Abstract
In this study we examined the effects of non-myeloablative total body irradiation (TBI) in combination with immunosuppressive chemotherapy on immune homeostasis in rhesus macaques. Our results show that the administration of cyclosporin A or tacrolimus without radiotherapy did not result in lymphopenia. The addition of TBI to the regimen resulted in lymphopenia as well as alterations in the memory/naive ratio following reconstitution of lymphocyte populations. Dendritic cell (DC) numbers in whole blood were largely unaffected, while the monocyte population was altered by immunosuppressive treatment. Irradiation also resulted in increased levels of circulating cytokines and chemokines that correlated with T cell proliferative bursts and with the shift towards memory T cells. We also report that anti-thymocyte globulin (ATG) treatment and CD3 immunotoxin administration resulted in a selective and rapid depletion of naive CD4 and CD8 T cells and increased frequency of memory T cells. We also examined the impact of these treatments on reactivation of latent simian varicella virus (SVV) infection as a model of varicella zoster virus (VZV) infection of humans. None of the treatments resulted in overt SVV reactivation; however, select animals had transient increases in SVV-specific T cell responses following immunosuppression, suggestive of subclinical reactivation. Overall, we provide detailed observations into immune modulation by TBI and chemotherapeutic agents in rhesus macaques, an important research model of human disease.
Keywords: anti-thymocyte globulin, CD3 immunotoxin, immune system, irradiation, Janus activated kinase inhibitor simian varicella virus, prednisone, rhesus macaque, tacrolimus
Introduction
The number of individuals undergoing irradiation and immunosuppression for the treatment of cancer and following organ transplant has increased during the past few decades. However, our understanding of disturbances in immune homeostasis and reconstitution following immunosuppressive treatments in the absence of confounding factors associated with neoplasms and non-syngeneic grafts remains incomplete. Here we address the impact of ionizing radiation and immunosuppressive drug treatment used routinely in the clinical setting on the frequency and phenotype of immune cell subsets, proliferation of both innate and adaptive immune cells and concentration of circulating immune signalling molecules in immune competent rhesus macaques (RM). Immunosuppressed individuals are more susceptible to new infections as well as reactivation of latent viral infections. Therefore, we also monitored reactivation of latent simian varicella virus (SVV) infection in rhesus macaques following ionizing radiation and immunosuppressive drug treatment.
In this study, three cohorts (n = 4/cohort) of RM were treated with non-bone marrow ablative ionizing radiation (2 or 4 Gy), which causes apoptosis in radiation-sensitive tissues, including lymphocytes (reviewed in [1]). Low-dose total body irradiation (TBI) from 2–8 Gy is used in a variety of clinical situations [2]; for example, in conjunction with chemotherapy to treat transplant patients, who cannot tolerate myeloablation due to age, performance status or co-morbidities [3–7].
In addition to TBI, the animals received different combinations of immunosuppressive drugs used commonly in transplant recipients (Fig. 1). Specifically, animals in cohort 1 were first pretreated for several weeks prior to TBI with the calcineurin inhibitors (CNI), cyclosporin A (CsA) or tacrolimus (FK506), which interfere with signal 2 of T cell activation, resulting in the inhibition of cytokine gene expression important for T cell activation, proliferation and survival, notably interleukin (IL)-2 [8–11]. In addition, cohort 1 was also treated with prednisone, a corticosteroid that primarily suppresses T cell activation by inhibiting production of cytokines such as IL-2 and interferon (IFN)-γ [12].
Fig. 1.
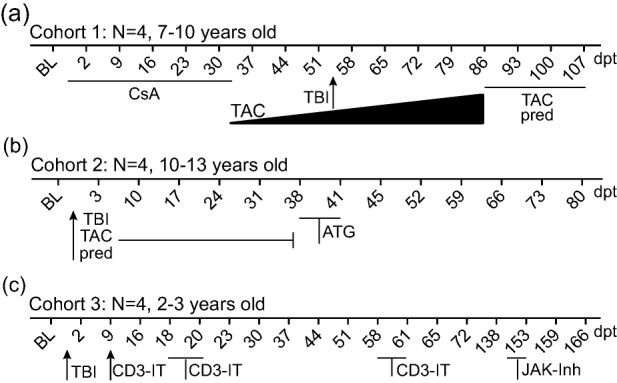
Treatment time-line. (a) Cohort 1 received 25 mg/kg/day cyclosporin A (CsA) at day 0. CsA ceased 32 days post-treatment (dpt) and replaced with 0·1-mg/kg/day tacrolimus (TAC). At 55 dpt animals were treated with a single dose of 2-Gy ionizing irradiation (TBI). TAC was increased to 0·3-mg/kg/day 59 dpt and again to 1 mg/kg/day 68 dpt. Prednisone (pred) at 1 mg/kg/day was added at 86 dpt. (b) Cohort 2 received at day 0 a single dose of 2 Gy TBI and 3 mg/kg/day tacrolimus and 1 mg/kg/day prednisone from 0 to 37 dpt. At 38 dpt animals received 1·5 mg/kg anti-thymocyte globulin (ATG) for 4 days. (c) Cohort 3 received a single dose of 4 Gy TBI at day 0. Recombinant anti-CD3 immunotoxin (CD3-IT) was given at 50 μg/kg on 9 and 18–21 dpt and a second dose from 58–61 dpt. Janus activated kinase (JAK) inhibitor (JAK-Inh) was given 15 mg/kg/day from 149–153 dpt. BL = baseline.
Animals in cohort 2 first underwent TBI and then received maintenance immunosuppression with tacrolimus and prednisone. Later in the study, cohort 2 also received a 4-day course of anti-thymocyte globulin (ATG), which is the purified immunoglobulin (Ig)G fraction of rabbits or horses that are immunized with human thymocytes or T cell lines. ATG depletes peripheral lymphocytes through complement-dependent lysis or activation-associated apoptosis [13–15]. ATG formulations have been used in human transplantation for decades [16]. Furthermore, in non-human primate (NHPs) ATG was also found to induce dose-dependent T cell depletion in the spleen and lymph nodes [17].
Lastly, cohort 3 first underwent TBI then received anti-CD3 immunotoxin (CD3-IT) and a Janus activated kinase (JAK) inhibitor, both of which are newer therapies aimed at depleting T cells or inhibiting lymphocyte activation without the adverse effects sometimes associated with CNIs and other currently available drugs (reviewed in [18]). CD3-IT is a recombinant fusion protein consisting of a truncated diphtheria toxin fused to affinity matured anti-CD3 antibody FN18, which is able to deplete T cells [19]. Anti-CD3 immunotoxins have been evaluated in clinical settings for T cell lymphoma [20,21] and in numerous transplant models [22–25]. JAK inhibitor (tofacitinib citrate, CP-690550 citrate) inhibits mainly JAK3, but also JAK1, JAK2 and, to a lesser degree, tyrosine kinase 2 (TYK2), resulting in the inhibition of cytokine signalling and functionally interfering with T helper type 1 (Th1) and Th2 differentiation as well as suppressing the generation of Th17 cells [26–28]. JAK inhibitor has been investigated in a NHP kidney transplant model [29,30] and evaluated further in human kidney transplant settings [31,32]. Furthermore, JAK inhibitors are also being developed for treatment of rheumatoid arthritis [33,34], psoriasis [35] and colitis [36].
In order to assess the impact of irradiation and immunosuppressive regimens on latent viral infection, RMs were infected with simian varicella virus (SVV) prior to treatment. Intrabronchial infection of rhesus macaques with SVV, a homologue of varicella zoster virus (VZV), recapitulates the hallmarks of VZV infection in humans [37]. Reactivation of VZV is a frequent complication of organ transplantation, and multiple studies suggest an association between the intensity of immunosuppression and the frequency/severity of herpes zoster episodes [38–42]. Indeed, the incidence of herpes zoster is higher in heart or lung transplant recipients compared to liver or renal transplant recipients [43]. In that study, renal transplant recipients received CsA or tacrolimus as well as azathioprine (AZA) or mycophenolate mofetil (MMF); liver transplant recipients received similar treatment to renal transplant patients, with the addition of solumedrol and prednisone; finally, heart and lung transplant patients received ATG, AZA or MMF, CsA or tacrolimus and prednisone. Recently, a greater rate of herpes zoster was also observed in patients treated with an oral JAK inhibitor (tofacitnib) for the treatment of rheumatoid arthritis [44]. Therefore, in this study we monitored SVV reactivation in latently infected RM undergoing immunosuppression using clinically relevant therapies.
Materials and methods
Animals and sample collection
All RMs were housed at the Oregon National Primate Research Center (ONPRC) and were handled in accordance with good animal practices as defined by the Office of Laboratory Animal Welfare. The study was approved by the ONPRC Institutional Animal Care and Use Committee. RMs (Macaca mulatta) were infected intrabronchially with 4 × 105 plaque-forming units (PFU) of wild-type simian varicella virus (SVV) in the form of intact infected telomerized rhesus fibroblasts. Peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage (BAL) cells were collected from RMs as described previously [37].
Immunosuppressive treatment
Cohort 1
Four female RMs 7–10 years old received 25 mg/kg CsA (Sandimmune®; Novartis, East Hanover, NJ, USA). This dose is the same as that used in a NHP model of xenograft transplant [45]. A dose range of 6–14 mg/kg/day is given to kidney transplant patients to prevent rejection and achieve therapeutic levels of 150–250 ng/ml [46,47]. Tacrolimus (AmerisourceBergen, Chesterbrook, PA, USA) treatment started at 0·1 mg/kg, increased to 0·3 mg/kg and increased again to 1 mg/kg. A previous study showed that RMs receiving 1 and 3 mg/kg/day of tacrolimus for 3 months did not result in renal lesions or haematological, serum biochemical or urinalysis changes [48]. In a clinical setting, tacrolimus was given at 1 mg daily and then adjusted to achieve a therapeutic level of 5–15 ng/ml in a bone marrow transplant setting [49] and 0·2 mg/kg/day to attain whole-blood trough levels of 10–15 ng/ml in a renal transplant setting [46]. Prednisone (Henry Schein, Carlsbad, CA, USA) was administered at 1 mg/kg daily in line with a previous NHP transplant study [45]. TBI was delivered as a single dose of 2 Gy ionizing radiation. All TBI treatments consisted of 6 MeV photons, with the dose split equally between anterior and posterior. A polystyrene spoiler was used to ensure adequate superficial dose. An irradiation dose of 2–4 Gy is within a range used in non-ablative allogeneic transplantation protocols [50–53].
Cohort 2
Four female RMs 10–13 years old received a single dose of 2 Gy TBI, 3 mg/kg tacrolimus, 1 mg/kg prednisone and 1·5 mg/kg anti-thymocyte globulin intravenously (thymoglobulin rabbit ATG; Genzyme, Cambridge, MA, USA). Preville et al. calculated that a dose of 1 mg/kg in cynomolgus macaques was sufficient to induce borderline immunosuppressive effects, while a dose of 5 mg/kg reflects ATG used in organ transplantation [17,54,55].
Cohort 3
Three female and one male RMs 2–3 years old received a single dose of 4 Gy TBI. Anti-monkey CD3 recombinant immunotoxin (CD3-IT) at 50 μg/kg was administered intravenously by slow bolus injection (NHP resource, material produced by the Massachusetts General Hospital–Dana Farber–Harvard Cancer Center Recombinant Protein Expression and Purification Core Facility) [19,23,24]. JAK inhibitor (tofacitinib citrate, CP-690550 citrate; Selleckchem, Houston, TX, USA) at 15 mg/kg diluted in 0·5% methylcellulose was given for 5 consecutive days by oral gavage [29]. Each cohort consisted of four animals, which is the minimum number of animals needed to achieve sufficient power to detect a statistically significant difference in various immunological parameters following SVV infection [37,56–59]. The necropsy schedules were based on the return of white blood cell (WBC) counts to baseline levels and the cessation of lymphocyte proliferation.
Complete blood analysis
Complete blood cell counts and differentials were obtained from whole blood using a complete blood count machine (Hemavet, Drew Scientific Group, Waterbury, CT, USA). Percentages of cell subsets from flow cytometric analysis were converted to absolute numbers using the lymphocyte number per microlitre of whole blood.
DNA extraction and quantitative real-time polymerase chain reaction (qPCR)
DNA was extracted from heparinized whole blood (WB) and BAL cells using ArchivePure DNA Cell/Tissue Kit (5 Prime, Gaithersburg, MD, USA), according to the manufacturer's protocol. SVV DNA viral loads in WB and BAL cells were measured by qPCR using Maxima Probe/ROX qPCR Master Mix (×2) (Fermentas, Glen Burnie, MD, USA) and primers/Taqman probe specific for SVV ORF21 [37]. Following an initial 10-min 95°C step, 40 cycles of 15 s at 95°C and 1 min at 60°C were completed using StepOnePlus (Life Technologies, Carlsbad, CA, USA). Plasmid containing the target amplicon or SVV BAC DNA was used as quantification standard [60].
Cytokine analysis
Plasma and BAL supernatant stored at −80°C were thawed and diluted 1 : 2 in serum matrix for analysis with Cytokine Monkey Magnetic 28-Plex Panel for the Luminex platform in duplicate, as per the manufacturer's instructions (Life Technologies). Concentrations for IL-1RA, IFN-inducible T cell alpha chemoattractant (I-TAC), basic fibroblast growth factor (FGF-basic), granulocyte–colony-stimulating factor (G-CSF), IL-15, eotaxin, monokine induced by IFN-γ (MIG), IL-6, IL-10, IL-12, macrophage inflammatory protein (MIP)-1α, IL-17, IL-8, epidermal growth factor (EGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), granulocyte–macrophage colony-stimulating factor (GM-CSF), tumour necrosis factor (TNF)-α, IL-1β, IL-2, IL-4, IL-5, regulated upon activation normal T cell expressed and secreted (RANTES), MIP-1β, monocyte chemoattractant protein (MCP)-1, IFN-γ, monodansylcadaverine (MDC) and macrophage migration inhibitory factor (MIF) were measured on a Magpix instrument (Luminex, Austin, TX, USA).
Enzyme-linked immunosorbent assay (ELISA)
ELISA plates were coated with SVV lysate overnight at 4°C, blocked with 5% milk in wash buffer (0·05% Tween in PBS) for 1 h at room temperature (RT), washed three times with wash buffer and incubated with heat-inactivated (55°C, 30 min) plasma samples in threefold dilutions in duplicate for 1 h. After washing three times with wash buffer, horseradish peroxidase (HRP)-conjugated anti-rhesus IgG (Nordic Immunology, Tilburg, the Netherlands) was added for 1 h, followed by the addition of chromagen o-phenylenediamine-2HCl (OPD) (Sigma, St Louis, MO, USA) substrate for 20 min to allow detection and quantitation of bound antibody molecules. The reaction was stopped with the addition of 1 M HCl. The optical density was measured at 490 nm using an ELISA plate reader (SpectraMax 190; Molecular Devices, Sunnyvale, CA, USA). End-point IgG titres were calculated using log–log transformation of the linear portion of the curve, with 0·1 optical density (OD) units as the cut-off. Titres were standardized using a positive control sample included with each assay.
Measurement of immune cell frequency and proliferation
Peripheral blood mononuclear cells (PBMC) were surface-stained with antibodies against: (1) CD4 (eBioscience, San Diego, CA, USA), CD8β (Beckman Coulter, Brea, CA, USA), CD28 (BioLegend, San Diego, CA, USA) and CD95 (BioLegend) to delineate the naive (CD28+CD95−), central memory (CD28+CD95+) and effector memory (CD28−CD95+) T cell subsets; (2) CD20 (Beckman Coulter), IgD (Southern Biotech, Birmingham, AL, USA) and CD27 (BioLegend) to delineate naive (CD20+IgD+CD27−), marginal zone (MZ)-like (CD20+IgD+CD27+) and memory (CD20+IgD−CD27+) B cell subsets; and (3) CD3 (BD Pharmingen, San Diego, CA, USA), CD20, human leucocyte antigen D-related (HLA-DR) (BioLegend), CD14 (BioLegend), CD123 (BioLegend) and CD11c (BioLegend) to delineate monocytes (CD3−CD20−CD14+HLA-DR+) and dendritic cells (DCs, CD3−CD20−CD14−HLA-DR+). DCs were further defined into myeloid (mDC, CD123−CD11c+), plasmacytoid (pDC, CD123+CD11c−) and less differentiated (ldDC, CD123−CD11c−). Cells were fixed and permeabilized before the addition of Ki67-specific antibody (BD Biosciences, San Jose CA, USA). The samples were analysed using the LSRII instrument (Beckton Dickinson Company, San Jose, CA, USA) and FlowJo software (TreeStar, Ashland, OR, USA).
Intracellular cytokine staining
PBMC were stimulated with SVV lysate (1 μg) for 1 h followed by addition of brefeldin A (Sigma) to block cytokine export for an additional 14 h. After stimulation cells were surface-stained with antibodies against CD4 and CD8β, as described above. Samples were fixed, permeabilized (BioLegend) and dual-stained using antibodies against IFN-γ (eBioscience) and TNF-α (eBioscience). Samples were analysed using the LSRII instrument and FlowJo software.
Statistical analysis
Statistical analysis and graphing was conducted with GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Significance values for Figs 6 utilized one-way repeated-measures analysis of variance (anova) with Tukey's post-test to explore differences between pre- and post-treatment values.
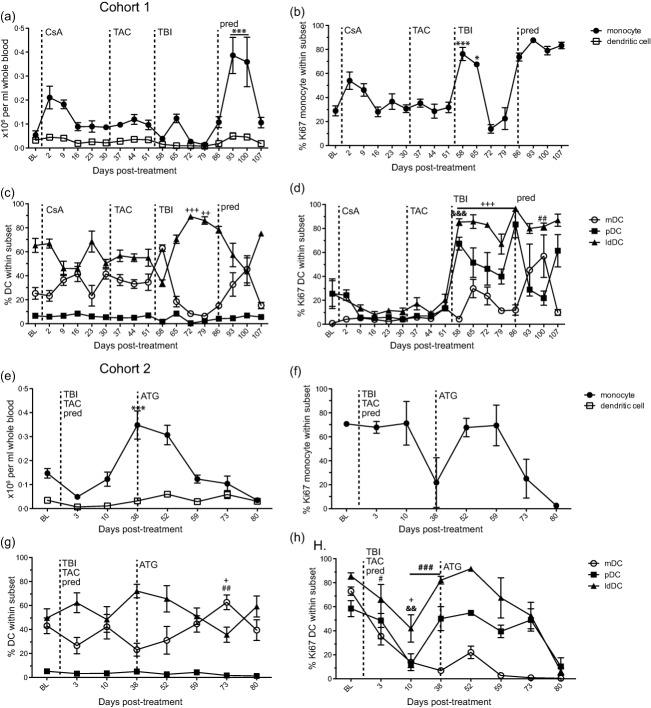
Fig. 6.
Effect of immunosuppression on monocyte and dendritic cell (DC) populations. Absolute number of monocytes (closed square) and DC (open square) were counted per ml of whole blood at various days post-infection in (a) cohort 1 and (e) cohort 2. Frequency of DC subsets: myeloid (mDC), plasmacytoid (pDC) and less-differentiated (ldDC) DCs in peripheral blood mononuclear cells (PBMC) measured by flow cytometry in (c) cohort 1 and (g) cohort 2. Percentage of proliferating monocytes in (b) cohort 1 and (f) cohort 2 and DC subsets in (d) cohort 1 and (f) cohort 2 from PBMC measured by flow cytometry based on the expression of Ki67. Average ± standard error of the mean (s.e.m.). Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT), and Janus activated kinase inhibitor (JAK-Inh). Statistical analysis was performed using one-way repeated-measures analysis of variance (anova) with Tukey's post-test to test differences between pre- and post-treatment values P < 0·001 ***monocyte, ###mDC, &&&pDC, +++ldDC; P < 0·01 &&pDC, ##mDC, ++ldDC; P < 0·05 *monocyte, #mDC, +ldDC.
Results
Immunosuppression treatment time-line
To measure the effects of ionizing radiation and immunosuppressive drug treatment on immune cell subsets of RMs latently infected with SVV, three cohorts of RMs were treated as follows. Animals in cohort 1 (Fig. 1a) were first treated with daily CsA. Then, on day 32 post-treatment (dpt), CsA was replaced with daily tacrolimus treatment. At 55 dpt animals were exposed to 2 Gy ionizing radiation (TBI) and the dose of tacrolimus was increased at 59 dpt and again at 68 dpt. Prednisone was added daily starting at 86 dpt. The animals were euthanized at 107 dpt. Animals in cohort 2 (Fig. 2b) received 2 Gy ionizing radiation on day 0 and daily tacrolimus and prednisone for 37 days. At 38 dpt, RMs received anti-thymocyte globulin (ATG) for 4 days. The animals were euthanized at 80 dpt. Animals in cohort 3 (Fig. 2c) were exposed to 4 Gy ionizing radiation at day 0 and then received a 4-day course of anti-monkey CD3 immunotoxin (CD3-IT) at 9 dpt. Animals received a second dose of CD3-IT 58–61 dpt. Approximately 3 months later, a Janus-activated kinase (JAK) inhibitor (JAK-Inh; tofacitinib citrate, CP-690550 citrate) was given for 5 days from 149–153 dpt and animals were euthanized at 166 dpt.
Fig. 2.
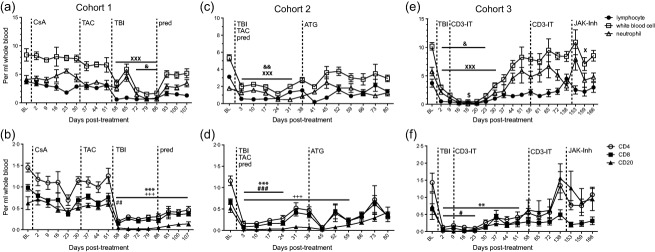
Whole blood cell counts in simian varicella virus (SVV) latently infected animals undergoing immunosuppressive treatment. Absolute numbers of lymphocytes (closed square), white blood cells (open square), neutrophils (open triangle) were counted per ml of whole blood at various days post-infection in (a) cohort 1, (c) cohort 2 and (e) cohort 3. Absolute number of CD4 T cells (open circle), CD8 T cells (closed square) and CD20 B cells (closed triangle) were calculated per ml of whole blood from flow cytometric analysis using the lymphocyte count in (b) cohort 1, (d) cohort 2 and (f) cohort 3. Average ± standard error of the mean (s.e.m.). Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh). Statistical analysis was performed using one-way repeated-measures analysis of variance (anova) with Tukey's post-test to test differences between pre- and post-treatment values P < 0·001 XXXlymphocyte, ***CD4 T cell, ###CD8 T cell, +++CD20 B cell; P < 0·01 &&white blood cell, **CD4 T cell, ##CD8 T cell; P < 0·05 and white blood cell, $neutrophil, #CD8 T cell.
The effect of immunosuppression on white blood cell, lymphocyte and neutrophil numbers in whole blood
We investigated the impact of irradiation and immunosuppressive drug treatment on total WBC, lymphocyte and neutrophil counts at various intervals during treatment (Fig. 2). We also calculated the absolute number of circulating CD4 and CD8 T cells and CD20 B cells throughout treatment (Fig. 2). In cohort 1, CsA or tacrolimus treatment alone had no significant impact on WBC, lymphocyte or neutrophil counts (Fig. 2a). In contrast, irradiation resulted in an initial decrease (58 dpt) in WBC count followed by an increase at 65 dpt and then another significant decrease from 72 to 86 dpt (P < 0·05) before returning to near baseline (BL) level by 93 dpt (Fig. 2a). The increase in WBC count at 65 dpt was due to a transient increase in neutrophils. In contrast, the significant decrease in lymphocyte counts post-irradiation (80%, P < 0·001, 58 dpt) persisted for approximately 1 month during daily tacrolimus treatment. Prednisone was added to the daily tacrolimus regimen at 86 dpt but had no effect on either lymphocyte or neutrophil numbers. By the end of the study, lymphocyte counts remained depressed by 65% compared to baseline (BL), while neutrophil counts were not significantly altered from baseline.
Within the lymphocyte subset, CsA or tacrolimus alone had no significant effect on the absolute counts of circulating CD4, CD8 and CD20 cells (Fig. 2b). Conversely, irradiation resulted in a significant decrease in the total number of CD4 and CD8 T cells and CD20 B cells (> 80% reduction, P < 0·001, P < 0·01 and P < 0·001, respectively, 58 dpt). At completion of the study, CD4 T cells remained significantly reduced by 63% and CD8 T cells were reduced by 50% compared to the pre-irradiation counts. Similarly, B cell counts did not recover by completion of the study and remained 78% reduced compared to the pre-irradiation count.
As described for cohort 1, irradiation resulted in a significant decrease in the number of WBCs in cohort 2 animals (63%, P < 0·01, 3 dpt), due chiefly to a decrease in lymphocytes (82%, P < 0·001, 3 dpt), as neutrophil counts were not altered significantly (Fig. 2c). Following TBI, animals received daily tacrolimus and prednisone to maintain immunosuppression. WBC counts, and more specifically lymphocyte numbers, remained significantly low for approximately 3 weeks before beginning to recover. At this time (38 dpt), the animals received a 4-day course of ATG resulting in a rapid but short-lived decrease at 41 dpt in lymphocyte numbers from 1·5 × 106 ± 0·2 to 0·2 × 106 ± 0·02 cells per ml WB. Lymphocyte numbers returned to pre-ATG levels 2 weeks after treatment, at 55% compared to baseline at the end of the study. Within the lymphocyte population (Fig. 2d), TBI produced a significant decrease in the absolute number of CD4 (86%, P < 0·001) and CD8 (88%, P < 0·001) T cells at 3 dpt. As described for cohort 1, TBI resulted in more profound and sustained B cell depletion (94% reduction, P < 0·001, 3 dpt). As expected, ATG administration produced a severe but brief reduction in CD4 and CD8 cell counts at 45 dpt but did not alter the already low number of circulating B cells.
Cohort 3 RMs were exposed to twice the dose of ionizing radiation compared to cohort 1 and 2 RMs and CD3-IT was introduced 9 days post-irradiation to further deplete T cells. Similarly to cohorts 1 and 2, this treatment regimen resulted in a significant decrease in WBC (71% reduction, P < 0·05, 2 dpt) and lymphocytes (86% reduction, P < 0·001, 2 dpt). In contrast to the earlier treatments, we also saw a significant reduction in neutrophil counts (35% reduction, P < 0·05, 2 dpt) (Fig. 2e). The significant reduction in WBC, lymphocyte and neutrophil counts lasted for another 2 and 3 weeks, respectively, after which counts increased. A second round of CD3-IT treatment was given 37 days after the first treatment, but had no significant effect on lymphocyte numbers. This is due most probably to the rise in antibodies directed against diphtheria immunotoxin, which then abrogated the efficacy of the CD3 immunotoxin (data not shown). We administered JAK inhibitor for 4 days (149–153 dpt), which decreased the lymphocyte count by 50% at 159 dpt compared to 138 dpt (P < 0·05). All subsets returned to approximate baseline counts at the end of the study.
Within the lymphocyte subset the increased dose of irradiation reduced (91%) the number of circulating CD4 T cells (P < 0·001) and CD8 T cells (P < 0·05) significantly compared to baseline (Fig. 2f). After irradiation and the first round of CD3-IT treatment at 9 and 18–21 dpt, the CD4 count remained reduced significantly for more than a month and the CD8 count for 2 weeks before starting to recover. JAK inhibitor reduced total CD4 and CD8 T cell counts. At the end of the study, CD4 T cell counts were reduced by 25%, while CD8 T cells were reduced by 52% compared to baseline. Similar to the first two cohorts, irradiation reduced the absolute number of circulating B cells for approximately 7 weeks before the CD20 cell count recovered to above BL levels (Fig. 2f).
The effect of immunosuppression on T cell subset frequency and proliferation
To investigate further the impact of ionizing radiation and immunosuppressive drug treatment on T cell homeostasis we measured the relative frequency of naive (CD28+CD95−), central memory (CM, CD28+CD95+) and effector memory (EM, CD28−CD95+) subsets within CD4 (Fig. 3) and CD8 (Fig. 4) T cell compartments by flow cytometry. We also characterized the kinetics and magnitude of proliferation by measuring the frequency of Ki67+ T cells within naive, CM and EM subsets during treatment by flow cytometry (Figs 3 and 4). Ki67 is a nuclear protein up-regulated when cells are actively proliferating [61].
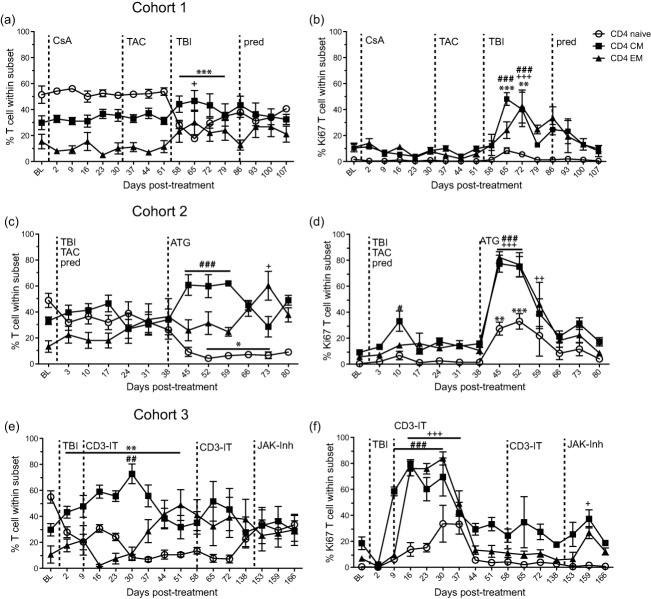
Fig. 3.
Effect of immunosuppression on CD4 T cell frequency and proliferation. The frequency of naive (open circle), central memory (CM, closed square) and effector memory (EM, closed triangle) CD4 T cells was measured by flow cytometry in peripheral blood mononuclear cells (PBMC) for (a) cohort 1, (c) cohort 2 and (e) cohort 3 rhesus macaques (RMs). The frequency of proliferating CD4 T cell subsets was measured by flow cytometry based on the expression of Ki67 in (b) cohort 1, (d) cohort 2 and (f) cohort 3 RMs. Average ± standard error of the mean (s.e.m.). Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh). Statistical analysis was performed using one-way repeated-measures analysis of variance (anova) with Tukey's post-test to test differences between pre- and post-treatment values P < 0·001 ***naive, ###CM, +++EM; P < 0·01 **naive, ##CM, ++EM; P < 0·05 *naive, #CM, +EM.
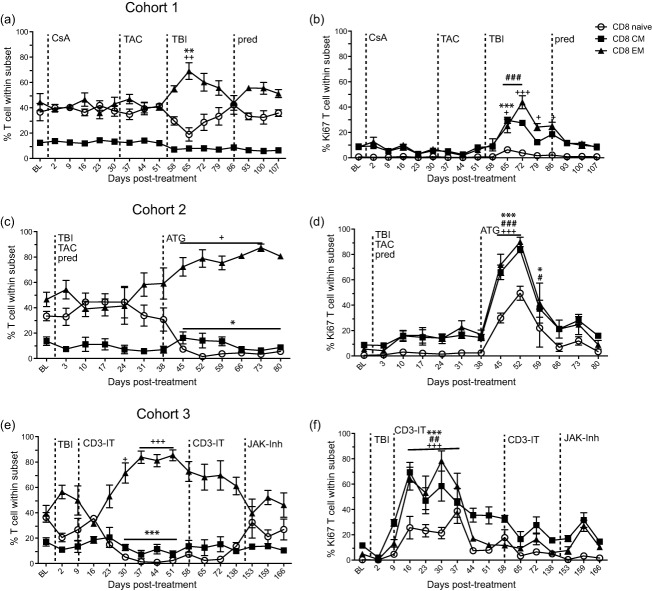
Fig. 4.
Effect of immunosuppression on CD8 T cell frequency and proliferation. The frequency of naive (open circle), central memory (CM, closed square) and effector memory (EM, closed triangle) CD8 T cells was measured by flow cytometry in peripheral blood mononuclear cells (PBMC) for (a) cohort 1, (c) cohort 2 and (e) cohort 3 rhesus macaques (RMs). The frequency of proliferating CD8 T cell subsets was measured by flow cytometry based on the expression of Ki67 in (b) cohort 1, (d) cohort 2 and (f) cohort 3 RMs. Average ± standard error of the mean (s.e.m.). Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh). Statistical analysis was performed using one-way repeated-measures analysis of variance (anova) with Tukey's post-test to test differences between pre- and post-treatment values P < 0·001 ***naive, ###CM, +++EM; P < 0·01 **naive, ##CM, ++EM; P< 0·05 *naive, #CM, +EM.
CsA or tacrolimus treatment alone (cohort 1) had no impact on CD4 T cell subset frequency (Fig. 3a) or proliferation (Fig. 3b). After irradiation, the relative frequency of CD4 memory cells, especially CD4 EM, increased at 65 dpt (P < 0·05). Conversely, the frequency of naive CD4 T cells was decreased significantly compared to the pre-TBI frequency and remained depressed for 3 weeks (P < 0·001). This change in memory/naive ratios coincided with a significant increase in proliferation of all CD4 T cell subsets between 10 and 17 days post-irradiation. The addition of prednisone did not impact significantly the relative frequency or proliferation of CD4 T cell subsets.
Similarly, in cohort 2 the frequency of naive CD4 cells decreased while the frequency of memory cells increased after irradiation and during daily tacrolimus and prednisone treatment (Fig. 3c). The frequency of naive CD4 cells was also reduced significantly after ATG treatment (P < 0·05), while the frequency of CD4 CM cells increased (P < 0·001, 45–59 dpt) compared to 38 dpt (Fig. 3c). CD4 EM T cells were increased significantly 5 weeks post-ATG treatment (P < 0·05, 73 compared to 38 dpt). This shift towards a memory phenotype was accompanied by increased T cell proliferation both after irradiation (CD4 CM) and after ATG treatment (all subsets) (Fig. 3d).
In cohort 3, the combined effect of irradiation and CD3-IT led to the decreased frequency of CD4 naïve (P < 0·01, 2–51 dpt) and CD4 EM cells, while the frequency of CD4 CM cells increased (P < 0·01, 30 dpt compared to BL) (Fig. 3e). Proliferation in memory subsets of CD4 T cells increased significantly after irradiation and the first treatment with CD3-IT, CM (P < 0·001) 9–30 dpt and EM (P < 0·001) 16–37 dpt compared to 120 dpt (Fig. 3e). JAK inhibitor did not have significant effects on the frequency of CD4 T cell subsets. Proliferation of CD4 EM T cells (P < 0·05) and CD4 CM T cells also increased at 159 dpt compared to 138 dpt after JAK inhibitor was utilized (Fig. 3f).
With regard to the CD8 T cell compartment, in cohort 1, CsA or tacrolimus treatment alone had no impact on CD8 T cell subset frequency (Fig. 4a) or proliferation (Fig. 4b). After irradiation, the frequency of CD8 EM cells increased significantly while the frequency of naive cells decreased at 65 dpt compared to 51 dpt (P < 0·01) (Fig. 4a). The addition of prednisone at 86 dpt resulted in a slight decrease in the frequency of CD8 naive and an increase in frequency of CD8 EM T cells, whereas the frequency of CD8 CM T cells remained steady throughout treatment (Fig. 4a). Changes in subset frequency were accompanied by a significant proliferative burst within all CD8 subsets after irradiation (Fig. 5b).
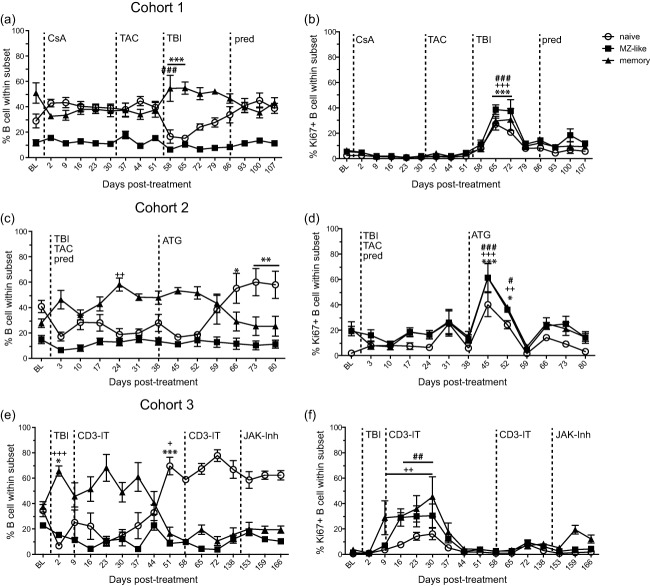
Fig. 5.
Effect of immunosuppression on the frequency and proliferation of B cell subsets. Frequency of naive (open circle), marginal zone-like (MZ-like, closed square), and memory (closed triangle) B cells in peripheral blood mononuclear cells (PBMC) measured by flow cytometry from (a) cohort 1, (c) cohort 2 and (e) cohort 3 rhesus macaques (RMs). Percentage of proliferating naive, MZ-like and memory B cell subsets was measured using flow cytometry based on the expression of Ki67 in (b) cohort 1, (d) cohort 2 and (f) cohort 3 RMs. Average ± standard error of the mean (s.e.m.). Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh). Statistical analysis was performed using one-way repeated-measures analysis of variance (anova) with Tukey's post-test to test differences between pre- and post-treatment values P < 0·001 ***naive, ###MZ-like, +++memory; P < 0·01 **naive, ##MZ-like, ++memory; P < 0·05 *naive, #MZ-like, +memory.
As described for CD4 T cells, irradiation, tacrolimus and prednisone treatment in cohort 2 had minimal effect on the relative frequency of CD8 naive or memory subsets (Fig. 4c). In contrast, ATG treatment resulted in a significant decrease in the frequency of naive CD8 T cells (P < 0·05, 45–80 compared to 38 dpt). ATG treatment also resulted in a significant increase in the frequency of CD8 EM T cells from days 45 to 73 dpt compared to 38 dpt (P < 0·05) (Fig. 4c). Proliferation in all subsets of CD8 T cells increased for 3 weeks post-ATG treatment (Fig. 4d).
In cohort 3, approximately 4 weeks after TBI and the first treatment with CD3-IT the frequency of CD8 naive T cells was reduced significantly from 30 to 51 dpt (P < 0·001) while the frequency of CD8 EM T cells increased significantly at 30–51 dpt (P < 0·05) compared to baseline (Fig. 4e). The frequency of CD8 CM T cells remained steady throughout treatment. All CD8 T cell subsets showed increased proliferation post-irradiation and the first treatment with CD3-IT on days 16–37 post-treatment (naive and EM P < 0·001 and CM P < 0·01) (Fig. 4f). The percentage of proliferating CD8 memory cells also increased after the second CD3-IT treatment (72 dpt) and after JAK inhibitor treatment (159 dpt) (Fig. 4f).
The effect of immunosuppression on B cell subset frequency and proliferation
To investigate the impact of ionizing radiation and immunosuppressive drug treatment on B cell homeostasis we measured the relative frequency of naive (CD27−IgD+), MZ-like (CD27+IgD+) and memory (CD27+IgD−) B cells by flow cytometry. B cell proliferation was also assessed based on expression of Ki67 using flow cytometry.
In cohort 1, CsA or tacrolimus treatment had no effect on the frequency or proliferation of B cell subsets (Fig. 5a,b). Irradiation reduced the total CD20 B cell number significantly (Fig. 2), and resulted in a redistribution of B cell subsets with a significant loss of naive B cells (P < 0·001, 58–65 dpt) and a relative increase in the frequency of memory B cells increased at 58 dpt (P < 0·001) (Fig. 5a), while the relative frequency of MZ-like B cell subsets remained stable. Proliferation in all subsets of B cells increased significantly at 65–72 dpt (P < 0·001) (Fig. 5b).
Similarly, in cohort 2, irradiation resulted in a reduction in the frequency of naive B cells and an increase in the frequency of memory B cells (Fig. 5c). During daily tacrolimus and prednisone treatment the frequency of memory B cells peaked at 24 dpt (P < 0·01). Four weeks post-ATG treatment, the frequency of memory B cells decreased while the frequency of naive B cells increased significantly to 66–80 dpt (P < 0·05) and no changes were detected in the frequency of MZ-like B cells throughout the study (Fig. 5c). In this cohort, B cell subsets did not proliferate after irradiation, tacrolimus and prednisone treatment. Rather, significant B cell proliferation in all subsets was not observed until 1 week post-ATG treatment (45 dpt, P < 0·001) (Fig. 5d).
Consistent with the previous cohorts, irradiation resulted in decreased frequency of naive (P < 0·05) B cells and increased frequency of memory B cells (P < 0·001) (Fig. 5e) in cohort 3. At 51 dpt, naive B cell frequency peaked (P < 0·001) and memory B cell frequency decreased (P < 0·05) compared to baseline, due most probably to new bone marrow output. No significant changes in B cell frequency were induced by JAK inhibitor treatment. Similar to previous cohorts, the frequency of MZ-like B cell subsets remained stable throughout treatment. All B cell subsets showed increased proliferation after irradiation and the first treatment with CD3-IT compared to baseline, especially MZ-like (P < 0·01) and memory B cells (P < 0·01) (Fig. 5f).
The effect of immunosuppression on monocytes and dendritic cells
We measured changes in the frequency and proliferation of peripheral blood monocytes and dendritic cell (DC) subsets including myeloid (mDCs, CD123−CD11c+), plasmacytoid (pDC, CD123+CD11c−) and less differentiated (ldDC, CD123−CD11c−) [62–67] by flow cytometry in response to immunosuppression. Due to limited blood sample volumes we were unable to perform these assays on a portion of cohort 2 and all cohort 3 time-points.
In cohort 1, we saw no major changes in the total DC cell count in whole blood throughout immunosuppressive treatment (Fig. 6a). In contrast, we detected a twofold increase in monocyte counts after CsA treatment, a 60% decrease after irradiation and a significant increase after prednisone treatment (threefold, P < 0·001) (Fig. 6a). The changes in the frequency of monocytes corresponded to increased proliferation after CsA treatment, after irradiation (P < 0·001 58 dpt compared to 51 dpt) and after the addition of prednisone (Fig. 6b). There were multiple changes in the frequency of mDC and ldDC subsets and no significant change in pDC frequencies throughout treatment (Fig. 6c). Specifically, the mDC frequency increased 1 week post-irradiation (P < 0·01, 58 dpt), while the ldDC frequency decreased. Proliferation of pDC and ldDC subsets increased at 58 dpt after irradiation and daily tacrolimus treatment and increased proliferation of mDCs and pDCs was also detected after prednisone treatment (Fig. 6d).
Similarly, in cohort 2 the number of total DCs in whole blood remained steady throughout treatment (Fig. 6e), whereas the number of monocytes increased significantly at 38 dpt (P < 0·001) after irradiation and daily tacrolimus and prednisone treatment before returning to baseline levels at 59 dpt (Fig. 6e). Similar to cohort 1, there were multiple changes in the frequency of mDC and ldDC subsets and no significant change in pDC frequencies throughout treatment (Fig. 6g). After ATG treatment the frequency of the mDC subset increased significantly (P < 0·01), while the ldDC subset decreased significantly (P < 0·05) at 73 dpt compared to 38 dpt. The proliferation of all DC subsets was reduced after irradiation and during tacrolimus and prednisone treatment and increased slightly at 52 dpt, 2 weeks post-ATG treatment (Fig. 6h).
The effect of immunosuppression on plasma chemokine and cytokine levels
We measured the concentrations of the following chemokines, cytokines and growth factors in plasma by multiplex technology: MCP-1, FGF-β, IL-1β, G-CSF, IL-10, IL-6, IL-12, RANTES, eotaxin, IL-17, MIP-1α, GM-CSF, MIP-1β, IL-15, EGF, IL-5, HGF, VEGF, IFN-γ, MDC, I-TAC, MIF, IL-1RA, TNF-α, IL-2, MIG, IL-4 and IL-8 (Fig. 7 and Supporting information, Fig. S2). Below we describe the results for a subset of the chemokines and cytokines that showed changes with treatment in cohorts 1 (Fig. 7a), 2 (Fig. 7b) and 3 (Fig. 7c).
Fig. 7.
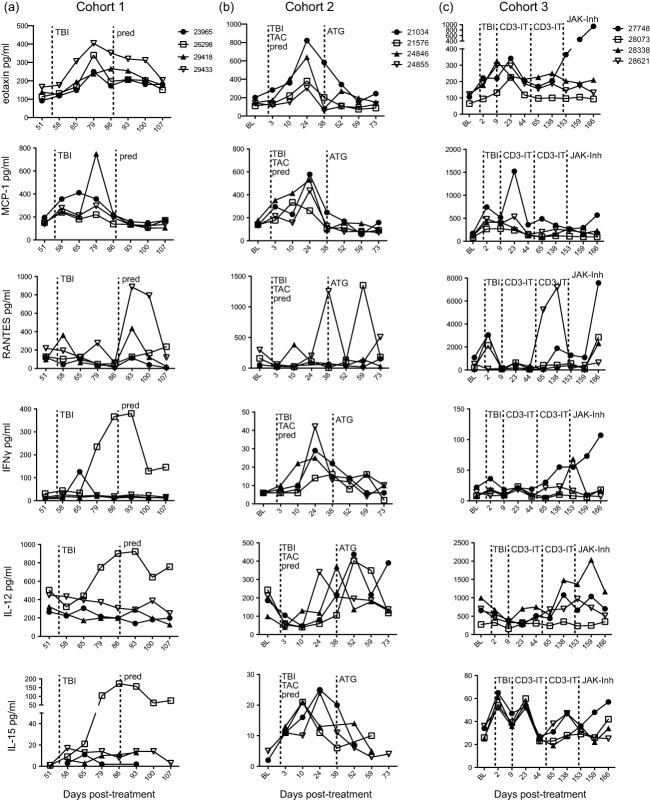
The effect of immunosuppression on chemokine and cytokine levels in the plasma. The concentration (pg/ml) of chemokines and cytokines in plasma were determined using multiplex technology in individual rhesus macaques (RMs) from (a) cohort 1, (b) cohort 2 and (c) cohort 3. Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh).
Eotaxin is an important chemotactic factor for eosinophils, basophils and Th2 T cells [68–70]. In cohort 1, at 79 dpt we measured an increase in eotaxin levels in all RMs approximately 3 weeks after irradiation and during daily tacrolimus treatment. Similar to cohort 1, all RMs in cohort 2 showed an increase in levels of eotaxin at 24 dpt, approximately 3 weeks post-irradiation and during daily tacrolimus and prednisone treatment. Within cohort 3, all RMs also displayed increased levels of eotaxin 2–3 weeks post-irradiation and the first treatment of CD3-IT, and one RM (22748) also had increased levels of eotaxin after JAK inhibitor treatment.
Plasma levels of the chemokine MCP-1 (CCL2), which can recruit monocytes, memory T cells and DCs to sites of inflammation [71], increased in cohort 1 after irradiation and during daily tacrolimus treatment, with the most dramatic increase detected in RM 29418 at 79 dpt. Increased levels of MCP-1 were also observed in all RMs in cohort 2 between 2 and 3 weeks post-irradiation and during daily tacrolimus and prednisone treatment. Within cohort 3, we measured two peaks of MCP-1 expression in the plasma of RM 27748, immediately after irradiation at 2 dpt and after the first treatment with CD3-IT at 23 dpt. The other RMs in cohort 3 showed minor increases in MCP-1 levels after irradiation and CD3-IT treatment.
Levels of the chemokine RANTES (CCL5), which plays a role in immune cell recruitment to inflammatory sites [72], increased in RM 29418 and 29433 (cohort 1) 1 week after the introduction of daily prednisone treatment. In cohort 2, RANTES levels increased in RM 24855 at 38 dpt after irradiation and during daily tacrolimus and prednisone treatment. Plasma levels of RANTES also peaked at 59 dpt, post-ATG treatment in RM 21576. Three RMs in cohort 3 showed increased RANTES levels at 2 dpt after irradiation. RM 28621 also showed peak levels of RANTES at 138 dpt after the second treatment with CD3-IT and RM 27748 showed a comparable increase in plasma RANTES at 166 dpt after JAK inhibitor treatment.
In cohort 1 levels of IFN-γ, a proinflammatory cytokine [73], increased in RM 26298 starting at 79 dpt after irradiation and tacrolimus treatment and peaking at 93 dpt after prednisone was added to the treatment regimen. RM 23965 also displayed a slight increase in IFN-γ at 65 dpt 10 days post-irradiation and tacrolimus treatment. In cohort 2 IFN-γ plasma levels increased 3 weeks after irradiation and during daily tacrolimus and prednisone treatment (Fig. 8b). In cohort 3, RM 27748 IFN-γ levels remained steady through treatment until 196 dpt, when levels began to increase until the end of the study. IFN-γ levels in RM 28338 peaked at 284 dpt corresponding to the last day of JAK inhibitor treatment.
Fig. 8.
The effect of immunosupression the frequency of simian varicella virus (SVV)-specific T cells. Percentage of SVV-specific CD4 and CD8 T cells in peripheral blood mononuclear cells (PBMC) producing interferon (IFN)-γ and/or tumour necrosis factor (TNF)-α was measured by intracellular cytokine staining following stimulation with SVV lysate in individual rhesus macaques (RMs) from (c) cohort 1, (d) cohort 2 and (e) cohort 3. Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh).
IL-12, a cytokine important for differentiation of naive T cells into CD4 Th1 cells [74], remained elevated in RM 26298 (cohort 1) between 79 and 107 dpt. IL-12 levels in cohort 2 RMs initially decreased after irradiation and during tacrolimus and prednisone treatment and then peaked at different times before and after ATG treatment. In cohort 3, IL-12 levels in all RMs except for 28073 increased after irradiation and CD3-IT treatments, while RM 28338 exhibited peak levels at 159 dpt after JAK inhibitor treatment.
IL-15, which is involved in the proliferation of T cells and natural killer (NK) cells [75], was also expressed differentially between RMs and treatments. In cohort 1, plasma levels of IL-15 in RM 26298 increased after irradiation and during daily tacrolimus treatment and remained elevated after prednisone was added to the treatment schedule. In cohort 2, plasma IL-15 levels peaked between 10 and 24 dpt after irradiation and during daily tacrolimus and prednisone treatment. In all cohort 3 RMs, IL-15 levels changed throughout treatment, increasing after irradiation and the first CD3-IT treatment. In RMs 27748 and 28621, IL-15 levels also increased after the second CD3-IT treatment while JAK inhibitor treatment produced increased levels in RM 27748.
The effect of immunosuppression on SVV viral loads and frequency of SVV-specific IgG titres and T cells
Additionally, we measured the impact of irradiation and immunosuppressive drug treatment on SVV reactivation by monitoring changes in viral loads, SVV-specific IgG titres and the frequency of SVV-specific CD4 and CD8 T cells. Prior to immunosuppression, RMs were infected intrabronchially with 4 × 105 PFU wild-type SVV, as described previously [37]. All RMs displayed viral exanthem within 7–14 days post-infection (dpi) and had measurable SVV viral loads in whole blood and BAL samples (Supporting information, Fig. S1). Peak viral loads were higher in BAL samples following acute infection than in whole blood, probably reflecting the route of infection. Viraemia resolved in all RMs prior to initiation of immunosuppressive treatment and viral loads remained below the threshold of detection throughout immunosuppression in all cohorts.
We measured SVV-specific IgG antibody end-point titres in plasma using standard ELISA during the course of infection and immunosuppression. After SVV infection all RMs show an increase in SVV-specific antibodies with a set-point IgG titre established by 14 dpi (Supporting information, Fig. S1b,d,f). Throughout immunosuppression, no significant changes were measured in the titres of SVV-specific antibodies in all cohorts.
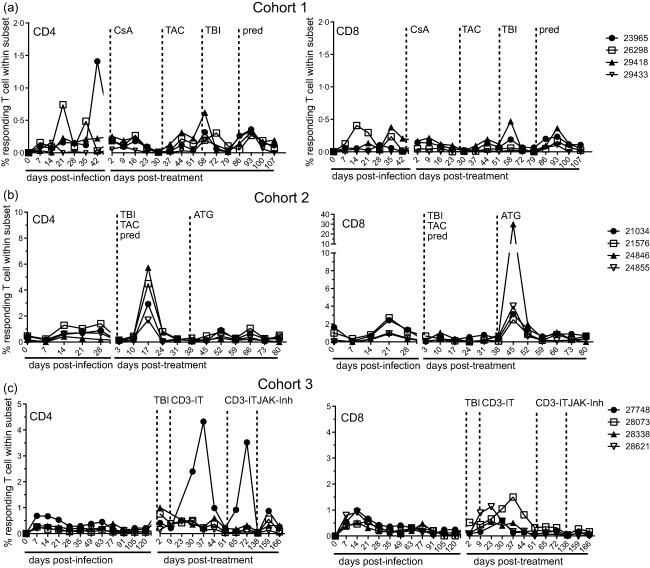
The frequency of SVV-specific CD4 and CD8 T cells was determined by quantifying the number of single- and double-positive IFN-γ- and TNF-α-producing cells using intracellular cytokine staining (ICS) after stimulation with SVV lysate. Cohort 1 RMs (Fig. 8a) show a small increase, similar to the initial response to SVV infection, in the percentage of SVV-specific CD4 and CD8 T cells after irradiation (58 dpt) and after the addition of prednisone (93 dpt). In cohort 2 (Fig. 8b), the percentage of SVV-specific CD4 T cells is increased in all RMs after irradiation and during tacrolimus and prednisone treatment at 17 dpt, whereas no significant change was detected in SVV-specific CD8 T cells. In contrast, an increase in SVV-specific CD8 T cells but not CD4 T cells was detected following ATG treatment at 45 dpt. In cohort 3 (Fig. 8c), RMs 28073 and 28621 exhibited a peak in the percentage of SVV-specific CD4 T cells at 37 and 23 dpt, respectively, after irradiation and CD3-IT treatment, levels similar to acute SVV infection. Also within cohort 3, RM 27748 showed a substantial increase in SVV-specific CD8 T cells on 37 dpt after irradiation and CD3-IT treatment and also at 72 dpt after the second treatment with CD3-IT. We also detected a small increase in SVV-specific CD8 T cells in all RMs after JAK inhibitor was administered (159 dpt), which is commensurate with the initial response to infection.
Discussion
Lymphodepletion and immunosuppression are routine in the treatment of haematological malignancy and organ transplantation. However, these regimens increase patients’ susceptibility to microbial infections, reactivation of latent pathogens and risk of secondary malignancy. The relative contribution of immunosuppressive regimens versus that of malignancy or the presence of a non-syngeneic organ to immune dysregulation remains incompletely understood due to the lack of studies in immune competent hosts. Therefore, the goal of this study was to investigate the effects of ionizing radiation as well as several clinically relevant chemotherapeutic agents on immune homeostasis in the absence of confounding factors utilizing immune competent adult rhesus macaques.
Our data provide a comprehensive analysis of changes in both adaptive and innate immunity, circulating factors and immune response to latent viral infection following several immune suppression regimens used commonly in the clinical setting. Furthermore, our data extend previous studies in RMs investigating immune cell reconstitution following high-dose radiation exposure (9–12 Gy) [76]. High-dose irradiation resulted in severe, acute myelosuppression and lymphopenia. Neutrophils recovered to baseline levels by 30 days post-treatment while the levels of CD3+ cells recovered to 65% and CD20+ cells recovered to 100% of baseline at 80 days post-treatment. Moreover, there was a decrease in the CD4: CD8 ratio compared to baseline and an overall failure of recent thymic emigrants and naive T cells subsets to recover to baseline values. Correspondingly, irradiation (2–4 Gy) caused the most dramatic restructuring in the absolute number and relative frequencies of immune cells in the blood of rhesus macaques. Overall, irradiation effectively depleted WBCs, with lymphocytes being more sensitive than neutrophils. At a 2-Gy dose (cohorts 1 and 2), lymphocyte numbers dropped dramatically within 3 days of irradiation while neutrophil numbers did not decrease significantly. However, the increased dose of irradiation (4 Gy) that cohort 3 received resulted in thrombocytopenia and neutropenia, in addition to lymphopenia, although the animals required no additional treatments such as transfusions or antibiotic support. Moreover, lymphocytes required several weeks to return to baseline levels.
Within the lymphocyte population, B cells were more radiosensitive than T cells except possibly at a higher dose (cohort 3), but those animals also received anti-CD3 immunotoxin to deplete T cells 9 days after irradiation. Our observations are in agreement with multiple rodent studies that describe B cells to be more radiosensitive than T cells [77–80]. Our studies expand upon these previous studies and show that, within the B cell population in RMs, the frequency of naive and switched memory B cells were the most responsive to ionizing radiation, while the MZ-like memory subset remained somewhat resistant to change. In all cases, naive B cell numbers rebounded 1–2 months after irradiation as haematopoietic cell progenitor production in the bone marrow recovered.
Within the T cell compartment, previous in-vitro and mouse studies reported that CD4 T cells were observed to be more radiosensitive than CD8 cells [81–83]. However, our in-vivo studies in RM do not show an appreciable difference in the sensitivity of CD4 and CD8 T cells to ionizing radiation. As described previously for B cells, naive T cells are more sensitive than memory subsets. During immune reconstitution the ratios of naive, EM and CM shift, resulting in the EM population dominating the CD8 T cell compartment and CM population dominating the CD4 T cell compartment. These changes are similar to those observed with ageing in RM, where EM T cells relatively outnumber the naive and CM T cells within the CD8 subset while CM T cells are the dominant memory population in the CD4 subset [84]. These changes are probably the result of increased levels of homeostatic proliferation [61,85]. Our data also support the proposed analogy between ionizing radiation exposure and ageing [86]. Similar observations were made in atomic bomb survivors from WW II, who exhibited an overall decrease in naive T cells and an increase in CD8 memory T cell subsets [87]. In future experiments it will be interesting to explore the relationship between TBI and immunosenescence, possibly exploring changes in T cell repertoire diversity before and after TBI in a well-characterized anti-viral T cell response such as that to rhesus cytomegalovirus.
Our studies enrich previous clinical studies using non-myeloablative conditioning for allogeneic haematopoietic cell transplantation investigating the recovery of total CD20, CD4 and CD8 cell populations as well as the rates of naive CD4 T cell recovery in older versus younger patients [88]. This group used long-term immunosuppression that resembled the regimens used in our study in many of these patients because of chronic graft-versus-host disease (GVHD), but were not able to evaluate the pre- versus post-treatment in memory/naive profiles. Their data suggest a difference in the compartment contributing to the reconstitution of the T cell compartment. In patients aged less than 60 years receiving non-myeloablative conditioning, thymic neo-generation of T cells occurred, while in patients aged more than 60 nearly all peripheral T cells resulted from the expansion of mature donor T cells contained in the graft. In future studies we will determine the compartment contributing to the expansion of T cells in young versus aged RMs. Another study investigating memory and naive profiles of patients receiving allogeneic transplants following myeloablative conditioning found that a poor recovery of naive and central memory T cell populations following transplant was associated with GVHD [89].
In contrast to the lymphocyte populations, total DC numbers in whole blood were mainly unaffected by immunosuppressive treatment, although we measured an increase in the frequency of ldDCs and a decrease in the frequency of mDCs post-irradiation. Interestingly, ionizing radiation during daily tacrolimus (cohorts 1 and 2) and prednisone treatment (cohort 2) initially produced a decrease in the number of monocytes, followed by an increase in their frequency in both cohorts. There is evidence that inflammation, like that observed here following TBI and drug treatment, may be related to the production of myeloid-derived suppressor cell populations that interfere with antigen-specific and innate immune responses to pathogens and tumour antigens [90,91]. Future directions for this work might explore the phenotype and function of the increase in myeloid-derived cells following TBI.
Multiple studies have reported that ionizing radiation leads to increased expression of proinflammatory cytokines [92–94] as well as inducing inflammasome pathway activation in immune cell populations [95]. Our analysis revealed several changes consistent with a heightened inflammatory milieu. For instance, in each cohort we measured an increased concentration in the plasma of the chemokines eotaxin and MCP-1 and the cytokines IFN-γ, IL-12 and IL-15 after irradiation. We also detected increased plasma IL-1β in all the animals in cohorts 2 and 3 of four animals from cohort 3 after irradiation as well as increased TNF-α levels in three of four animals in cohort 3 after irradiation (Supporting information, Fig. S2). Changes in chemokine levels correlate with lymphocyte proliferation. Increases in IL-12 and IL-15, which play a critical role in T cell differentiation and survival, correlate with the shift towards CM and EM T cells.
Using doses consistent with previous NHP and clinical studies [45,46,48,49], CsA or tacrolimus alone (cohort 1 RMs) did not induce any significant change in either cell counts or the lymphocyte memory/naive ratio. Circulating monocyte numbers increased ∼twofold after cyclosporin was introduced. Similarly, the addition of prednisone to the highest dose of tacrolimus treatment resulted in a significant increase (∼threefold) in circulating monocytes. Again, it would be interesting to explore here the phenotype and function of these new monocytes with regard to stimulation or inhibition of novel immune responses.
Administration of ATG (cohort 2) resulted in a severe but short-lived decrease in both CD4 and CD8 T cell counts 7 days later. This loss was most significant for naive T cells and was accompanied by a very robust proliferative burst and a reciprocal increase in the frequency of CD4 CM T cells and CD8 EM T cells. These observations are similar to those reported for patients undergoing induction therapy with ATG or alemtuzumab for renal transplantation where naive T cells and CD4+CD25+ T cells were absent following depletion and that CD4+ T cells with a surface phenotype consistent with effector memory cells predominate following either depletion strategies [96]. The proliferative burst also correlated with an increased frequency of SVV-specific CD8 T cells, but not CD4 T cells expressing IFN-γ and TNF-α by ICS after stimulation with SVV lysate.
Similar to a previous study utilizing the same dosage of CD3 immunotoxin in a rhesus macaque renal transplant model [24], after the first CD3-IT administration we observed a decrease in naive T cells and an increase in CD4 CM and CD8 EM T cells. After both CD3-IT treatments we measured, in RM 27748, an increase in the SVV-specific CD8 T cell response via ICS that was substantially higher than the initial SVV infection response (Fig. 8). Although we used a drug with a truncated diphtheria toxin, which was thought to be effective in the face of pre-existing anti-diphtheria antibodies, the second course of CD3-IT was not effective in our animals. Notably, a CD3 immunotoxin is currently in a Phase II clinical trial for patients with cutaneous T cell lymphoma receiving 7·5 μg/kg twice a day for 4 days by intravenous (i.v.) injection. An earlier Phase I trial investigated dose response to the CD3 immunotoxin with patients receiving doses between 2·5 and 11·25 μg/kg per dose. The study observed responses to treatment at all dose levels up to 7·5 μg/kg per dose (http://ClinicalTrials.gov Identifier: NCT00611208).
We also treated cohort 3 RMs with a JAK inhibitor at a similar dose used previously in a NHP model of kidney transplantation [29], approximately 3 months after the second CD3-IT treatment. JAK inhibitor treatment was shown to prolong survival of kidney transplants in cynomolgus monkeys, reduce total peripheral lymphocytes and inhibit the transcription of granzyme B, FasL, RANTES, MIG and IP-10 [97]. In naive, non-transplanted animals the authors showed no effects on B cells, CD4+ T or CD8+ T cells, but decreases were found in NK cells after 4 weeks of treatment. Another group detected changes in circulating CD8+ T cell numbers in cynomolgus monkeys treated with JAK inhibitor for 3 weeks [98]. Similarly to this last study, we measured no changes in the frequency of CD4 T cells but we detected a decrease in CD8 EM T cells. These differences are due most probably to the lower dose and shorter treatment course that we deployed in this study.
Rhesus macaques infected with SVV develop varicella and viraemia that resolves approximately 21 days post-infection. SVV establishes latency exclusively within the sensory ganglia [37]. In all cohorts, we applied treatment after the resolution of viraemia. RMs in our study did not experience zoster rash, nor did we measure an increase in SVV DNA in whole blood or BAL or an increase in SVV-specific IgG antibody titres following immune suppression (Supporting information, Fig. S1). However, in select RMs we observed an increase in the SVV-specific T cell response via ICS following stimulation with SVV lysate. The increase in the frequency of SVV-specific T cells following immunosuppression could be due to subclinical reactivation of SVV or to increased homeostatic proliferation of antigen-specific T cells or a preferential survival of SVV-specific T cells following immunosuppressive treatment. The increased frequency of SVV-specific T cells coincided with proliferative bursts, which suggests that it may be driven by homeostatic proliferation rather than viral reactivation. In future experiments, it would be important to look at T cell responses to multiple antigens, both persistent and cleared viral pathogens, in order to determine if increases in antigen-specific immunity are the result of viral reactivation or homeostatic proliferation.
In summary, our studies documented changes in the lymphocyte and myeloid cell population in immune-competent RMs following ionizing radiation and chemotherapeutic immunosuppression reminiscent of therapy given to patients receiving solid organ or bone marrow transplantation. Our results reveal alterations in memory versus naive T and B cell ratios following immune reconstitution. We also observed the expansion of a myeloid cell population following lymphodepletion that may have immunosuppressive effects on the remainder of the immune compartment. Lastly, we document the up-regulation of multiple inflammatory cytokines and chemokines, following immunosuppression, that may play a role in the homeostatic proliferation of the remaining immune compartment and the ability of this newly reconstituted compartment to respond to novel or reactivated pathogens. These studies provide insight into the effects of these immune suppressive modalities in the absence of confounding factors such as cancer or an ongoing allogeneic immune response and will help clinicians gauge more effectively the consequences of treatment modalities on the immune fitness of patients.
Acknowledgments
We would like to thank the Division of Animal Resources (DAR) at the Oregon National Primate Research Center for expert animal care, especially Alfred Legasse, Miranda Fischer and Shannon Planer for collection of blood samples. This work was supported by American Heart Association career development grant 0930234N, NIH R01AG037042, 2T32AI007472-16, NIH 8P51 OD011092-53 and the Brookdale Foundation.
Author contributions
Study design: I. M., C. M. and C. R. T.; data collection: C. M., J. D., F. E., J. W., W. L. and S. P.; data interpretation and manuscript preparation: I. M., C. M., J. W. and C. R. T.
Disclosure
The authors have no competing interests to declare.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's Web site:
Fig. S1. Simian varicella virus (SVV) infection, viral load and immunoglobulin (Ig)G titre. All animals in (a) cohort 1, (b) cohort 2 and (c) cohort 3 were infected intrabronchially with SVV at day 0. SVV DNA viral load was measured in whole blood (closed square) and bronchoalveolar lavage cells (BAL, open circle) by quantitative polymerase chain reaction (PCR) using primers and probe specific for SVV ORF21. Average copy number per 100 ng of DNA. (b) SVV-specific IgG antibody end-point titres were measured by standard enzyme-linked immunosorbent assay (ELISA) in (b) cohort 1 (open triangle), (d) cohort 2 (closed triangle) and (f) cohort 3 (closed square) rhesus macaques (RMs). Average ± standard error of the mean (s.e.m.). Solid line indicates limit of detection. Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh).
Fig. 2. The effect of immunosuppression on multiple factors in the plasma. Levels of chemokines, cytokines and growth factors in plasma were determined using multiplex technology in individual rhesus macaques (RMs) from (a) cohort 1, (b) cohort 2 and (c) cohort 3. Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh).
References
- 1.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–28. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 2.Barrett J, Childs R. Non-myeloablative stem cell transplants. Br J Haematol. 2000;111:6–17. doi: 10.1046/j.1365-2141.2000.02405.x. [DOI] [PubMed] [Google Scholar]
- 3.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 4.Hegenbart U, Niederwieser D, Sandmaier BM, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24:444–53. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 5.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–55. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–45. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–7. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP–FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 10.O'Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O'Neill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–4. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- 12.Almawi WY, Lipman ML, Stevens AC, Zanker B, Hadro ET, Strom TB. Abrogation of glucocorticoid-mediated inhibition of T cell proliferation by the synergistic action of IL-1, IL-6, and IFN-gamma. J Immunol. 1991;146:3523–7. [PubMed] [Google Scholar]
- 13.Genestier L, Fournel S, Flacher M, Assossou O, Revillard JP, Bonnefoy-Berard N. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–8. [PubMed] [Google Scholar]
- 14.Michallet MC, Preville X, Flacher M, Fournel S, Genestier L, Revillard JP. Functional antibodies to leukocyte adhesion molecules in antithymocyte globulins. Transplantation. 2003;75:657–62. doi: 10.1097/01.TP.0000053198.99206.E6. [DOI] [PubMed] [Google Scholar]
- 15.Zand MS, Vo T, Huggins J, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79:1507–15. doi: 10.1097/01.tp.0000164159.20075.16. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE, Marchioro TL, Hutchinson DE, Porter KA, Cerilli GJ, Brettschneider L. The clinical use of antilymphocyte globulin in renal homotransplantation. Transplantation. 1967;5(Suppl):1100–5. doi: 10.1097/00007890-196707001-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–8. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 18.Srinivas TR, Meier-Kriesche HU. Minimizing immunosuppression, an alternative approach to reducing side effects: objectives and interim result. Clin J Am Soc Nephrol. 2008;3(Suppl. 2):S101–16. doi: 10.2215/CJN.03510807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GB, Wang Z, Liu YY, et al. A fold-back single-chain diabody format enhances the bioactivity of an anti-monkey CD3 recombinant diphtheria toxin-based immunotoxin. Protein Eng Des Sel. 2007;20:425–32. doi: 10.1093/protein/gzm040. [DOI] [PubMed] [Google Scholar]
- 20.Woo JH, Lee YJ, Neville DM, Frankel AE. Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials. Methods Mol Biol. 2010;651:157–75. doi: 10.1007/978-1-60761-786-0_10. [DOI] [PubMed] [Google Scholar]
- 21.Frankel AE, Zuckero SL, Mankin AA, et al. Anti-CD3 recombinant diphtheria immunotoxin therapy of cutaneous T cell lymphoma. Curr Drug Targets. 2009;10:104–9. doi: 10.2174/138945009787354539. [DOI] [PubMed] [Google Scholar]
- 22.Fechner JH, Jr, Dong Y, Hong X, et al. Graft survival in a rhesus renal transplant model after immunotoxin-mediated T-cell depletion is enhanced by mycophenolate and steroids. Transplantation. 2001;72:581–7. doi: 10.1097/00007890-200108270-00005. [DOI] [PubMed] [Google Scholar]
- 23.Matar AJ, Pathiraja V, Wang Z, et al. Effect of pre-existing anti-diphtheria toxin antibodies on T cell depletion levels following diphtheria toxin-based recombinant anti-monkey CD3 immunotoxin treatment. Transplant Immunol. 2012;27:52–4. doi: 10.1016/j.trim.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page EK, Page AJ, Kwun J, et al. Enhanced de novo alloantibody and antibody-mediated injury in rhesus macaques. Am J Transplant. 2012;12:2395–405. doi: 10.1111/j.1600-6143.2012.04074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wamala I, Matar AJ, Farkash E, Wang Z, Huang CA, Sachs DH. Recombinant anti-monkey CD3 immunotoxin depletes peripheral lymph node T lymphocytes more effectively than rabbit anti-thymocyte globulin in naive baboons. Transplant Immunol. 2013;29:60–3. doi: 10.1016/j.trim.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186:4234–43. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 28.Meyer DM, Jesson MI, Li X, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond) 2010;7:41. doi: 10.1186/1476-9255-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paniagua R, Si MS, Flores MG, et al. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation. 2005;80:1283–92. doi: 10.1097/01.tp.0000177643.05739.cd. [DOI] [PubMed] [Google Scholar]
- 30.Borie DC, Changelian PS, Larson MJ, et al. Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates. Transplantation. 2005;79:791–801. doi: 10.1097/01.tp.0000157117.30290.6f. [DOI] [PubMed] [Google Scholar]
- 31.Busque S, Leventhal J, Brennan DC, et al. Calcineurin-inhibitor-free immunosuppression based on the JAK inhibitor CP-690,550: a pilot study in de novo kidney allograft recipients. Am J Transplant. 2009;9:1936–45. doi: 10.1111/j.1600-6143.2009.02720.x. [DOI] [PubMed] [Google Scholar]
- 32.Vincenti F, Tedesco Silva H, Busque S, et al. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant. 2012;12:2446–56. doi: 10.1111/j.1600-6143.2012.04127.x. [DOI] [PubMed] [Google Scholar]
- 33.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–60. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 34.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 35.Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668–77. doi: 10.1111/j.1365-2133.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–24. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 37.Messaoudi I, Barron A, Wellish M, et al. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLOS Pathog. 2009;5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Archives Inter Med. 1995;155:1605–9. [PubMed] [Google Scholar]
- 39.Guinee VF, Guido JJ, Pfalzgraf KA, et al. The incidence of herpes zoster in patients with Hodgkin's disease. An analysis of prognostic factors. Cancer. 1985;56:642–8. doi: 10.1002/1097-0142(19850801)56:3<642::aid-cncr2820560334>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 40.Mandal BK. Herpes zoster in the immunocompromized populations. Indian J Dermatol. 2006;51:235–43. [Google Scholar]
- 41.Ragozzino MW, Melton LJ, III, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Rifkind D. The activation of varicella-zoster virus infections by immunosuppressive therapy. J Lab Clin Med. 1966;68:463–74. [PubMed] [Google Scholar]
- 43.Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK. Herpes zoster infection following solid organ transplantation: incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant. 2004;4:108–15. doi: 10.1046/j.1600-6143.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 44.Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675–84. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rijkelijkhuizen JK, Haanstra KG, Wubben J, et al. T-cell-specific immunosuppression results in more than 53 days survival of porcine islets of Langerhans in the monkey. Transplantation. 2003;76:1359–68. doi: 10.1097/01.TP.0000085290.60182.6B. [DOI] [PubMed] [Google Scholar]
- 46.Jurewicz WA. Tacrolimus versus cyclosporin immunosuppression: long-term outcome in renal transplantation. Nephrol Dial Transplant. 2003;18(Suppl. 1):i7–11. doi: 10.1093/ndt/gfg1028. [DOI] [PubMed] [Google Scholar]
- 47.Vincenti F, Laskow DA, Neylan JF, Mendez R, Matas AJ. One-year follow-up of an open-label trial of FK506 for primary kidney transplantation. A report of the U.S. Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;61:1576–81. doi: 10.1097/00007890-199606150-00005. [DOI] [PubMed] [Google Scholar]
- 48.Kindt MV, Kemp R, Allen HL, Jensen RD, Patrick DH. Tacrolimus toxicity in rhesus monkey: model for clinical side effects. Transplant Proc. 1999;31:3393–6. doi: 10.1016/s0041-1345(99)00835-0. [DOI] [PubMed] [Google Scholar]
- 49.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laukkanen MO, Kuramoto K, Calmels B, et al. Low-dose total body irradiation causes clonal fluctuation of primate hematopoietic stem and progenitor cells. Blood. 2005;105:1010–5. doi: 10.1182/blood-2004-04-1498. [DOI] [PubMed] [Google Scholar]
- 51.Huhn RD, Tisdale JF, Agricola B, Metzger ME, Donahue RE, Dunbar CE. Retroviral marking and transplantation of rhesus hematopoietic cells by nonmyeloablative conditioning. Human Gene Ther. 1999;10:1783–90. doi: 10.1089/10430349950017464. [DOI] [PubMed] [Google Scholar]
- 52.Rosenzweig M, MacVittie TJ, Harper D, et al. Efficient and durable gene marking of hematopoietic progenitor cells in nonhuman primates after nonablative conditioning. Blood. 1999;94:2271–86. [PubMed] [Google Scholar]
- 53.Kang EM, Hanazano Y, Frare P, et al. Persistent low-level engraftment of rhesus peripheral blood progenitor cells transduced with the fanconi anemia C gene after conditioning with low-dose irradiation. Mol Ther. 2001;3:911–9. doi: 10.1006/mthe.2001.0337. [DOI] [PubMed] [Google Scholar]
- 54.Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of thymoglobulin versus atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–8. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 55.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of thymoglobulin versus atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66:29–37. doi: 10.1097/00007890-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 56.Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLOS Pathog. 2011;7:e1002367. doi: 10.1371/journal.ppat.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer C, Dewane J, Haberthur K, et al. Bacterial artificial chromosome derived simian varicella virus is pathogenic in vivo. Virol J. 2013;10:278. doi: 10.1186/1743-422X-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer C, Engelmann F, Arnold N, et al. Abortive intrabronchial infection of rhesus macaques with varicella-zoster virus provides partial protection against simian varicella virus challenge. J Virol. 2015;89:1781–93. doi: 10.1128/JVI.03124-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer C, Kerns A, Haberthur K, et al. Attenuation of the adaptive immune response in rhesus macaques infected with simian varicella virus lacking open reading frame 61. J Virol. 2013;87:2151–63. doi: 10.1128/JVI.02369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray WL, Zhou F, Noffke J, Tischer BK. Cloning the simian varicella virus genome in E. coli as an infectious bacterial artificial chromosome. Archives Virol. 2011;156:739–46. doi: 10.1007/s00705-010-0889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitcher CJ, Hagen SI, Walker JM, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 62.Aldebert D, Diallo M, Niang M, et al. Differences in circulating dendritic cell subtypes in peripheral, placental and cord blood in African pregnant women. J Reprod Immunol. 2007;73:11–9. doi: 10.1016/j.jri.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Diallo M, Aldebert D, Moreau JC, Ndiaye M, Jambou R. Decrease of lymphoid dendritic cells in blood from malaria-infected pregnant women. Int J Parasitol. 2008;38:1557–65. doi: 10.1016/j.ijpara.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Dombrecht EJ, Aerts NE, Schuerwegh AJ, et al. Influence of anti-tumor necrosis factor therapy (Adalimumab) on regulatory T cells and dendritic cells in rheumatoid arthritis. 2006;24:31–7. Clin Exp Rheumatol. [PubMed] [Google Scholar]
- 65.Efron PA, Matsumoto T, McAuliffe PF, et al. Major hepatectomy induces phenotypic changes in circulating dendritic cells and monocytes. J Clin Immunol. 2009;29:568–81. doi: 10.1007/s10875-009-9291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagendorens MM, Ebo DG, Schuerwegh AJ, et al. Differences in circulating dendritic cell subtypes in cord blood and peripheral blood of healthy and allergic children. Clin Exp Allergy. 2003;33:633–9. doi: 10.1046/j.1365-2222.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- 67.McAuliffe PF, Efron PA, Scumpia PO, et al. Varying blood monocyte and dendritic cell responses after laparoscopic versus open gastric bypass surgery. Obes Surg. 2005;15:1424–31. doi: 10.1381/096089205774859362. [DOI] [PubMed] [Google Scholar]
- 68.Forssmann U, Uguccioni M, Loetscher P, et al. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185:2171–6. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–72. doi: 10.1006/bbrc.1993.2599. [DOI] [PubMed] [Google Scholar]
- 70.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 71.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 73.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 74.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–7. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 75.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 76.MacVittie TJ, Bennett AW, Cohen MV, Farese AM, Higgins A, Hankey KG. Immune cell reconstitution after exposure to potentially lethal doses of radiation in the nonhuman primate. Health Phys. 2014;106:84–96. doi: 10.1097/HP.0b013e3182a2a9b2. [DOI] [PubMed] [Google Scholar]
- 77.Chambers KA, Harrington NP, Ross WM, Filion LG. Relative alterations in blood mononuclear cell populations reflect radiation injury in mice. Cytometry. 1998;;31:45–52. [PubMed] [Google Scholar]
- 78.Garg S, Boerma M, Wang J, et al. Influence of sublethal total-body irradiation on immune cell populations in the intestinal mucosa. Radiat Res. 2010;173:469–78. doi: 10.1667/RR1742.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harrington NP, Chambers KA, Ross WM, Filion LG. Radiation damage and immune suppression in splenic mononuclear cell populations. Clin Exp Immunol. 1997;107:417–24. doi: 10.1111/j.1365-2249.1997.272-ce1158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pecaut MJ, Nelson GA, Gridley DS. Dose and dose rate effects of whole-body gamma-irradiation: I. Lymphocytes and lymphoid organs. In Vivo. 2001;15:195–208. [PubMed] [Google Scholar]
- 81.Crompton NE, Ozsahin M. A versatile and rapid assay of radiosensitivity of peripheral blood leukocytes based on DNA and surface-marker assessment of cytotoxicity. Radiat Res. 1997;147:55–60. [PubMed] [Google Scholar]
- 82.Kajioka EH, Andres ML, Li J, et al. Acute effects of whole-body proton irradiation on the immune system of the mouse. Radiat Res. 2000;153:587–94. doi: 10.1667/0033-7587(2000)153[0587:aeowbp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 83.Seki H, Iwai K, Kanegane H, et al. Differential protective action of cytokines on radiation-induced apoptosis of peripheral lymphocyte subpopulations. Cell Immunol. 1995;163:30–6. doi: 10.1006/cimm.1995.1095. [DOI] [PubMed] [Google Scholar]
- 84.Asquith M, Haberthur K, Brown M, et al. Age-dependent changes in innate immune phenotype and function in rhesus macaques (Macaca mulatta. Pathobiol Aging Age Relat Dis. 2012;2 doi: 10.3402/pba.v2i0.18052. [S.l.], June 2012. Available at: http://www.pathobiologyofaging.net/index.php/pba/article/view/18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. Am J Transplant. 2012;12:1079–90. doi: 10.1111/j.1600-6143.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 86.Sasaki H, Kodama K, Yamada M. A review of forty-five years study of Hiroshima and Nagasaki atomic bomb survivors. Aging. J Radiat Res. 1991;32(Suppl):310–26. doi: 10.1269/jrr.32.supplement_310. [DOI] [PubMed] [Google Scholar]
- 87.Chernyshov VP, Vykhovanets EV, Slukvin II, Antipkin YG, Vasyuk AN, Strauss KW. Analysis of blood lymphocyte subsets in children living on territory that received high amounts of fallout from Chernobyl accident. Clin Immunol Immunopathol. 1997;84:122–8. doi: 10.1006/clin.1997.4369. [DOI] [PubMed] [Google Scholar]
- 88.Castermans E, Hannon M, Dutrieux J, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011;96:298–306. doi: 10.3324/haematol.2010.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yakoub-Agha I, Saule P, Magro L, et al. Immune reconstitution following myeloablative allogeneic hematopoietic stem cell transplantation: the impact of expanding CD28negative CD8+ T cells on relapse. Biol Blood Marrow Transplant. 2009;15:496–504. doi: 10.1016/j.bbmt.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 90.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–81. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrer IR, Hester J, Bushell A, Wood KJ. Induction of transplantation tolerance through regulatory cells: from mice to men. Immunol Rev. 2014;258:102–16. doi: 10.1111/imr.12158. [DOI] [PubMed] [Google Scholar]
- 92.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci USA. 1989;86:10104–7. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ishihara H, Tanaka I, Nemoto K, et al. Immediate-early, transient induction of the interleukin-1 beta gene in mouse spleen macrophages by ionizing radiation. 1995;36:112–24. doi: 10.1269/jrr.36.112. J Radiat Res. [DOI] [PubMed] [Google Scholar]
- 94.Nemoto K, Ishihara H, Tanaka I, et al. Expression of IL-1 beta mRNA in mice after whole body X-irradiation. 1995;36:125–33. doi: 10.1269/jrr.36.125. J Radiat Res. [DOI] [PubMed] [Google Scholar]
- 95.Stoecklein VM, Osuka A, Ishikawa S, Lederer MR, Wanke-Jellinek L, Lederer JA. Radiation exposure induces inflammasome pathway activation in immune cells. 2015;;194:1178–89. doi: 10.4049/jimmunol.1303051. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. 2005;5:465–74. doi: 10.1111/j.1600-6143.2005.00759.x. Am J Transplant. [DOI] [PubMed] [Google Scholar]
- 97.Changelian PS, Flanagan ME, Ball DJ, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. 2003;302:875–8. doi: 10.1126/science.1087061. Science. [DOI] [PubMed] [Google Scholar]
- 98.Conklyn M, Andresen C, Changelian P, Kudlacz E. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. 2004;76:1248–55. doi: 10.1189/jlb.0504282. J Leukocyte Biol. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Simian varicella virus (SVV) infection, viral load and immunoglobulin (Ig)G titre. All animals in (a) cohort 1, (b) cohort 2 and (c) cohort 3 were infected intrabronchially with SVV at day 0. SVV DNA viral load was measured in whole blood (closed square) and bronchoalveolar lavage cells (BAL, open circle) by quantitative polymerase chain reaction (PCR) using primers and probe specific for SVV ORF21. Average copy number per 100 ng of DNA. (b) SVV-specific IgG antibody end-point titres were measured by standard enzyme-linked immunosorbent assay (ELISA) in (b) cohort 1 (open triangle), (d) cohort 2 (closed triangle) and (f) cohort 3 (closed square) rhesus macaques (RMs). Average ± standard error of the mean (s.e.m.). Solid line indicates limit of detection. Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh).
Fig. 2. The effect of immunosuppression on multiple factors in the plasma. Levels of chemokines, cytokines and growth factors in plasma were determined using multiplex technology in individual rhesus macaques (RMs) from (a) cohort 1, (b) cohort 2 and (c) cohort 3. Dashed line indicates onset or change in immunosuppressive treatment; total body irradiation (TBI), cyclosporin A (CsA), tacrolimus (TAC), prednisone (pred), anti-thymocyte globulin (ATG), anti-CD3 immunotoxin (CD3-IT) and Janus activated kinase inhibitor (JAK-Inh).