal pregnant and pre-eclamptic women in the first trimester of pregnancy, but significantly higher when features of pre-eclampsia were present. As would be expected, birth weight was lower in the offspring from pre-eclamptic pregnancies. Patient body mass index at the first midwife ‘booking’ appointment (approximately 8 weeks) was not significantly different between the two groups (normal versus pre-eclamptic women). Patient details are described in Table 1.
Table 1.
Patient characteristics of non-pregnant, normal pregnant and pre-eclamptic women
| Non-pregnant (n = 13) | Normal pregnant (n = 13) | Pre-eclamptic (n = 13) | P-value | |
|---|---|---|---|---|
| Age (years) | 29·2 (5·3) | 29·5 (5·1) | 29·1 (5·2) | n.s. |
| Nulliparity | 11 | 11 | 11 | n.s. |
| Gestation at plasma sample (days) | 236·0 (17·9) | 235·5 (17·9) | n.s. | |
| Gestation at delivery (days) | 281·7 (10·0) | 241·4 (19·8) | P < 0·0005 | |
| Booking BP (mmHg) | 115·5 (9·8)/71·1 (8·7) | 120·2 (10·3)/70·1 (9·3) | n.s. | |
| Maximum BP (mmHg) | 129·3 (6·9)/81·9 (10·0) | 169·6 (6·4)/108·4 (6·4) | P < 0·0005/P < 0·0005 | |
| Birth weight (g) | 3438·0 (522·3) | 1856·5 (596·1) | P < 0·0005 |
BP = blood pressure; n.s.: not significant.
Detection of IL-1 family members and regulatory factors by ELISA
The following ELISAs were used to assess the levels of human analytes in plasma samples or perfusion supernatants; the ranges of detection are also shown: IL-37 (AdipoGen, Incheon, Korea); 1–0·016 ng/ml, IL-18BP (R&D Systems, Minneapolis, MN, USA; 6–0·09375 ng/ml), sIL-1RAcP (USCN Life Science Inc., Wuhan, China; 6–0·09375 ng/ml) and IL-36Ra (Cusabio Biotech Inc., Wuhan, China; 0·5–0·0078 ng/ml), samples with higher values than the upper limit of detection were diluted according to the manufacturer's instructions. For each assay both standards and samples were tested in duplicate.
Detection of IL-1 family members and regulatory factors by immunoblotting
For Western blotting, a 0·5-cm3 sample of placenta, syncytiotrophoblast EVs or cell line pellet was resuspended in HEPES lysis buffer containing mini complete protease inhibitors (Sigma Aldrich, Poole, UK). Placenta lysates from normal (n = 7) or pre-eclamptic pregnancies (n = 7), or lysates from various trophoblast cell lines (JEG-3, BeWo, SGHPL-5, AC1M59, JAR), were prepared and 20 µg placental lysate protein, 10 µg cell lysate or 10 µg EV protein lysate [as determined by bicinchoninic acid assay (BCA) assay; Pierce, Rockford, IL, USA] resolved by sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer to polyvinylidine difluoride (PVDF) membranes for Western blotting, as described previously [25]. The membranes were incubated overnight with the appropriate primary antibody at 4°C. Primary antibodies used were: murine anti-IL-37 (ab57187), goat polyclonal anti-IL-18 (ab106939) and murine anti-β-actin (Abcam, Cambridge, UK); rabbit anti-IL-1RAcP (polyclonal) (Sino Biological Inc., Beijing, China); mouse anti-IL-18 (25-2G) (MBL International Corporation, Woburn, MA, USA); murine anti-IL-18BP (2A9) (Abnova, Taipei, Taiwan); murine anti-IL-36α (162122), murine anti-IL-36β (162601), goat anti-IL-36γ (278706), goat anti-IL-36Ra (3A12) (IL-1F5) and rat anti-IL-38/IL-1F10 (316709) (R&D Systems). Reactions were visualized by incubating the membranes with the appropriate anti-mouse, anti-rabbit, anti-rat or anti-goat secondary antibody conjugated to horseradish peroxidase (Dako, Glostrup, Denmark) for 1 h and detected using an enhanced chemiluminescence system (Pierce, Thermo Scientific, Rockford, IL, USA). Band density was determined using Image J version 1·45s software (NIH, Bethesda, MD, USA) and proteins were normalized for protein loading by comparison to β-actin.
Detection of IL-1 family members and regulatory factors by immunohistochemistry
Tissue samples from normal term (n = 3) and pre-eclampsia placentas (n = 3; 30 ± 1, 34 ± 6 and 39 ± 1 gestation) were fixed in 10% formalin and processed into paraffin blocks. Serial sections (5 μm) were cut, placed onto coated slides and dried. Slides were deparaffinized in Histo-Clear (National Diagnostics, Merck, Eurolab S.A., Fontenay-sous-bois, France), rehydrated in a graded alcohol series, and sodium citrate antigen retrieval performed. Sections were pre-blocked with 10% fetal calf serum/phosphate-buffered saline (FCS/PBS) for 1 h at room temperature. Endogenous peroxidase was quenched with 3% hydrogen peroxide. The sections were incubated overnight at 4°C with negative control mouse immunoglobulin (Ig)G antibody (Dako) or negative control polyclonal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti-IL-18BP (EP1088Y), murine anti-IL-37 (ab57187) (Abcam); rabbit anti-IL-1RAcP (polyclonal) (Sino Biological Inc.); and rabbit anti-IL-36RN (IL-36Ra) (polyclonal) (Sigma Aldrich). Bound antibodies were detected using a diaminobenzidine (DAB) peroxidase staining kit staining (Vector Laboratories, Burlingame, CA, USA), following the manufacturer's instructions. The sections were finally counterstained with haematoxylin.
Placental perfusion model and syncytiotrophoblast extracellular vesicle preparation
To determine if factors were released from the villous surface of the placenta into the maternal circulation a modified dual placental perfusion system was used, as described previously [41]. Placentas were obtained from normal pregnant, healthy women (n = 8) or women with pre-eclampsia (n = 8). Pre-eclampsia cases ranged from 30 to 39 weeks in gestation and all normal pregnant placentas were delivered at term between 38 and 40 weeks gestation. The maternal side (syncytiotrophoblast) perfusates were centrifuged to remove cellular debris in a Beckman J6-M centrifuge at 600 g for 10 min at 4°C, and aliquots of the supernatants frozen at −80°C until analysed. Syncytiotrophoblast EVs were prepared by ultracentrifugation of the perfusate at 150 000 g for 1 h at 4°C in a Beckman L8-80M ultracentrifuge. Analytes in supernatants were measured by ELISA, as described below. Those in syncytiotrophoblast EVs were analysed by Western blotting; briefly, 10 μg EV protein lysate was loaded per well and Western blotting performed as described above for trophoblast lysates.
Treatment of BeWo cells by hypoxia or proinflammatory stimuli
BeWo cells were maintained in full growth medium [Dulbecco's modified Eagle's medium/Ham's F12 supplemented with 2 mM L-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin (Sigma Aldrich) and 10% (v/v) FCS (Sera Laboratories International, Haywards Heath, UK)]. Cells were grown as a monolayer at a density of 107 cells per 75 mm2 flask at 37°C in 95% air and 5% CO2. For passages, cells were detached with trypsin/EDTA (Life Technologies, Paisley, UK) at 37°C.
BeWo cells were plated at 7·5 × 104/ml in 24-well plates and allowed to adhere for 24 h before being transferred to a 1% O2 incubator for hypoxia treatment or cells were cultured at 20% O2 for normoxia treatment. After 24 h, 10 ng/ml IL-1β (Peprotech, Rocky Hill, NJ, USA) or 20 ng/ml TNF-α (R&D Systems) were added and cells cultured at each oxygen concentration for a further 20 h before supernatants were removed and cells were lysed. SDS-PAGE and Western blot analysis were performed for IL-37, IL-18BP and β-actin, as described previously. Densitometry was performed and IL-37 and IL-18BP levels were normalized to β-actin. This experiment was performed on four separate occasions.
Statistical analysis
Protein expression levels in normal versus pre-eclampsia placenta lysates or syncytiotrophoblast EVs were compared using a Mann–Whitney U-test. Differences in circulating levels of analytes between non-pregnant versus normal pregnant and normal pregnant versus pre-eclamptic were compared using Wilcoxon's signed-rank test. BeWo cell treatments were compared to normoxia condition control cells using an unpaired Student's t-test.
Results
Maternal plasma levels of sIL-1RAcP, IL-37, IL-18BP and IL-36Ra in normal pregnancy and pre-eclampsia
We first sought to determine whether sIL-1RAcP, IL-37, IL-18BP and IL-36Ra were detectable in the plasma of pregnant women and whether the levels were changed in women diagnosed with pre-eclampsia (Fig. 2). Controls were plasmas from women with normal pregnancies at the same gestation as the pre-eclamptic women (also matched for maternal age and parity, which may affect circulating cytokine levels), or from non-pregnant women with matched maternal age and parity; patient details are shown in Table 1 (n = 13 in each group). sIL-1RAcP was detectable in all plasma samples, and was significantly higher in pre-eclamptic women compared to normal pregnancy (P < 0·05). IL-37 was found in all three groups, but there were no significant differences between them. IL-18BP and IL-36Ra were also detectable in plasma samples from all women, and levels were higher in normal pregnancy compared to non-pregnant controls (P < 0·001, P < 0·05, respectively); both had lower mean values in pre-eclampsia but this was not significant.
Fig. 2.
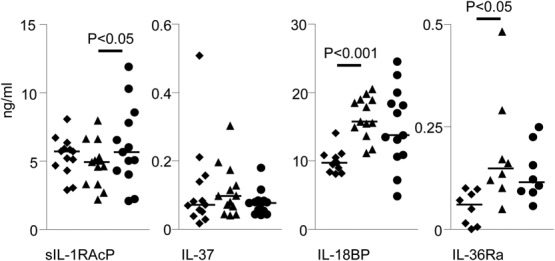
Detection of IL-1 family members in the circulation of non-pregnant, normal pregnant and pre-eclamptic women (n = 13). Plasma samples were taken from pre-eclamptic women (•) at the time of diagnosis and controls acquired from matched normal pregnant women (▲) and non-pregnant women (♦). ELISAs were performed for sIL-1RAcP, IL-37, IL-18BP and IL-36Ra. Bars = mean values.
Normal and pre-eclampsia placenta expression of IL-1 family members and regulatory factors
We then investigated whether or not the placenta could be the source of these factors by Western blotting. Lysates of normal (n = 7) and pre-eclampsia placentas (n = 7) were analysed for expression of sIL-1RAcP, IL-37, IL-18BP, IL-36Ra and IL-38 (an antibody for Western blotting was available) and β-actin (Fig. 3a). Normal placental samples were from elective caesarean sections with gestational ages between 38 ± 4 and 39 ± 2 (weeks ± days); pre-eclampsia placentas were from women undergoing emergency caesarean section, without labour, ranging from 27 ± 0 to 39 ± 0 weeks, and the results displayed in ascending order of gestational age. All factors were detected in the placenta. For the pre-eclampsia placentas protein expression did not depend upon gestational age. Protein levels were normalized by comparison to β-actin and densitometry performed (Fig. 3b). Levels of IL-37 and IL-18BP were increased significantly in pre-eclampsia placentas compared to normal pregnancies. IL-37 levels changed by more than any other factor; pre-eclampsia placentas expressed on average five times greater levels than normal placentas.
Fig. 3.
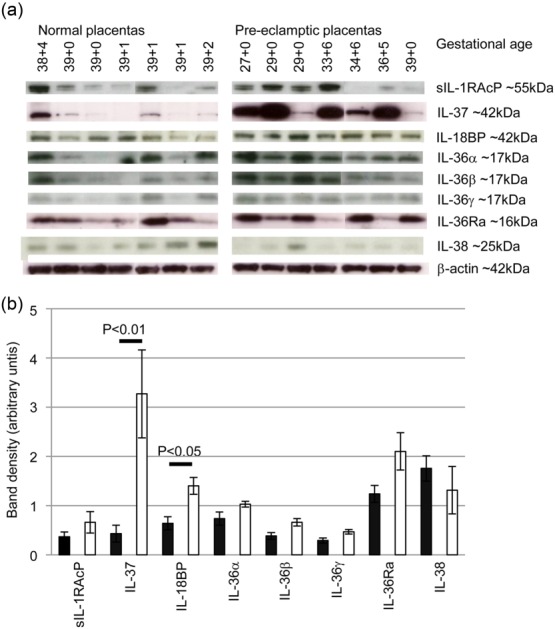
Normal and pre-eclampsia placenta expression of IL-1 family members. (a) Lysates of placenta from term normal pregnancies (n = 7) or pre-eclamptic pregnancies (n = 7) from varying gestations were analysed by Western blotting for expression of IL-1 family cytokines and IL-1 regulatory proteins. (b) Densitometry analysis of protein expression normalised to β-actin comparing levels of protein expression between normal (black bars) and pre-eclampsia placentas (white bars). Bars = mean ± standard error of the mean.
Localization of IL-1 family members and regulatory factors within normal and pre-eclampsia placentas
Sections of placenta from normal (n = 3) or pre-eclamptic (n = 3) women were stained to identify protein localization; representative images are shown in Fig. 4. Staining was positive for IL-1RAcP, IL-37, IL-18BP and IL-36Ra. An antibody validated for immunohistochemistry towards IL-38 was not available, and the antibody that was used for Western blotting did not give positive results. IL-1RAcP staining was found primarily in the syncytiotrophoblast (N.B.: antibodies may be detecting either the receptor IL-1RAcP or soluble IL-1RAcP). IL-37 staining varied between placentas, reflecting the Western blotting results. Some had low levels of expression (see normal placenta), whereas some placentas displayed much higher levels of protein (for example, see pre-eclampsia placenta) and expression was noted in all placental cell types and included strong labelling of the cell nucleus. IL-18BP was distributed patchily within the syncytiotrophoblast. Finally, IL-36Ra expression was low/negligible within trophoblast cells; the strongest expression was perivascular surrounding fetal blood vessels. There were no notable differences in expression between pre-eclamptic and normal placentas.
Fig. 4.
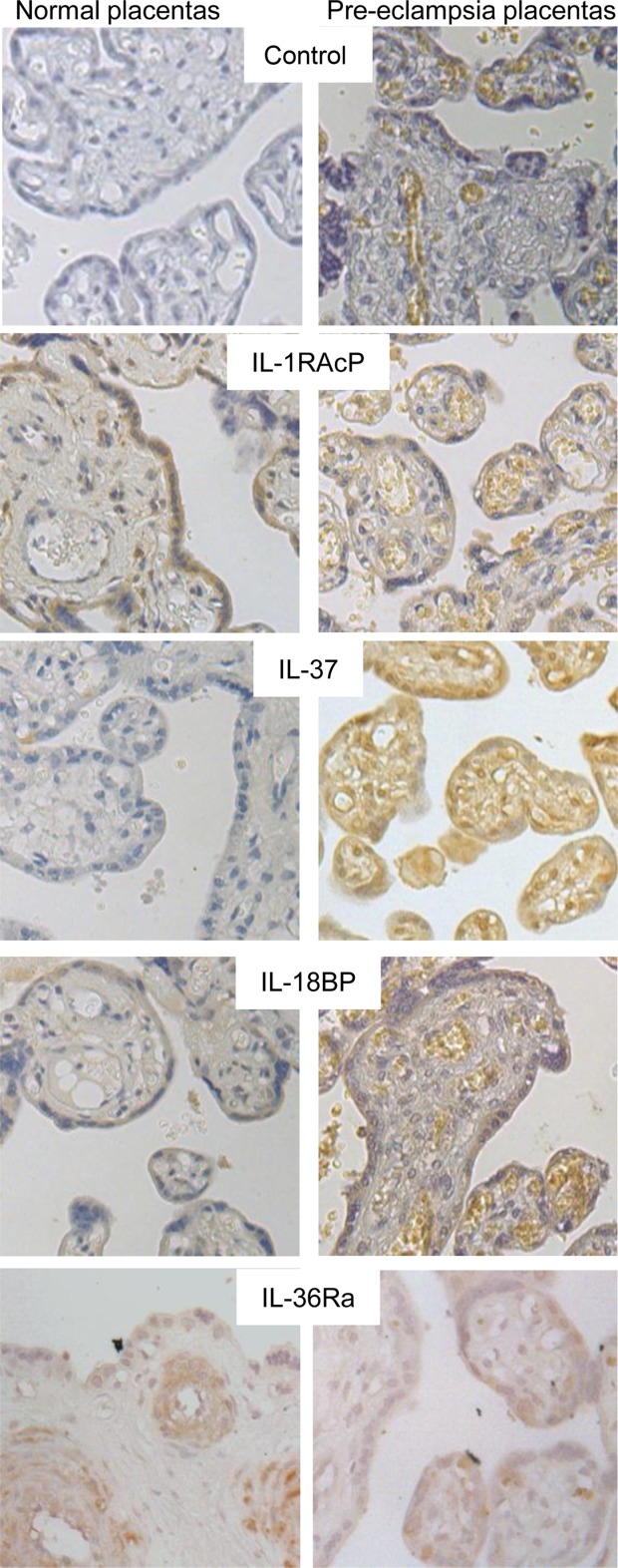
Localization of IL-1 family members in normal and pre-eclampsia placentas. Representative immunohistochemistry from normal and pre-eclampsia placental sections with antibody staining for IL-1RAcP, IL-37, IL-18BP and IL-36Ra, or control staining with an isotype control antibody [example shown for rabbit IgG control for normal placenta and for mouse IgG for pre-eclampsia placenta]; ×40 magnification.
Release of IL-1 family members and regulatory factors by the placenta
Having found that several factors (IL-1RAcP, IL-37 and IL-18BP) are expressed in the placenta, we sought to determine whether they might be secreted or shed from the placenta into the maternal blood. To model this, we used a dual-placental perfusion system to study the release of these factors from the syncytiotrophoblast placental surface. After delivery by caesarean section without labour, normal (n = 8) and pre-eclampsia placentas (n = 8) were perfused as described above. We analysed the maternal perfusate for sIL-1RAcP, IL-37, IL-18BP and IL-36Ra by ELISA (Fig. 5a). sIL-1RAcP and IL-18BP were secreted consistently by all the placentas. IL-37 was secreted by only a minority of placentas, and IL-36Ra was not detected in any maternal perfusates. Statistical tests were not carried out as the size of the placental lobe perfused in each preparation is not comparable, so that the area of syncytiotrophoblast perfused cannot be determined.
Fig. 5.
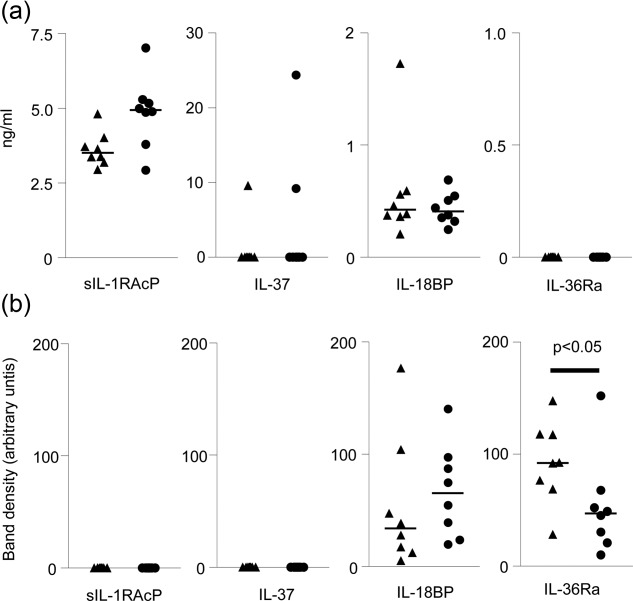
Release of IL-1 family members by normal and pre-eclampsia placentas. (a) Supernatants were collected from the maternal side of a dual placental perfusion from normal (▲) and pre-eclampsia (•) placentas (n = 8). ELISAs were performed for sIL-1RAcP, IL-37, IL-18BP and IL-36Ra. Bars = mean values. (b) Syncytiotrophoblast EVs were prepared from the maternal side perfusion media by ultracentrifugation; 10 μg EV protein lysate was loaded per lane, and SDS-PAGE and Western blotting performed. IL-18BP and IL-36Ra were detected and densitometry performed to determine relative analyte concentration between normal and pre-eclampsia syncytiotrophoblast EVs. Bars = mean values.
To determine whether or not IL-1 family members and associated regulatory factors were associated with syncytiotrophoblast EVs, they were isolated by ultracentrifugation from the maternal perfusate. EVs from normal (n = 8) and pre-eclamptic (n = 8) placentas were studied by Western blotting (Fig. 5b). Of the four analytes described in Fig. 2, only IL-36Ra and IL-18BP were contained within the EVs; IL-36Ra was expressed at lower levels (per μg EV protein) in EVs from pre-eclampsia placentas compared to normal placenta EVs (P < 0·05). No correlation between gestational age of the syncytiotrophoblast EV preparation and expression level of the proteins were seen (data not shown).
IL-37 expression is induced by hypoxia
IL-37 and IL-18BP expression was increased in pre-eclampsia placentas compared to those from normal pregnancies (Fig. 3). In an attempt to model the factors which induced their expression in vitro we first screened five trophoblast cell lines (JEG-3, BeWo, SGHPL-5, AC1M59, JAR), using human umbilical vein endothelial cells (HUVEC) and monocyte (U937) cells as controls, and found that JEG-3, JAR, BeWo and AC1M59 cells express both these molecules (Supporting information, Fig. S 1). Major stresses on the pre-eclampsia placenta are hypoxia, oxidative and inflammatory stress; therefore, we treated BeWo cells, which are used extensively to model trophoblast function with IL-1β, TNF-α and hypoxic conditions to model this aspect of the disease in vitro (Fig. 6). Cells cultured in 1% oxygen showed a twofold higher expression of IL-37 than cells cultured in 20% oxygen, P < 0·05 (n = 4). Treatment with IL-1β or TNF-α did not increase IL-37 expression significantly (Fig. 6a). Intracellular IL-18BP levels (Fig. 6b) did not change in response to hypoxic conditions or treatment with IL-1β or TNF-α. A representative Western blot is shown in Fig. 6c.
Fig. 6.
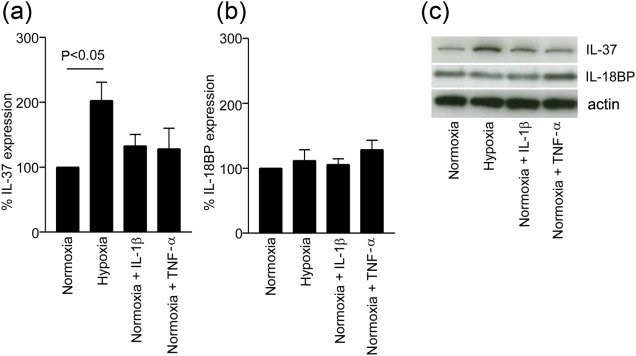
Hypoxic conditions stimulate intracellular trophoblast cell IL-37 expression. BeWo cells were cultured under normoxic (20% O2) conditions with or without additional IL-1β or TNF-α, or under hypoxic (1% O2) conditions for 24 h. Cell lysates were analysed for IL-37 (a) and IL-18BP (b) expression by SDS-PAGE followed by Western blotting, and densitometry of protein bands analysed (n = 4). Bars = mean ± standard error of the mean. (c) representative Western blot for IL-37, IL-18BP and actin.
Discussion
IL-18BP and IL-36Ra are increased significantly in plasma from normal pregnant women compared to the non-pregnant state, and while sIL-1RAcP is not increased in normal pregnancy, pre-eclamptic women have higher circulating levels. It is tempting to speculate that the placenta contributes directly to some, if not all, the increased plasma levels by secreting these factors into the maternal circulation. We used a range of techniques to characterize placental expression and release of the IL-1 family cytokines and associated molecules. Six newly described members of the IL-1 cytokine family (IL-36Ra, IL-36α, IL-37, IL-36β, IL-36γ and IL-38) and the regulatory factors IL-18BP and sIL-1RAcP were detected by immunoblotting. Placental expression of IL-1α, IL-1β, IL-1Ra and IL-18 is well documented [10,23,31], and we and others have recently demonstrated placental IL-33 expression [25,26]. We also screened five trophoblast cell lines (JEG-3, BeWo, SGHPL-5, AC1M59, JAR) for each of the eight factors investigated in this paper (Supplementary Fig. S 1). Expression of all the IL-1 family cytokines or regulatory factors was found in the majority of the five trophoblast cell lines, further supporting their trophoblast origin. Therefore, all known members of the IL-1 cytokine family are expressed by placenta from normal pregnancies, highlighting the complex network of cytokine regulation in pregnancy.
In terms of the IL-1 family regulatory factors, we have previously shown placental expression of sST2 which inhibits IL-33 [25]. Here we show that other IL-1 cytokine family inhibitory molecules – sIL-1RAcP, IL-36Ra, IL-38 and IL-18BP – are also expressed by placentas. We compared total analyte expression between normal term placentas and pre-eclampsia placentas, and found significantly more IL-37 and IL-18BP in pre-eclampsia placentas. However, an important limitation of interpreting this finding is that pre-term pre-eclampsia placentas cannot be matched for gestational age with normal placentas.
Several of the factors studied are expressed by the syncytiotrophoblast layer (Fig. 4), as has been shown previously for IL-1β, IL-1Ra and sST2 [23,25]. Syncytiotrophoblast factors may be secreted or shed directly into the maternal blood in vivo as either soluble factors or associated with EVs [40]. Using an ex-vivo perfusion system we studied these factors derived from both normal and pre-eclampsia placentas. Using ELISA, we could detect consistent release of sIL-1RAcP and IL-18BP, and three of 16 placentas released IL-37 (Fig. 5a). None released detectable IL-36Ra despite expression in the syncytiotrophoblast (Fig. 4), although others have shown that JEG-3 trophoblast cells constitutively secrete IL-36Ra [42]. IL-36Ra was, however, present within syncytiotrophoblast EVs – possibly the quantity directly released into the perfusate was beneath the detection limit of the ELISA used here. IL-18BP was also expressed by EVs but not sIL1-RAcP or IL-37. ELISAs for IL-36α, IL-36β, IL-36γ and IL-38 were not available, so we were unable to determine if they are secreted as soluble factors but, as discussed in the Results section, they are not present in EVs (data not shown). As discussed above, it is impossible to compare secretion of factors between placentas in the perfusion model because the placentas were from varying gestational ages and the size of the placenta lobe perfused was variable; therefore, we could not determine which factors were secreted more or less readily by pre-eclampsia compared to normal placentas.
IL-18BP was released from placentas as both a soluble and EV-associated factor. Pre-eclampsia placentas contained significantly more IL-18BP than normal placentas, but this was not reflected in plasma concentrations, which were increased in normal but not in pre-eclamptic women compared to non-pregnant women. IL-18 triggers IL-18R signalling and production of type 1 cytokines, therefore the increased levels of IL-18BP in the plasma in normal pregnancy are likely to help to prevent excessive inflammation.
Similarly, IL-36Ra is elevated in normal pregnancy plasma, which would inhibit type 1 inflammation and recruitment of immune cells. Several IL-36 proteins were first identified through screening for homologous genes using placental tissues [43,44]. IL-36R and its ligands are also highly expressed in the skin [45], epithelia exposed to pathogens (lung, oesophagus), the brain, gut and kidney [34,46–48]. IL-36γ triggers inflammation in Jurkat cells transfected to express IL-36R, a process that can be blocked by IL-36Ra [49], and IL-36 acts with IL-12 to induce Th1 cell polarization [50] and regulates dendritic cell activation [51]. Loss of function mutations in IL-36Ra are associated with a severe episodic inflammatory skin disease, generalized pustular psoriasis which may present in pregnancy, often associated with placental insufficiency [52,53].
Circulating antagonists IL-18BP and IL-36Ra were increased significantly in late normal pregnancy relative to non-pregnancy but only plasma sIL-1RAcP was different (higher: P < 0·05) in pre-eclamptic women, compared to normal pregnancy controls. This factor combines with IL-1Ra and sST2 (two other factors that are known to be increased in the circulation of pre-eclamptic women), to directly inhibit the proinflammatory effects of IL-1α/β or IL-33-driven type 2 cytokine production, respectively. The latter observation fits with the hypothesis that maternal systemic inflammation in pre-eclampsia is skewed towards excessive type 1 cytokine production over type 2 cytokines. As sIL1-RAcP also combines with IL-1Ra to enhance IL-1α/β-driven inhibition this will further curtail inflammation, therefore the increased levels found in pre-eclampsia are at first appearance counterintuitive. However, these factors are produced in response to inflammatory stimuli, and as pre-eclamptic women have exacerbated inflammation, the increased levels of these factors may reflect that. Functionally it is likely that they are dampening inflammation without them inflammation could be greater and more damaging.
Plasma IL-37 levels are unchanged by pregnancy, but this is perhaps not surprising given that we cannot detect consistent secretion of this cytokine. Many tissues have been shown to express IL-37, including testis, thymus and uterus [35], inflamed epithelium [54], keratinocytes [44], tonsils (mainly B cells), breast and colon cells and the placenta, although reverse transcription–polymerase chain reaction (RT–PCR) suggested low placental expression [34,55]. Raised IL-37 serum levels are associated with septic shock [18,33] and liver damage in patients with chronic hepatitis B infection [56]. IL-37 has a protective role in a murine model of concanavalin A-induced hepatitis [57]. Along with the proposed extracellular functions of IL-37, IL-37 also functions as an intracellular factor. Within macrophage-like cells IL-37 translocates to the nucleus upon lipopolysaccharide (LPS) stimulation, after its precursor has been cleaved by caspase-1 [37]. Over-expression of IL-37 reduces responses to proinflammatory stimuli, and blockade of IL-37 expression in peripheral blood mononuclear cells (PBMC) results in higher inflammation in response to LPS [33]. IL-37 is therefore thought to limit inflammation through a feedback loop. Here we have found that IL-37 is up-regulated in trophoblast cell lines by hypoxic conditions.
The IL-1 family is linked closely with ischaemic–reperfusion injury in other organ systems [58], as IL-1α/IL-1β knock-out animals display reduced ischaemic-induced inflammation. IL-37 limits damage in an in-vivo ischaemia–reperfusion-induced hepatitis [59] and myocardial injury [60]. Therefore, some of the increased IL-37 found within pre-eclampsia placentas may be due to hypoxia. It is important to remember, however, that as pre-eclampsia is a syndrome caused by a multitude of factors it is difficult to model this in vitro, and we do not claim that this simple model of hypoxic insult on BeWo trophoblast cells is representative of the condition. IL-37 needs to be investigated using a more complex model of pre-eclampsia in further studies, which are beyond the scope of this paper.
We have found that it is not the proinflammatory IL-1 family cytokines that are up-regulated in pre-eclampsia rather it is the regulatory receptors or anti-inflammatory factors that are expressed at higher levels in the placenta. This was surprising as it is often presumed that the placenta is proinflammatory in pre-eclampsia, but here we only detect up-regulation of two anti-inflammatory factors. This observation has been seen with other members of this superfamily, while there is an abundance of contradictory data regarding the levels of IL-1β in pre-eclampsia (reviewed in [23]), IL-1Ra is elevated [20–22]; similarly, circulating levels of the anti-inflammatory sST2 are highly elevated in pre-eclampsia while levels of its proinflammatory ligand are not [25]. It appears that placental mechanisms are down-regulating excessive systemic inflammation in pre-eclampsia. It is not clear whether the placenta regulation fails or if without these placenta-derived anti-inflammatory factors the pre-eclampsia would be more severe.
Acknowledgments
We would like to thank our research midwives, Carol Simms, Nicola Higgins, Hazel Meacher, Linda Holden, Ali Chevassut and Tessa Finlayson for recruiting patients and collection of samples for this study. We would also like to thank Dionne Tannetta for the help with placental experiments and for providing placental perfusion samples. Great thanks go to Eleni Fotaki for performing western blotting on EVs.
Author contributions
This work was supported by the Wellbeing of Women (grant number RG1326). J. H. S. performed the experiments; J. H. S. and I. G. designed the study; J. H. S., C. W. G. R., I. L. S. and I. G. wrote the paper.
Disclosure
The authors have no conflicts of interests.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's Web site:
Fig. S1. Trophoblast cell lines express interleukin (IL)-1 family cytokines. Cell lysates from JEG-3, BeWo, SGHPL-5, AC1M59 and JAR cells were subjected to SDS-PAGE and Western blotting to detect sIL-1RAcP, IL-37, IL-18BP, IL-36α, IL-36β, IL-36γ, IL-36Ra, IL-38 and β-actin.
References
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Redman CW. Preeclampsia: a multi-stress disorder. Rev Med Interne. 2011;32((Suppl 1):S41–4. doi: 10.1016/j.revmed.2011.03.331. [DOI] [PubMed] [Google Scholar]
- 4.Hwang I. Cell–cell communication via extracellular membrane vesicles and its role in the immune response. Mol Cell. 2013;36:105–11. doi: 10.1007/s10059-013-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 6.Santner-Nanan B, Peek MJ, Khanam R, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–30. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 7.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–82. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 9.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 10.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–12. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 11.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 12.Kocyigit Y, Atamer Y, Atamer A, Tuzcu A, Akkus Z. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol. 2004;19:267–73. doi: 10.1080/09513590400018108. [DOI] [PubMed] [Google Scholar]
- 13.Kalinderis M, Papanikolaou A, Kalinderi K, et al. Elevated serum levels of interleukin-6, interleukin-1beta and human chorionic gonadotropin in pre-eclampsia. Am J Reprod Immunol. 2011;66:468–75. doi: 10.1111/j.1600-0897.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 15.Polentarutti N, Rol GP, Muzio M, et al. Unique pattern of expression and inhibition of IL-1 signaling by the IL-1 receptor family member TIR8/SIGIRR. Eur Cytokine Netw. 2003;14:211–8. [PubMed] [Google Scholar]
- 16.Wald D, Qin J, Zhao Z, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 18.Boraschi D, Lucchesi D, Hainzl S, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22:127–47. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- 19.Smith DE, Hanna R, Della F, et al. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity. 2003;18:87–96. doi: 10.1016/s1074-7613(02)00514-9. [DOI] [PubMed] [Google Scholar]
- 20.Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84:937–40. [PubMed] [Google Scholar]
- 21.Kimya Y, Akdis C, Cengiz C, et al. Plasma interleukin-1alpha, interleukin-1beta and interleukin-1 receptor antagonist levels in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1997;73:17–21. doi: 10.1016/s0301-2115(97)02698-5. [DOI] [PubMed] [Google Scholar]
- 22.Szarka A, Rigo J, Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amash A, Holcberg G, Sapir O, Huleihel M. Placental secretion of interleukin-1 and interleukin-1 receptor antagonist in preeclampsia: effect of magnesium sulfate. J Interferon Cytokine Res. 2012;32:432–41. doi: 10.1089/jir.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granne I, Southcombe JH, Snider JV, et al. ST2 and IL-33 in pregnancy and pre-eclampsia. PLOS ONE. 2011;6:e24463. doi: 10.1371/journal.pone.0024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fock V, Mairhofer M, Otti GR, et al. Macrophage-derived IL-33 is a critical factor for placental growth. J Immunol. 2013;191:3734–43. doi: 10.4049/jimmunol.1300490. [DOI] [PubMed] [Google Scholar]
- 27.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Palmer G, Lipsky BP, Smithgall MD, et al. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine. 2008;42:358–64. doi: 10.1016/j.cyto.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Huang H, Dong M, Yao Q, Wang H. Serum and placental interleukin-18 are elevated in preeclampsia. J Reprod Immunol. 2005;65:77–87. doi: 10.1016/j.jri.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–36. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 33.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Hanning CR, Brigham-Burke MR, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 35.Pan G, Risser P, Mao W, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13:1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 36.Bufler P, Azam T, Gamboni-Robertson F, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA. 2002;99:13723–8. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, Kulk N, Nold MF, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180:5477–82. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 38.van de Veerdonk FL, Stoeckman AK, Wu G, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci USA. 2012;109:3001–5. doi: 10.1073/pnas.1121534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–88. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- 40.Redman CW, Tannetta DS, Dragovic RA, et al. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33((Suppl):S48–54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLOS ONE. 2011;6:e20245. doi: 10.1371/journal.pone.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barton JL, Herbst R, Bosisio D, Higgins L, Nicklin MJ. A tissue specific IL-1 receptor antagonist homolog from the IL-1 cluster lacks IL-1, IL-1ra, IL-18 and IL-18 antagonist activities. Eur J Immunol. 2000;30:3299–308. doi: 10.1002/1521-4141(200011)30:11<3299::AID-IMMU3299>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, McDonnell PC, Lehr R, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem. 2000;275:10308–14. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 44.Busfield SJ, Comrack CA, Yu G, et al. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics. 2000;66:213–6. doi: 10.1006/geno.2000.6184. [DOI] [PubMed] [Google Scholar]
- 45.Mulero JJ, Pace AM, Nelken ST, et al. IL1HY1: A novel interleukin-1 receptor antagonist gene. Biochem Biophys Res Commun. 1999;263:702–6. doi: 10.1006/bbrc.1999.1440. [DOI] [PubMed] [Google Scholar]
- 46.Towne JE, Sims JE. IL-36 in psoriasis. Curr Opin Pharmacol. 2012;12:486–90. doi: 10.1016/j.coph.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Smith DE, Renshaw BR, Ketchem RR, Kubin M, Garka KE, Sims JE. Four new members expand the interleukin-1 superfamily. J Biol Chem. 2000;275:1169–75. doi: 10.1074/jbc.275.2.1169. [DOI] [PubMed] [Google Scholar]
- 48.Ichii O, Otsuka S, Sasaki N, et al. Local overexpression of interleukin-1 family, member 6 relates to the development of tubulointerstitial lesions. Lab Invest. 2010;90:459–75. doi: 10.1038/labinvest.2009.148. [DOI] [PubMed] [Google Scholar]
- 49.Debets R, Timans JC, Homey B, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–6. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- 50.Vigne S, Palmer G, Martin P, et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120:3478–87. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- 51.Vigne S, Palmer G, Lamacchia C, et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118:5813–23. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 52.Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432–7. doi: 10.1016/j.ajhg.2011.07.022. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–8. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 54.Imaeda H, Takahashi K, Fujimoto T, et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172:410–6. doi: 10.1111/cei.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor SL, Renshaw BR, Garka KE, Smith DE, Sims JE. Genomic organization of the interleukin-1 locus. Genomics. 2002;79:726–33. doi: 10.1006/geno.2002.6752. [DOI] [PubMed] [Google Scholar]
- 56.Li C, Ji H, Cai Y, et al. Serum interleukin-37 concentrations and HBeAg seroconversion in chronic HBV patients during telbivudine treatment. J Interferon Cytokine Res. 2013;33:612–8. doi: 10.1089/jir.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bulau AM, Fink M, Maucksch C, et al. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. ScientificWorldJournal. 2011;11:2480–90. doi: 10.1100/2011/968479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wanderer AA. Ischemic–reperfusion syndromes: biochemical and immunologic rationale for IL-1 targeted therapy. Clin Immunol. 2008;128:127–32. doi: 10.1016/j.clim.2008.03.514. [DOI] [PubMed] [Google Scholar]
- 59.Sakai N, Van Sweringen HL, Belizaire RM, et al. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27:1609–16. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu B, Meng K, Ji Q, et al. Interleukin-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin Exp Immunol. 2014;176:438–51. doi: 10.1111/cei.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Trophoblast cell lines express interleukin (IL)-1 family cytokines. Cell lysates from JEG-3, BeWo, SGHPL-5, AC1M59 and JAR cells were subjected to SDS-PAGE and Western blotting to detect sIL-1RAcP, IL-37, IL-18BP, IL-36α, IL-36β, IL-36γ, IL-36Ra, IL-38 and β-actin.


