The results of our study in an acute terminal ileitis model in pigs suggest that US with dual-selectin–targeted microbubbles can be translated from small- to large-animal imaging with current clinical US equipment.
Abstract
Purpose
To evaluate the feasibility and reproducibility of ultrasonography (US) performed with dual-selectin–targeted contrast agent microbubbles (MBs) for assessment of inflammation in a porcine acute terminal ileitis model, with histologic findings as a reference standard.
Materials and Methods
The study had institutional Animal Care and Use Committee approval. Acute terminal ileitis was established in 19 pigs; four pigs served as control pigs. The ileum was imaged with clinical-grade dual P- and E-selectin–targeted MBs (MBSelectin) at increasing doses (0.5, 1.0, 2.5, 5.0, 10, and 20 × 108 MB per kilogram of body weight) and with control nontargeted MBs (MBControl). For reproducibility testing, examinations were repeated twice after the MBSelectin and MBControl injections. After imaging, scanned ileal segments were analyzed ex vivo both for inflammation grade (by using hematoxylin-eosin staining) and for expression of selectins (by using quantitative immunofluorescence analysis). Statistical analysis was performed by using the t test, intraclass correlation coefficients (ICCs), and Spearman correlation analysis.
Results
Imaging signal increased linearly (P < .001) between a dose of 0.5 and a dose of 5.0 × 108 MB/kg and plateaued between a dose of 10 and a dose of 20 × 108 MB/kg. Imaging signals were reproducible (ICC = 0.70), and administration of MBSelectin in acute ileitis resulted in a significantly higher (P < .001) imaging signal compared with that in control ileum and MBControl. Ex vivo histologic grades of inflammation correlated well with in vivo US signal (ρ = 0.79), and expression levels of both P-selectin (37.4% ± 14.7 [standard deviation] of vessels positive; P < .001) and E-selectin (31.2% ± 25.7) in vessels in the bowel wall of segments with ileitis were higher than in control ileum (5.1% ± 3.7 for P-selectin and 4.8% ± 2.3 for E-selectin).
Conclusion
Quantitative measurements of inflammation obtained by using dual-selectin–targeted US are reproducible and correlate well with the extent of inflammation at histologic examination in a porcine acute ileitis model as a next step toward clinical translation.
© RSNA, 2015
Introduction
Inflammatory bowel disease (IBD) is a group of chronic relapsing disorders, including Crohn disease and ulcerative colitis, with inflammatory changes in the small and large bowel (1,2). Approximately 1.4 million patients are affected in the United States, and the global incidence of the disease is increasing (3). Current therapies for IBD with immunosuppressants and immunomodulators are not disease specific and may have major side effects (2,4). Therefore, ongoing research explores inexpensive, widely available, safe, and noninvasive methods for the quantitative assessment of disease extent that allow better individualization of treatment regimens through earlier differentiation of nonresponders from responders and minimization of drug doses in patients responding to a certain treatment (5). Currently, there is no single test available that fulfills these requirements.
Ultrasonography (US) is an accessible, noninvasive, and safe imaging modality. In particular, in children and young adults with good acoustic windows, US is considered to be one of the first-line imaging modalities for assessing bowel diseases. In IBD, after the diagnosis is confirmed with endoscopy and histologic examination and the extent of bowel involvement is assessed by using magnetic resonance imaging or computed tomographic (CT) enterography, US can be used as a complementary, noninvasive, and relatively inexpensive test to longitudinally monitor inflammation and to differentiate responders from nonresponders early in treatment without radiation exposure. However, the accuracy and reproducibility of current US technology in the quantification of inflammation are limited (6,7).
Molecular imaging with US utilizes contrast agent microbubbles (MBs) that have been modified to enable molecular targeting to proteins upregulated in the inflamed vasculature. This technique has been used to quantify inflammation in small-animal models of IBD (8,9). Leveraging the natural pathway of leukocyte rolling on inflamed vascular endothelial cells, a clinically translatable, dual-targeted contrast agent (10,11) specific for the leukocyte adhesion molecules P- and E-selectin has been shown to enable accurate quantification of inflammation in a murine model of chemically induced colitis (12). Furthermore, in an intra-animal cross-modality comparison (12), dual-selectin–targeted US signal correlated well quantitatively with fluorodeoxyglucose uptake at positron emission tomography/CT in murine colitis. These data suggest that US with a dual-selectin–targeted contrast agent may be further developed as a quantitative tool for monitoring inflammation in IBD. It is critical to address the question of whether selectin-targeted US can be transferred from a murine to a large-animal model of IBD with the use of clinically available US equipment, which would be required for an eventual future translation of this imaging approach to patients with IBD.
The purpose of our study was to evaluate the feasibility and reproducibility of US with dual-selectin–targeted MBs for assessment of inflammation in a porcine acute terminal ileitis model, with histologic findings as a reference standard.
Materials and Methods
Bracco Suisse (Geneva, Switzerland) provided the clinically translatable contrast agent used in this study. The authors who were not employees of Bracco Suisse (H.W., S.A.F., S.M., I.G., R.L., L.T., A.M.L., J.K.W.) had control over inclusion of any data and information that might have presented a conflict of interest for the author who was an employee of Bracco Suisse (T.B.).
Animals
This study was approved by the institutional Animal Care and Use Committee of Stanford University. Twenty-three 11–13-week-old female pigs (Yorkshire; Pork Power Farms, Turlock, Calif; mean weight, 31 kg; range, 25–37 kg) were used in this study. All pigs were housed in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited and U.S. Department of Agriculture–registered facility. Pigs were fed a commercially prepared balanced ration (Nature’s Match Sow and Pig Complete; Purina Mills, St Louis, Mo) ad libitum, with exception of the times 12 hours prior to surgery and 12 hours prior to US. Water was made available at all times.
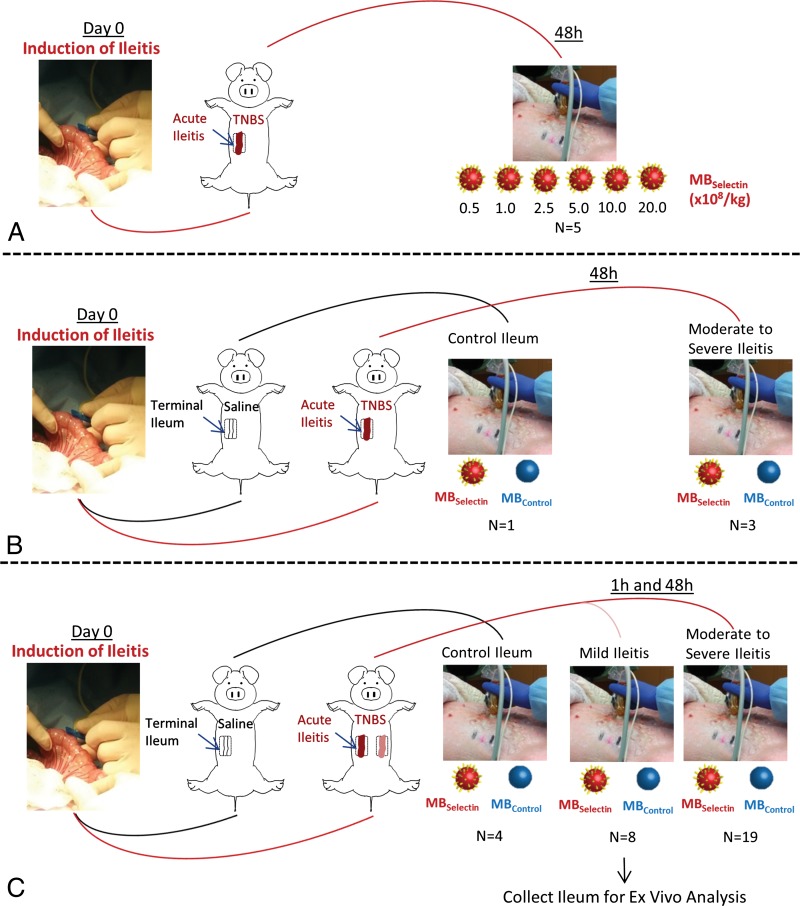
Figure 1 summarizes the overall study design, which included a dose-escalation study of increasing contrast agent doses in a subgroup of five animals, a reproducibility study in a subgroup of four animals, and quantitative correlation of selectin-targeted US with histologic findings in all 23 animals.
Figure 1:
A–C, Overview of experimental design for in vivo dual-selectin–targeted US in pigs shows, A, the testing of different MB doses, B, the testing for reproducibility, and, C, the correlation of imaging findings with pathologic findings. MBControl = control MBs, MBSelectin = dual-selectin–targeted MBs, TNBS = 2, 4, 6-trinitrobenzene sulfonic acid. (For more details on the animal model, please see Appendix E1 [online].)
The acute terminal ileitis model in pigs (Fig E1 [online]) and the preparation of dual-selectin–targeted MBs are described in Appendix E1 (online).
In Vivo US Protocol
All 23 pigs were anesthetized as described in Appendix E1 (online) and were placed in the supine position. One hour before US scanning, 2 L of a highly diluted barium sulfate suspension (VoLumen; E-Z-Em, Lake Success, NY) was administered through a nasogastric tube placed into the stomach to improve small bowel distention and to minimize intraluminal air, according to standard protocols for clinical cross-sectional small-bowel imaging (13–15). Pigs were premedicated with acetylsalicylic acid (Aspirin; Wedgewood, Swedesboro, NJ) administered either orally (3.5 mg per kilogram of body weight, every 12 hours beginning 24 hours prior to US; n = 12) or intravenously (3.5 mg/kg; 5 minutes prior to intravenous contrast agent injection; n = 11) as previously described (16–19).
In all pigs, imaging was performed by an abdominal radiologist (J.K.W., with 14 years of experience) using a clinical US scanner (Acuson Sequoia 512; Siemens Healthcare, Malvern, Pa) and a 15L8W clinical transducer 48 hours after the induction of ileitis. In a subgroup of eight pigs, imaging was also performed 1 hour after induction of ileitis to assess whether selectin-targeted US allows the detection of early changes after inflammation induction. In these eight animals, the same segments of ileum before inflammation induction served as intra-animal control tissue, and the segment was scanned again at 48 hours.
First, the terminal ileum was identified in brightness mode. Transverse selectin-targeted US data sets of acute ileitis were then acquired in contrast pulse sequencing mode (center frequency, 7 MHz; lateral and axial resolution, 200 µm each; transmit power, −18 dB; mechanical index, 0.23; and dynamic range, 80 dB) 4 minutes after intravenous bolus injection of 5 × 108/kg MBSelectin or MBControl through a 20–22-gauge intravenous catheter placed in the auricular vein and followed by a 10-mL saline chaser. Four minutes after the injection of the contrast agents, 160 imaging frames were acquired, which detected signals from both molecularly attached targeted MBs and freely circulating MBs. Then, a 5-second destruction pulse (full transmit power; mechanical index, 1.9) was applied to destroy all MBs in the same field of view. After the destruction pulse, another 160 imaging frames were acquired in the same imaging plane to obtain the signal from freely circulating MBs only. The injection order of MBSelectin or MBControl was randomized in all pigs, and a pause of at least 30 minutes was allowed between injections to allow the clearance of MBs from the preceding injection. To maximize intra-animal data acquisition and to minimize the overall number of animals needed, we imaged between one and five locations (at least 1 cm apart) of either control or inflamed bowel segments per pig. A total of 34 bowel segments with moderate-to-severe ileitis, eight segments with mild ileitis (proximal ileum segments), and 15 segments with no ileitis (normal control bowel) were scanned.
Dose-Escalation Study of Selectin-targeted MBs in Acute Terminal Ileitis
In five pigs with moderate-to-severe acute terminal ileitis, an intra-animal dose-escalation study of the dual-selectin–targeted contrast agent was performed. For this purpose, US with the protocol described above was performed after intravenous injection of increasing doses of MBselectin (0.5, 1.0, 2.5, 5.0, 10, and 20 × 108/kg) with a waiting time of at least 30 minutes between each injection to allow clearance of all MBs from preceding injections.
Reproducibility of Selectin-targeted US
To evaluate the reproducibility of dual-selectin–targeted US, an additional three animals with moderate-to-severe acute terminal ileitis and one control animal were imaged twice, consecutively, after both MBSelectin and MBControl injections (5.0 × 108/kg each) in randomized order in the same session by using the imaging protocol described above. Again, there was a pause of at least 30 minutes between the different injections.
Image Analysis
All US data sets were analyzed offline by one reader (H.W.; a radiologist with 3 years of experience) in a random order using software created in-house with MatLab, version 7.01 R14 (MathWorks, Natick, Mass). The reader was blinded to the bowel segment scanned (ileitis vs normal control bowel), to the MB types (MBSelectin vs MBControl), and to the MB doses. A region of interest (ROI) (mean size, 127 mm2; range, 72–236 mm2) was drawn over the wall of the ileum on transverse images. The magnitude of the imaging signal was calculated as the intensity ratio, defined as the ratio between the average linearized imaging signal amplitude before destruction and the average linearized imaging signal amplitude within the ROI after destruction. This parameter is not influenced by combinations of system settings (eg, gain, which can vary slightly among animals) and the attenuation of acoustic waves through tissue (which can also vary among pigs because of their different body weights).
Ex Vivo Analysis of Ileum Tissue
For each bowel segment imaged, the US locations were marked on the skin of each pig by using a waterproof marker (Sharpie, Oak Brook, Ill) to ensure that about the same area of the bowel segment could be correlated between US and histologic examination. Immediately after US, all pigs were sacrificed by means of intravenous injection of pentobarbital sodium (BeuthanasiaD; Schering-Plough Animal Health, Union, NJ), and the imaged bowel segments were harvested for ex vivo analysis, including hematoxylin-eosin staining (for inflammation grading) and immunofluorescence staining (for assessment of P- and E-selectin expression levels). Details of staining are in Appendix E1 (online).
Statistical Analysis
To assess the reproducibility of selectin-targeted US, we calculated intraclass correlation coefficients (ICCs) along with 95% confidence intervals. An ICC of 0–0.20 suggested no agreement; an ICC of 0.21–0.40, poor agreement; an ICC of 0.41–0.60, moderate agreement; an ICC of 0.61–0.80, good agreement; and an ICC of greater than 0.80, excellent agreement (20). The random-effects model was used for comparisons in multiple groups (control ileum, ileitis at 1 hour, and ileitis at 48 hours). A pig-specific random intercept was used to account for within-pig correlations. Similarly, the trend association between dose level and imaging signal was calculated with linear mixed-effect regression, while accounting for the within-subject correlation by using a random intercept. The signal intensity at different dose levels was estimated, and the incremental value of dose escalation was used to select the optimal dose. Specifically, the improved signal intensity per units of increased dose was estimated from 0.5 to 1.0, from 1.0 to 2.5, from 2.5 to 5.0, from 5.0 to 10, and from 10 to 20 × 108 MB/kg. For assessing associations between US signals and inflammation grades at hematoxylin-eosin staining, the Spearman correlation coefficient was estimated, and the corresponding P values were obtained by using the Fisher transformation. All statistical analyses were performed with statistical software (R software, version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria). The significance level was set at .05.
Results
Dose-Escalation Study of Selectin-targeted MBs
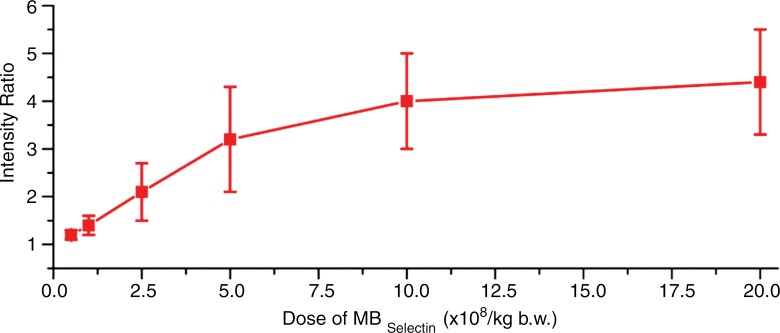
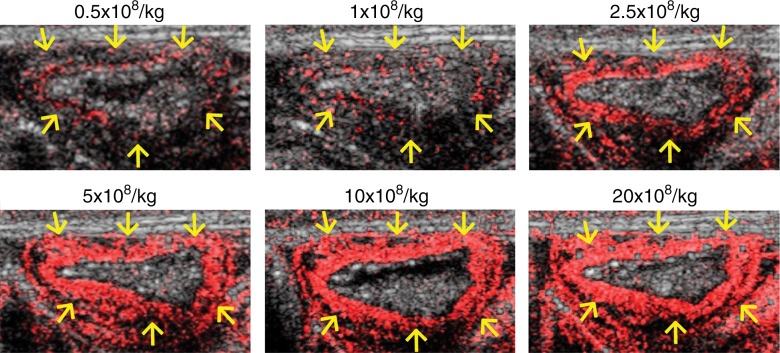
In the subgroup of pigs imaged after administration of different doses of MBSelectin, overall in vivo imaging signal intensities significantly (P < .001) increased with higher contrast agent doses in an approximately linear fashion up to a dose of 5.0 × 108 MB/kg (Fig 2). Signal intensities did not significantly further increase when the dose was increased from 10 to 20 × 108/kg (P = .20), suggesting plateauing of the imaging signal at higher doses.
Figure 2a:
(a) Graph summarizes quantitative US signal intensities (represented as signal intensity ratios) observed in five pigs with acute terminal ileitis with increasing doses (0.5, 1.0, 2.5, 5.0, 10, and 20 × 108/kg) of dual-selectin–targeted contrast MBs (MBSelectin). Error bars = standard deviations, b.w. = body weight. (b) Representative transverse US images obtained six times in approximately the same location of an inflamed ileum segment (arrows) in one pig show increasing imaging signal (overlaid in red onto the anatomic B-mode images) with increasing MBSelectin doses, with the signal plateauing between a dose of 10 and a dose of 20 × 108/kg. Note that US with MBSelectin depicts inflammation not only in the bowel wall but also in mesenteric fat adjacent to the ileum. The different US scans were each separated by a 30-minute pause to allow contrast agent clearance.
Figure 2b:
(a) Graph summarizes quantitative US signal intensities (represented as signal intensity ratios) observed in five pigs with acute terminal ileitis with increasing doses (0.5, 1.0, 2.5, 5.0, 10, and 20 × 108/kg) of dual-selectin–targeted contrast MBs (MBSelectin). Error bars = standard deviations, b.w. = body weight. (b) Representative transverse US images obtained six times in approximately the same location of an inflamed ileum segment (arrows) in one pig show increasing imaging signal (overlaid in red onto the anatomic B-mode images) with increasing MBSelectin doses, with the signal plateauing between a dose of 10 and a dose of 20 × 108/kg. Note that US with MBSelectin depicts inflammation not only in the bowel wall but also in mesenteric fat adjacent to the ileum. The different US scans were each separated by a 30-minute pause to allow contrast agent clearance.
Reproducibility of Selectin-targeted US
Overall, the reproducibility of selectin-targeted molecular contrast enhancement after two consecutive contrast agent injections was good (ICC = 0.70; 95% confidence interval: 0.50, 0.83), and US signal was not significantly different (P = .27) after two consecutive injections.
In Vivo Selectin-targeted US
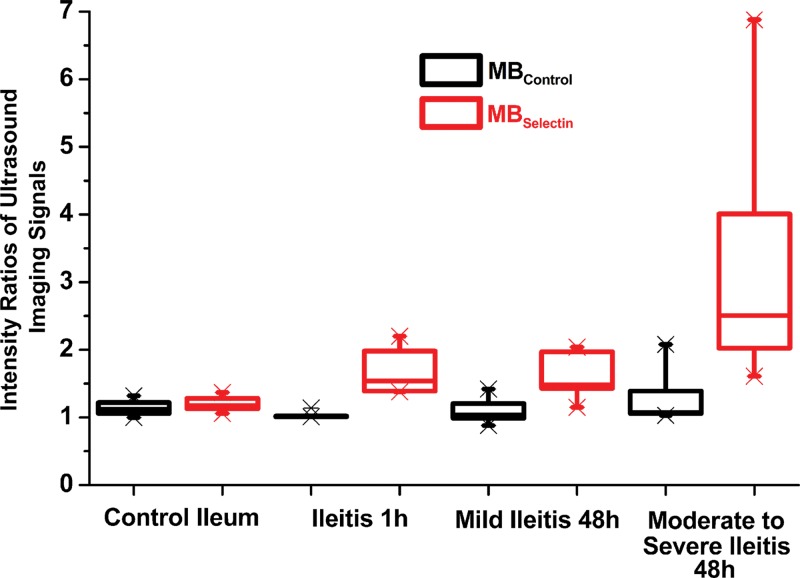
In normal ileum in control animals, the mean imaging signal ratios were only minimal after administration of both MBControl (1.1 ± 0.1 [standard deviation]) and MBSelectin (1.2 ± 0.1), and the imaging signal ratios were not significantly different when either MB type was used (P = .18) (Tables 1, 2; Figs 3, 4). One hour after the induction of inflammation, the dual-selectin–targeted contrast agent–enhanced US imaging signal ratio (1.9 ± 0.4) was significantly increased (P = .01) compared with that in baseline normal ileum in the same animals. Imaging signal ratios (mean, 3.0 ± 1.3) further significantly increased (P = .03) at 48 hours compared with those in normal ileum in control animals (Tables 1, 2; Figs 3, 4). Administration of MBControl resulted in significantly lower imaging signal ratios in the bowel wall in both mildly inflamed bowel (1.1 ± 0.1; P = .003) and moderately to severely inflamed ileum (1.2 ± 0.3; P < .001) (Tables 1, 2; Figs 3, 4).
Table 1.
US Signal Ratios in Control Ileum and Ileitis Obtained in 23 Pigs by Using MBSelectin and MBControl
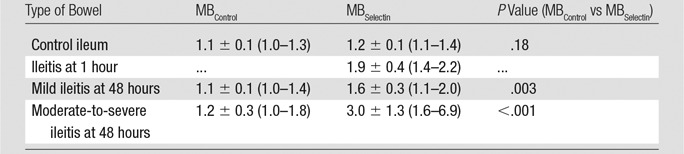
Note.—Data are means ± standard deviations, with ranges in parentheses. The same dose (5.0 × 108 MB/kg) was used for both MBSelectin and MBControl. Imaging signal from attached MBs was calculated as the intensity ratio, defined as the ratio between the average linearized imaging signal amplitude before destruction and the that after destruction within the region of interest. Note that US studies at 1 hour were performed in intra-animal comparison experiments, and histologic inflammation grades were therefore not available because the same bowel segment was also scanned at 48 hours.
Table 2.
P Values for Comparisons of US Signal Ratios in Study Groups
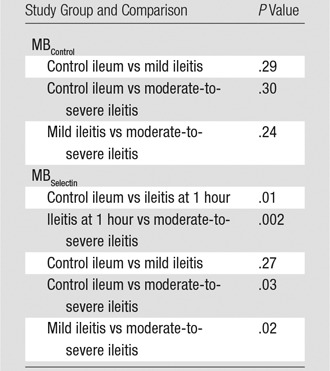
Note.—The same dose (5.0 × 108 MB/kg) was used for both MBSelectin and MBControl. Overall, the imaging signal ratios with MBControl among the groups of control ileum, mild ileitis at 48 hours, and moderate-to-severe ileitis at 48 hours were not significantly different (P = .28), while the imaging signal ratios with MBSelectin were significantly different among the groups of control ileum, ileitis at 1 hour, mild ileitis at 48 hours, and moderate-to-severe ileitis at 48 hours (P < .001).
Figure 3:
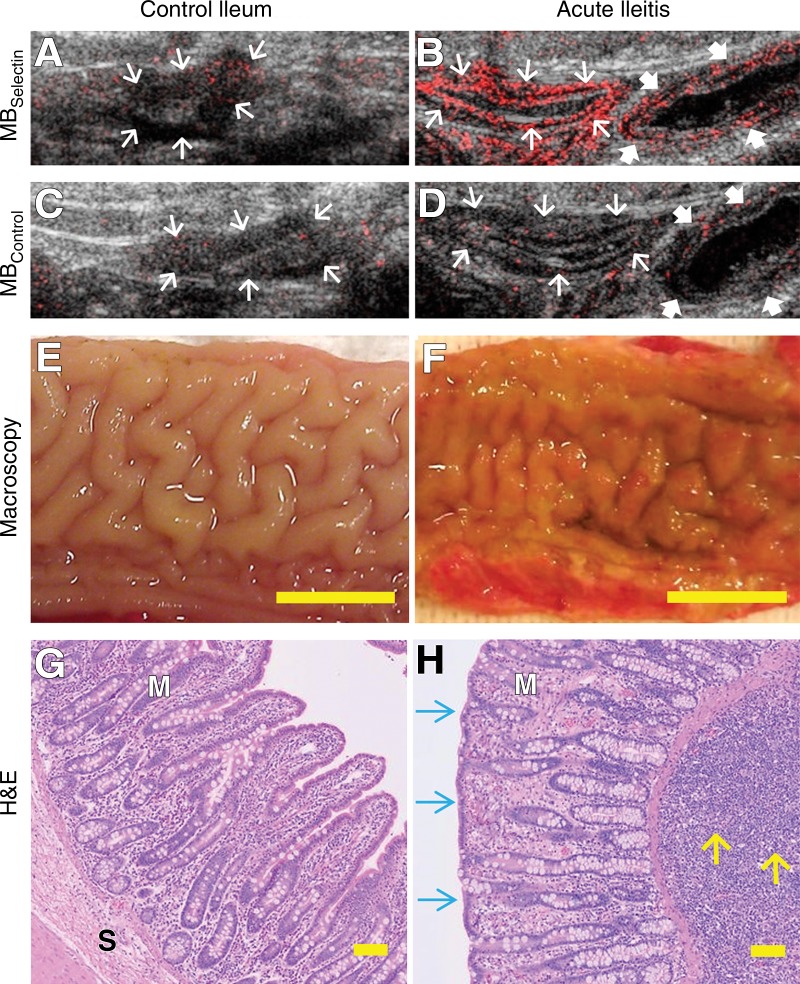
A–D, Representative transverse US molecular images of control ileum and acutely inflamed terminal ileum imaged by using both MBSelectin and MBControl show background signal (arrows) only in, A, control ileum but increased signal in the bowel wall (thin arrows) in, B, severely inflamed bowel after injection of MBSelectin, while injection of MBControl resulted in background signal (thin and thick arrows) in both, C, control and, D, inflamed bowel. Note that an adjacent bowel segment with mild inflammation (thick arrows in B and D ) was also scanned and showed less imaging signal than did the severely inflamed segment. E, F, Macroscopic views of the lumen of the ileum show intact anatomy in, E, normal ileum and red and thickened mucosa in, F, acute ileitis. Scale bar = 1 cm. G, H, Photomicrographs of corresponding ileum tissue slices stained with hematoxylin-eosin (H&E) show normal mucosal (M) and submucosal (S) microanatomy in, G, control terminal ileum and severe inflammation in, H, acute ileitis with alteration of ileal microanatomy, including the presence of blunt and thicker villi (blue arrows) and expansion of the lamina propria and submucosa with a large number of neutrophils (yellow arrows). Scale bar = 100 µm.
Figure 4:
Boxplot shows intensity ratios of US signals obtained by using dual-selectin–targeted MBs (MBSelectin) and control MBs (MBControl) in control ileum and in various degrees of ileitis at different time points (1 hour and 48 hours) after the induction of inflammation. Each box = 25th and 75th quartiles, the line inside each box = the median, and the whiskers = 5th and 95th percentiles, excluding the outliers.
Ex Vivo Analysis
Histologic examination confirmed the presence of moderate-to-severe inflammation in all segments that were directly exposed to TNBS/ethanol (mean inflammation score, 3.2 ± 0.8; range, 2–4; Fig 3). Histologic inflammation grades were significantly lower in segments proximal to the directly exposed segments, with, on average, mild inflammation (mean score, 1.5 ± 0.8; range, 1–4; P = .03). In control animals without TNBS/ethanol exposure, there was background neutrophil inflammation only (1.0 ± 0; P < .001). Overall, there was good correlation between inflammation grades as assessed at histologic examination and selectin-targeted US signal (ρ = 0.79, P < .001).
Consistent with the US findings, quantitative immunofluorescent staining of the ileum showed that both P-selectin (37.4% ± 14.7 of vessels positive; P < .001) and E-selectin (31.2% ± 25.7) expression levels in vessels in the bowel wall of ileitis segments were higher than those in control ileum (5.1% ± 3.7 for P-selectin and 4.8% ± 2.3 for E-selectin). Selectin-positive vessels were often observed in close proximity to neutrophils present in the submucosa and the base of the mucosa (Fig E2 [online]).
Discussion
Our study results show that dual-selectin–targeted US using a clinical US system and clinical-grade contrast agent MBs can be translated to a large-animal porcine acute terminal ileitis model and that US findings correlate well with different grades of inflammation in the bowel wall at reference-standard histologic examination, suggesting that this imaging approach could become a useful tool for noninvasive monitoring of inflammation in IBD.
Pigs are increasingly being recognized as important large-animal models for IBD (21–24). We modified published protocols of chemically induced ileitis using TNBS/ethanol installation (22,23) to create a reproducible and well-tolerated porcine terminal ileitis model. In this model, ethanol breaks the physical barrier of the intestine, allowing TNBS to haptenize intestinal autologous or microbiota proteins. This results in a T-cell–dependent mucosal immune response and transmural infiltration of neutrophils (10,25). We observed that the volume of ethanol can be substantially reduced to 0.8 mL (compared with 40 mL, as published previously [22,23]), which caused moderate-to-severe TNBS-mediated inflammation with minimal discomfort to the animals. Another detail worth noting is the need to premedicate pigs with aspirin, indomethacin, or a specific thromboxane receptor blocker before injecting lipid-based particles such as MBs (16–18), because pigs have abundant intrapulmonary macrophages (26,27). These macrophages rapidly clear intravenously injected particles and immediately release substances such as vasoconstrictor thromboxane A2, with subsequent pulmonary arterial hypertension, systemic hypotension, and decreased cardiac output (19,28).
After optimizing the animal model, we performed a dose-escalation study and showed in intra-animal comparisons that imaging signal increased in a dose-dependent linear manner up to a dose of MBSelectin of 5.0 × 108/kg and then leveled off between a dose of 10 and a dose of 20 × 108/kg. A possible explanation for the signal plateauing at higher doses includes steric hindrance of further MB binding and the masking of still-available receptor binding sites by already attached MBs because of their relatively large size. For all subsequent experiments, we chose a dose of 5.0 × 108/kg, which allowed the measurement of different selectin expression levels in different inflammation grades without a signal plateauing effect. This dose corresponds to 2.6 µL injected gas per kilogram and is very similar to the U.S. Food and Drug Administration–recommended dose for a nontargeted contrast agent MB used in patients in the United States. The recommended dose of the nontargeted perflutren lipid MB Definity (Lantheus Medical Imaging, North Billerica, Mass) is 1.5 µL/kg (corresponding to 10-µL/kg MB suspension) per bolus injection in human patients [29]). At this dose, US was well reproducible in consecutive examinations, and the magnitude of in vivo US signal correlated well with ex vivo histologic findings over a spectrum of inflammation grades.
We also tested how early after an inflammation stimulus a substantial increase in the dual-selectin–targeted US signal can be measured. We observed that as early as 1 hour after acute terminal ileitis induction, US signal was significantly increased. This rapid increase is likely due to the rapid expression of P-selectin on vascular endothelial cells following an acute inflammation stimulus, with efficient attachment of the dual-selectin–targeted MBs to the P-selectin receptor (30,31). P-selectin is contained in Weibel-Palade bodies within vascular endothelial cells and is immediately released, with subsequent appearance on the cell surface within only a few minutes of the stimulus (30,31). This rapid surface expression makes this protein a promising molecular target for imaging hyperacute inflammation, which could be further exploited in, for example, future imaging of transient ischemic events in the bowel. The second target of the MB, E-selectin, is under transcriptional control, and its expression on vascular endothelial cells can be measured approximately 2 hours after acute stimulation (30,32,33). In our study, US signal substantially increased from 1 hour to 48 hours after acute ileitis induction, which is likely a result of increased expression levels of both selectins at the later time point with increased attachment of MBs to both selectins. Immunofluorescence staining at 48 hours confirmed upregulation of both selectins in inflamed bowel tissue. Similar to previous results in mice (12), both selectins were expressed in the capillaries of the mucosa and submucosa in inflamed ileum in pigs.
We acknowledge the following limitations of our study: First, the porcine model was adapted from rodent models (12,34). Although at histologic examination, acute TNBS/ethanol exposure resulted in a phenotype that resembled acute inflammation in patients with IBD, the acute nature of the model did not reflect the chronicity of the disease, which often results in acute-on-chronic changes. Second, in this proof-of-principle study, the imaged bowel was attached to the abdominal wall to allow reliable quantitative comparison between in vivo imaging findings and ex vivo histologic findings. Future studies are warranted to assess how US allows quantification of inflammation in nonattached bowel segments. Third (according to the company’s information), the only commercially available antihuman E-selectin antibody with cross-reactivity against pigs can also reveal human and pig P-selectin. Therefore, the quantitative immunofluorescent staining results for E-selectin may be in part confounded by concurrent P-selectin binding of the antibody. Fourth, we did not assess whether US allows accurate quantification of inflammation longitudinally (eg, during anti-inflammatory treatment).
Practical applications: The results of our study suggest that selectin-targeted US with dual-selectin–targeted MBs can be translated from small- to large-animal imaging in an acute terminal ileitis model in pigs with current clinical US equipment. This is a critical further step toward future clinical translation of this imaging approach for accurate and objective quantification of inflammation in the abdomen. Because US is widely accessible, relatively inexpensive, and does not involve ionizing radiation, selectin-targeted US may have substantial future clinical utility as a complementary imaging modality for diagnosis in and treatment of patients with IBD.
Advances in Knowledge
■ US with a clinical-grade dual P- and E-selectin–targeted US contrast agent was feasible and reproducible (intraclass correlation coefficient = 0.70) and enabled accurate assessment of inflammation of various degrees in a chemically induced acute terminal ileitis model in pigs, with histologic findings as a reference standard.
■ Dual-selectin–targeted US signal in acute terminal ileitis increased linearly between a dose of 0.5 and a dose of 5.0 × 108 microbubbles (MBs) per kilogram of body weight (P < .001) and plateaued between a dose of 10 and a dose of 20 × 108 MB/kg.
■ Inflamed porcine ileum tissue shows strong selectin expression, mostly in capillaries of the submucosa and the base of the mucosa.
APPENDIX
SUPPLEMENTAL FIGURES
Received October 22, 2014; revision requested December 9; revision received December 31; accepted January 16, 2015; final version accepted February 5.
Funding: This research was supported by the National Institutes of Health (grant R01DK092509-01A1).
H.W. supported by the Stanford Dean Fellowship award. J.K.W. supported by Broad Medical Research Program grant IBD-0333 from the Broad Foundation.
Abbreviations:
- IBD
- inflammatory bowel disease
- ICC
- intraclass correlation coefficient
- MB
- microbubble
- TNBS
- 2, 4, 6-trinitrobenzene sulfonic acid
See also Science to Practice in this issue.
Disclosures of Conflicts of Interest: H.W. disclosed no relevant relationships. S.A.F. disclosed no relevant relationships. S.M. disclosed no relevant relationships. I.G. Activities related to the present article: none to disclose. Activities not related to the present article: is an employee of Siemens Healthcare. Other relationships: none to disclose. R.L. disclosed no relevant relationships. T.B. Activities related to the present article: none to disclose. Activities not related to the present article: is an employee of Bracco Suisse (since 2001). Other relationships: none to disclose. L.T. disclosed no relevant relationships. A.M.L. disclosed no relevant relationships. J.K.W. Activities related to the present article: none to disclose. Activities not related to the present article: is a consultant for Triple Ring Technologies and Bracco; institution has received grants from Siemens, Philips, and GE. Other relationships: none to disclose.
References
- 1.Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126(6):1504–1517. [DOI] [PubMed] [Google Scholar]
- 2.Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61(6):918–932. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46–54, e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 4.Ahluwalia JP. Immunotherapy in inflammatory bowel disease. Med Clin North Am 2012;96(3):525–544, x. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher JG, Fidler JL, Bruining DH, Huprich JE. New concepts in intestinal imaging for inflammatory bowel diseases. Gastroenterology 2011;140(6):1795–1806. [DOI] [PubMed] [Google Scholar]
- 6.Nylund K, Hausken T, Gilja OH. Ultrasound and inflammatory bowel disease. Ultrasound Q 2010;26(1):3–15. [DOI] [PubMed] [Google Scholar]
- 7.Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: ultrasound. World J Gastroenterol 2011;17(27):3192–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann C, Klibanov AL, Olson TS, et al. Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 2006;130(1):8–16. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande N, Lutz AM, Ren Y, et al. Quantification and monitoring of inflammation in murine inflammatory bowel disease with targeted contrast-enhanced US. Radiology 2012;262(1):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettinger T, Bussat P, Tardy I, et al. Ultrasound molecular imaging contrast agent binding to both E- and P-selectin in different species. Invest Radiol 2012;47(9):516–523. [DOI] [PubMed] [Google Scholar]
- 11.Hyvelin JM, Tardy I, Bettinger T, et al. Ultrasound molecular imaging of transient acute myocardial ischemia with a clinically translatable P- and E-selectin targeted contrast agent: correlation with the expression of selectins. Invest Radiol 2014;49(4):224–235. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Machtaler S, Bettinger T, et al. Molecular imaging of inflammation in inflammatory bowel disease with a clinically translatable dual-selectin-targeted US contrast agent: comparison with FDG PET/CT in a mouse model. Radiology 2013;267(3):818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maconi G, Radice E, Bareggi E, Porro GB. Hydrosonography of the gastrointestinal tract. AJR Am J Roentgenol 2009;193(3):700–708. [DOI] [PubMed] [Google Scholar]
- 14.Masselli G, Gualdi G. MR imaging of the small bowel. Radiology 2012;264(2):333–348. [DOI] [PubMed] [Google Scholar]
- 15.Ilangovan R, Burling D, George A, Gupta A, Marshall M, Taylor SA. CT enterography: review of technique and practical tips. Br J Radiol 2012;85(1015):876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaim SF, Hazard DR, Hogan J, Peters RM. Characterization and mechanism of side-effects of Oxygent HT (highly concentrated fluorocarbon emulsion) in swine. Artif Cells Blood Substit Immobil Biotechnol 1994;22(4):1511–1515. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZT, Li SL, Cai EQ, Wu WL, Jin JS, Zhu B. LPS induces pulmonary intravascular macrophages producing inflammatory mediators via activating NF-kappaB. J Cell Biochem 2003;89(6):1206–1214. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DB, Duchene DA, Taylor GD, Pearle MS, Cadeddu JA. Contrast-enhanced ultrasound evaluation of radiofrequency ablation of the kidney: reliable imaging of the thermolesion. J Endourol 2005;19(2):248–252. [DOI] [PubMed] [Google Scholar]
- 19.Kaul S, Wei K. When you have eliminated the impossible, whatever remains, however improbable, must be the truth. Eur J Echocardiogr 2009;10(6):713–715. [DOI] [PubMed] [Google Scholar]
- 20.Faria JR, Aarão AR, Jimenez LM, Silva OH, Avelleira JC. Inter-rater concordance study of the PASI (Psoriasis Area and Severity Index). An Bras Dermatol 2010;85(5):625–629. [DOI] [PubMed] [Google Scholar]
- 21.Pouillart PR, Dépeint F, Abdelnour A, et al. Nutriose, a prebiotic low-digestible carbohydrate, stimulates gut mucosal immunity and prevents TNBS-induced colitis in piglets. Inflamm Bowel Dis 2010;16(5):783–794. [DOI] [PubMed] [Google Scholar]
- 22.Negaard A, Loberg EM, Naess PA, Eriksen M, Klow NE. Feasibility of MRI in experimentally induced inflammatory small bowel disease: a pilot study in a porcine model. Dig Dis Sci 2010;55(1):14–20. [DOI] [PubMed] [Google Scholar]
- 23.Merritt AM, Buergelt CD, Sanchez LC. Porcine ileitis model induced by TNBS-ethanol instillation. Dig Dis Sci 2002;47(4):879–885. [DOI] [PubMed] [Google Scholar]
- 24.Rémond D, Buffière C, Godin JP, et al. Intestinal inflammation increases gastrointestinal threonine uptake and mucin synthesis in enterally fed minipigs. J Nutr 2009;139(4):720–726. [DOI] [PubMed] [Google Scholar]
- 25.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007;2(3):541–546. [DOI] [PubMed] [Google Scholar]
- 26.Walday P, Tolleshaug H, Gjøen T, et al. Biodistributions of air-filled albumin microspheres in rats and pigs. Biochem J 1994;299(Pt 2):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brain JD, Molina RM, DeCamp MM, Warner AE. Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species. Am J Physiol 1999;276(1 Pt 1):L146–L154. [DOI] [PubMed] [Google Scholar]
- 28.Szebeni J, Alving CR, Rosivall L, et al. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res 2007;17(2):107–117. [DOI] [PubMed] [Google Scholar]
- 29.Wei K, Main ML, Lang RM, et al. The effect of Definity on systemic and pulmonary hemodynamics in patients. J Am Soc Echocardiogr 2012;25(5):584–588. [DOI] [PubMed] [Google Scholar]
- 30.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res 1996;79(3):560–569. [DOI] [PubMed] [Google Scholar]
- 31.Homeister JW, Zhang M, Frenette PS, et al. Overlapping functions of E- and P-selectin in neutrophil recruitment during acute inflammation. Blood 1998;92(7):2345–2352. [PubMed] [Google Scholar]
- 32.Henseleit U, Steinbrink K, Goebeler M, et al. E-selectin expression in experimental models of inflammation in mice. J Pathol 1996;180(3):317–325. [DOI] [PubMed] [Google Scholar]
- 33.Labow MA, Norton CR, Rumberger JM, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity 1994;1(8):709–720. [DOI] [PubMed] [Google Scholar]
- 34.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 2007;59(11):1073–1083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.