Abstract:
Liver is a vital organ of the human body performing myriad of essential functions. Liver-related ailments are often life-threatening and dramatically deteriorate the quality of life of patients. Management of acute liver diseases requires adequate support of various hepatic functions. Thus far, liver transplantation has been proven as the only effective solution for acute liver diseases. However, broader application of liver transplantation is limited by demand for lifelong immunosuppression, shortage of organ donors, relative high morbidity, and high cost. Therefore, research has been focused on attempting to develop alternative support systems to treat liver diseases. Earlier attempts have been made to use nonbiological therapies based on the use of conventional detoxification procedures such as filtration and dialysis. However, the absence of liver cells in such techniques reduced the overall survival rate of the patients and led to inadequate essential liver-specific functions. As a result, there has been growing interest in the development of biological therapy-based extracorporeal liver support systems as a bridge to liver transplantation or to support the ailing liver. A bioartificial liver support is an extracorporeal device through which plasma is circulated over living and functionally active hepatocytes packed in a bioreactor with the aim to aid the diseased liver until it regenerates or until a suitable graft for transplantation is available. This review article gives a brief overview of efficacy of various liver support systems that are currently available. Also, the development of advanced liver support systems, which has been analyzed for improving the important system component such as cell source and other culture and circulation conditions for the maintenance of the liver-specific functions, have been described.
Keywords: liver failure, hepatocytes, liver support devices, bioartificial liver, bioreactor
Liver controls and affects the metabolic and physiological regulatory processes in the body, including protein synthesis, carbohydrate and fat metabolism, blood clotting, immune system, hormonal responses, detoxification (alcohol, chemical toxins, and drugs), and waste removal. Thus, the liver is a vital organ that plays multifunctional roles for the survival of human life. However, during acute liver failure, supportive administration is required for the large number of metabolic functions of the liver. Currently, the only available treatment is liver transplantation. However, unavailability of a donor liver when required and high cost for transplantation make this process practically difficult. Because of the limited availability of the organ and also constraints for liver transplantation, it is necessary to find alternate ways to temporarily assist the diseased organ until a suitable graft arrives (“bridging transplantation”) or, ideally, until the liver is able to regenerate and regain its full function (“bridging to regeneration”).
Several artificial approaches such as plasmapheresis, hemodialysis, plasma exchange, and hemoperfusion have been used but these approaches showed limited success as a result of the insufficient replacement of all the metabolic functions of the liver. On the other hand, extracorporeal biological treatments have shown some beneficial results. The bioartificial liver (BAL) device uses metabolically active liver cells to address liver-specific functions. They are incorporated in a bioreactor where they are cultured and induced to perform the hepatic functions by processing the blood or plasma of patients with liver failure. Matsumura et al. (1) reported the first clinical use of a liver support system with isolated hepatocytes, in which an artificial liver was developed using a dialyzer with the addition of cryopreserved rabbit liver cells. However, they are difficult to implement in a clinical setting because they are quite expensive and can be used for only a short duration (on the order of hours). Such systems also do not offer a long-term substitute for an individual’s liver and hence are less effective. The performance of currently available bioartificial liver devices that are under clinical trials is also limited by ineffective mass exchange, plasma flow rate in the system, and cell source. In all the available BAL devices, cells do not come in direct contact with patient plasma or blood; however, it is separated by one or even two capillary membranes, which limit proper mass exchange. Also, the flow in a bioartificial liver device is limited because of technical and rheological reasons, e.g., plasma separation rates and shear stress.
LIVER, AN ESSENTIAL ORGAN
The liver is the largest vital organ of our body weighing approximately 1500 g in a healthy adult. It is a reddish wedge-shaped, four-lobed organ strategically located in the abdominal cavity (2). Internally, liver lobes are made up of microscopic units called lobules, which are roughly hexagonal in shape. These lobules constitute central point radiated rows of liver cells, i.e., hepatocytes, which comprise approximately 70% of the liver mass. The rows of hepatic cells are in close contact with blood-filled sinusoids from one side and the other side exposes toward canaliculi into which bile is secreted. The phagocytic Kupffer cells are present at the lining of sinusoids and help in filtering out debris and bacteria from the blood (3). At the corner of each lobule, the portal triad is present, which is composed of the hepatic artery, hepatic portal vein, and bile duct. The hepatic artery (from the heart) provides oxygenated blood and hepatic portal vein (from intestine) provides deoxygenated blood to the liver. Impure blood passes through sinusoids where the hepatic cells detoxify it and is finally drained out through the central vein, which unites with the hepatic vein. Metabolic wastes and drug products may form part of the bile, which moves from the bile duct to the gallbladder and finally to the duodenum followed by the excretion from the body through the feces (4–7).
Liver Functions
The liver is a complex metabolic bioreactor of the body performing a myriad of functions. It is multifunctional and is estimated that it performs over 200 functions (8,9). Even, until now, many of the liver-specific processes are not yet fully understood. Some of the vital ones are as follows:
Synthesis:
Some of the essential components of blood are manufactured by the liver including approximately 95% of plasma proteins, blood clotting substances (fibrinogen, prothrombin, etc.). Albumin synthesized by the liver is responsible for maintenance of osmotic pressure of plasma. It is also involved in the synthesis of lipoproteins.
Metabolism:
Liver cells assimilate carbohydrates, fats, and proteins. They convert excess concentration of glucose into glycogen and mainly stored in the liver, which can reconvert into glucose when required by the body. Gluconeogenesis (production of glucose from sources other than carbohydrates) is also carried out by the liver. Digested proteins (amino acids) are further broken down by deamination. Bile acids secreted by the liver aid in adequate digestion and absorption of fats.
Detoxification:
The liver transforms potentially dangerous metabolites, toxins, and excess hormones into biologically harmless water-soluble compounds. This transformation is carried out by the expression of a class of enzymes called cytochrome-oxidase P450. For example, toxic ammonia, a waste product of protein breakdown, is transformed into the less toxic urea, which is further removed through body fluids.
Storage:
The liver is a major storage organ of various essential nutrients like glycogen, vitamins, and minerals including vitamins A, D, K, and B12.
REGENERATION, A UNIQUE PHENOMENA
The extraordinary ability of the liver to regenerate from resection or severe injury has fascinated researchers, physicians, and secular from several decades. Theogony of Hesiod in the 8th to 7th century B.C. first described the regenerative capacity of the liver. According to Greek mythology, Prometheus, a Titan, angered Zeus by stealing a glowing ember with a piece of reed. So, as punishment, Prometheus was chained to a cliff in the Caucasus Mountains where Zeus anguished him by sending an eagle each day to guttle his liver. Nightly, his damaged liver regenerated leaving him fit and prepare for the next day’s torture. The ability of the human liver to regenerate was not scientifically documented until 1890 (10). Despite the large metabolic burden, the liver is a largely dormant organ where cell proliferation along with only .01–.001% hepatocytes goes under mitosis division at any given time. This normative biological event in healthy liver tissue, however, is influenced during severe liver injury or a surgical process, which results in sudden, massive hepatocyte proliferation, actuates recuperation of functional liver mass within 2 weeks even after the loss of up to two-thirds of the liver (11–14).
Profile of Liver Diseases
The development of liver failure is a slow process and its progression usually goes unnoticed until it becomes life-threatening. The maladies that affect the liver are many. The etiology of the acute liver failure (ALF) is because of the various heterogeneous noxious agents. These could be viral (hepatitis viruses A, B, C, D, and E and other viruses like adeno, yellow fever, and cytomegaly), toxic (drug-induced, poison), vascular (Budd-Chiari syndrome), systemic (autoimmune hepatitis, copper metabolism-induced Wilson’s disease), etc. The increase in the levels of numerous endogenous substances such as ammonia, bilirubin, lactate, glutamate, mercaptans, free fatty acids, phenols, aromatic amino acids, etc., shows toxicity in the pathogenesis of liver failure (15). It occurs in persons of all ages, but it is most common among 25-to 65-year age group. The causes of liver damage include drug overdoses, surgical complications, metabolic disorders, and trauma. These cause impairment of vital liver functions such as lack of nutrients secreted by the liver and accumulation of toxins in the body, which culminates in multi organ failure (16). Saturation in the detoxification pathway occurs when the level of these toxins reaches a higher range, which leads to the production of cytokines and hepatocellular apoptosis and necrosis results in impairment of the patient’s liver. In most cases, liver cirrhosis is caused by hepatitis B or C virus infection, alcohol abuse (the most common reason in the United States), and higher concentration of iron in the body (hemochromatosis) (17,18). Some of the common ones are hepatic encephalopathy (affecting the brain functions when the liver does not remove toxins from blood), coagulopathy (clotting disorders resulting from the defect in the body’s coagulation mechanism), hepatitis (inflammation of the liver as a result of viral infection or alcohol abuse), liver cancer (like metastatic liver cancer, hepatocellular carcinoma), autoimmune hepatitis (body’s immune cells attack to liver cells), cholestasis (obstruction of flow of bile from the liver to the duodenum), kidney failure, etc., which can be either acute or chronic. The hepatitis B or C-affected patients are at higher risk for liver cancer, even if they do not have scarring of liver (19). Currently common methods to examine the liver diseases are abdominal ultrasound, liver biopsy, abdominal computed tomography scan, serum alpha fetoprotein analysis, liver function tests, etc.
Alcoholic liver disease, a major liver disease, is generally caused by drinking too much alcohol. In general, the alcohol is absorbed into the bloodstream from the intestine and stomach, which first goes to the liver where liver enzymes metabolize the alcohol into other chemicals and later convert into carbon dioxide and water, which goes out from the lung and in the urine. The capacity of processing of alcohol by liver cells is limited to a certain volume per time. So, uptake of a high volume of alcohol can lead to three types of liver conditions, i.e., cirrhosis, fatty liver, and hepatitis (20).
The hepatitis is the inflammation of liver which could be acute and chronic. A group of viruses cause hepatitis worldwide. Hepatitis B and C spread mainly through contaminated intravenous needles, sexual contact, and contaminated blood and blood products, which may cause cirrhosis, chronic hepatitis, liver cancer, liver failure, and death. The kinetics of hepatitis C have been studied, which suggested risk of reinfection after cadaver liver transplantation (19).
The most life-threatening syndrome is hepatic encephalopathy wherein intracranial pressure increases and leading to central nervous system complications like brain herniation (compression of the brain as a result of one of the bony prominences within the skull) and hepatic coma, which is often the cause of death. Acute liver failure can be either fulminant hepatic failure or subfulminant hepatic failure depending on the appearance of hepatic encephalopathy (21,22). The mortality rate of patients with liver diseases is up to 90%. Chronic liver disease is the tenth leading cause of death in the United States as per the American Liver Foundation report, which costs approximately $10 billion in annual healthcare costs. According to the World Health Organization database, approximately 20 million people worldwide have liver cirrhosis and/or liver cancer out of approximately 500 million peoples (almost 10% of the world population) who are stricken with persistent hepatitis B or hepatitis C viral infections. It is estimated worldwide that approximately one to two million people die each year as a result of liver-related diseases and among them, approximately 50,000 deaths per year are accounted only in the United States (23).
Therapeutic Treatment Modalities
Because of the multifunctionality of the liver, the management of ALF requires support of a large number of metabolic functions performed by the organ. Until now, the only proven treatment modality is liver transplantation (24). However, liver transplantation is beset by a scarcity of donor livers and a lack of prompt availability. Statistical analysis in the United States alone reveals that approximately 4,000 donor livers are available per year, whereas approximately 17,000 patients stay on the liver waiting list (25). These analyses demonstrate that a significant number of patients with liver failure are left waiting for biocompatible liver transplantation from cadaveric or, in rare cases, from living donors. In addition, the number of liver transplants does not function properly in the postoperative period. Conclusively, liver transplantation is a highly expensive operation, which ranges from $150,000–$250,000. However, approximately $230,000 is required for the immediate operations with the estimated annual follow-up costs approximately $15,000–$25,000 reported by American Liver Foundation (26). The growing disparity among the availability of liver from donors, number of patients waiting for transplantation, and high costs calls for the need of alternatives (26). Hepatocyte transplantation at the target site is an alternate. However, implanted hepatocytes have chances of aggregation in distal portal branches, sinusoids, and central vein resulting in portal hypertension and necrosis (27). Another treatment approach is the use of implantable constructs with the seeded hepatocytes. This will essentially be the replacement of the native organ with the construct and thus require mimicking the native architecture of liver, which is a complex cellular structure. Also, there will always be a delay because of lag between implantation and onset of function result in the chances of immune rejection (28). Transgenic animals can also be harvested and their livers can be used as a xenograft for transplantation. However, there are high chances of transmission of animal zoonosis (infection) and immune rejection. Xenotransplantation also raises ethical issues because people are skeptical about using an animal organ as a spare part and thus it requires the consent of the patient’s family member (29). A very effective treatment procedure involves the use of extracorporeal devices, which can be used to perfuse the patient’s blood or plasma through them outside the body and give time for the liver to regenerate and clinically allow stabilization of the patient’s condition until the transplant is available (30).
Several extracorporeal devices for liver support have been investigated or developed, which can be split into two major categories, nonbiological and biological support systems.
Nonbiological Liver Support Systems:
The nonbiological systems are based on artificial kidney dialysis principles and rely mainly on blood detoxification (30). Hemodialyses, hemoperfusion over charcoal or resins or immobilized enzymes, plasmapheresis, and plasma exchange have all been explored (31–33). For many years, it was assumed that the cause of hepatic coma resulting from the accumulation of small (less than 5 kDa) dialyzable toxins in the blood and many strategies were designed to remove these toxins (34). However, the liver is a complex organ that is capable not only of performing detoxification, but also of carrying out biotransformation, synthesis of many important plasma proteins, and regulation of multiple metabolic pathways. However, the success of these nonbiological approaches is limited as a result of the role of the liver (synthetic and metabolic functions) is inadequately replaced by these systems.
Biological Liver Support Systems:
The biological systems use viable liver cells as their active components. Among the available biological liver support systems, use of extracorporeal liver support devices is very effective (35). This approach exploits the fact that the liver has an indigenous property to regenerate and in many patients, if short-term hepatic support is provided, the damaged state of the liver can be reverted. The primary aim of extracorporeal liver support devices is to aid the liver until it regenerates, thereby bridging to regeneration (36). In critical cases in which the liver is severely damaged, transplantation remains the only option, whereas an extracorporeal liver support device can assist the diseased liver until a suitable graft arrives and thus bridging to transplantation (37). Various available cell-free extracorporeal techniques like hemofiltration, hemoperfusion, hemodialysis, etc., primarily are involved with only detoxification and no synthetic and metabolic functions. Within Duke University Medical Center, researchers have designed a venovenous circuit for ex-vivo perfusion using pig liver and suggested the possibility of a diagnosis of liver failure with liver perfusion outside the body. In the experiment, a pig liver was removed and transported on ice with three connected catheters in place. These catheters were placed in the patient’s body as per the approved protocol. The series of pig livers for the treatment of different age group patients (from 22–56 years old) were used for the treatment of human liver failure. During these medical practices, only a 22-year-old male patient remains well with normal liver function after an extensive cycle of pig liver perfusion followed by liver transplantation (38). The main aim of BAL treatment is to maintain liver function for a relatively short duration, but it does not provide a long-term replacement for an individual’s liver (39). Therefore, the candidates for BAL therapy are expected relatively short duration support (Figure 1). The bioartificial liver (incorporated with hepatocytes) constituted with an extra-corporeal blood circuit and bioreactor is a developing novel medical technology still in its infancy (40). Currently nine bioartificial liver support devices are undergoing various stages of clinical trials (41).
Figure 1.

A proposed set up of bioartificial liver (BAL) system. The system comprises the filtration of patient venous blood passes through plasma separator unit connected to oxygenator unit under a controlled temperature. Furthermore, the plasma is then passed through hepatocytes activated bioreactor and return back to the patient body along with the blood cells.
Engineering the Bioartificial Liver
Designing the bioartificial liver system involves optimization of various parameters, a biological component, bioreactor design, and perfusion systems. A bioartificial liver should ideally be configured with certain important components, which are discussed subsequently.
Cellular Component of a BAL: Choice of Hepatocytes
The choice of the cellular component plays an important function in the execution of a BAL device. The selected cells for this purpose must retain an as-yet-unidentified differentiated function necessary to attenuate the intracranial ramifications of liver failure (42). Both primary xenogenic hepatocytes and human cell lines have been used in the development.
Primary Hepatocytes:
Isolated liver cells exhibit a variety of specific functions; on the other hand, types of transformed cells typically lack subsets of these functions. Most of the BAL devices use primary porcine hepatocytes because a porcine liver has physiological similarities to the human liver, which are undergoing preclinical and clinical evaluation (43). The use of porcine hepatocytes have some drawbacks that the porcine proteins produced by these cells may induce immunogenic reactions and that primary hepatocytes often retain their differentiated functions for only a short period of time. Another possible risk associated with the use of porcine liver cells is that the transfer of viral pathogens from the xenograft donor to the recipient, like porcine endogenous retrovirus, porcine cytomegalovirus, and porcine lymphotropic herpesvirus (44,45).
Cell Lines:
The continuous cell lines are also used by various research groups (46–48), which often lose functions that cell possess in vivo. In addition, the cells should not be tumorigenic or shed viral particle (49). The most commonly used cell line for such purpose is human hepatoblastoma cell line (HepG2). The advantages of the HepG2 cell type are that it is readily available and importantly it produces human proteins. It also appears that the device with the continuous cell line allows a longer operating time in comparison to the devices that use primary hepatocytes. Besides some advantages, there is concern that HepG2 are tumor-derived cells, which may not perform fully differentiated metabolic functions or undergo spontaneous mutations or changes in gene expression may occur during culture that can lead to become a theoretical threat of patient seeding (50).
The differentiated liver function of isolated primary hepatocytes quickly loses in culture if not supplemented with suitable attachment substrate. The culture of hepatocytes in the form of spheroids is a promising mode of providing anchorage. They remain viable for several weeks in a suitable culture system and also exhibit differentiated liver function. Unlike isolated hepatocyte growth on culture substratum, the culturing of hepatocytes in the form of spheroids (aggregates cell mass) has been found to effectively enhance liver functions. Thus, the use of hepatocyte spheroids in the bioartificial liver should facilitate the improved performance of artificial liver support systems (51).
Human-Derived Stem Cells:
Hepatocytic cells can also develop by the induction of undifferentiated embryonic stem cells under specific culture conditions in vitro. These cells would be a potential candidate for developing BALs (52).
Quantity of Cells Required:
For a device to become a clinical reality, the scaling up is an essential and important parameter that provides effective therapy. The studies suggested that the estimated 10–30% of liver mass is needed to sustain life, which accounts for 150–450 g of normal liver, which is equivalent to 1 × 1010–3 × 1010 liver cells (normal cell count is 1 × 1011) (53).
Hepatocyte Preservation and Storage
Cryopreservation of hepatocytes makes the logistics of extracorporeal liver support easier, simpler, and less costly. When needed in emergency on short notice, they can come in handy and circumvent ad hoc conditions. However, cryopreservation results in loss of viable cells (approximately 10–20%). Also, cryopreserved cells are not metabolically active like freshly isolated cells (54). So, there is great need to improve survival for such cryopreserved cells.
Bioreactor Design
Progressive innovation in engineering and material science has created designs tailored for use with hepatocytes. Available bioreactors are based on hollow fiber, flat membrane, perfused beds, or packed bed technology (Figure 2) with their inherent advantages and disadvantages.
Figure 2.

Available bioreactor designs: (A) hollow fiber reactor; (B) flat membrane reactor; (C) perfused bed reactor; and (D) packed bed bioreactor, which are currently being evaluated for use in BAL [Figure reproduced with permission from Allen JW, et al. Hepatology 2001;34:447–55].
Hollow Fiber Design:
Six of the available bioreactors are based on hollow fiber technology. In such modules, cells are either seeded inside or outside the fiber lumen and the fluid flows on the other side. Hollow fibers provide a large surface area for cells to adhere and cells face less shear stress. However, the hollow fiber membrane is typically characterized by its molecular weight cutoff. Thus, the hollow fibers prevent the exposure of the immune component to bioreactor cells and also obstruct the release of larger xenogenic substances into circulation. Membrane molecular weight cutoff ranging between 100 and 150 kDa is generally sufficient, but it results in the inefficient mass transfer. Also, because the BALs are meant for single-time use and are a disposable system, the use of hollow fiber technology is quite expensive for such purpose (55–57).
Flat Membrane Design:
In this design, cells are seeded on flat plates and blood is made to pass on it. This flat system ensures uniform seeding of cells but such systems are difficult to scale up. Also, there is large dead volume leading to less surface area for cells to grow (58).
Perfused Bed Design:
This configuration is inspired from the native architecture of liver wherein plasma passes radially from the center to the periphery of the modules. This process effectively transfers the nutrients and toxins. However, because there is a lack of extensive sinusoidal system like liver, there is nonuniform perfusion of plasma (59).
Packed Bed Design:
In this module, liver cells are encapsulated and then packed in a fixed bed column through which blood or plasma is passed. This system ensures a uniform microenvironment for cells to grow, but it is limited with the inefficient mass transfer resulting from the restriction of cells by membrane.
Another major limitation of current BAL designs is the common deficiency of functional biliary excretion into an isolated compartment, which can lead to accumulation of detoxified products at later stages (60).
Blood vs. Plasma Perfusion Systems
In the BAL system, the perfusion of whole blood has an advantage because of the presence of erythrocytes, which serve as oxygen-delivery vehicles. However, the damage of cells and leukocyte activation may take place (61). On the other hand, the viability of hematopoietic cells maintained during the plasma perfusion and plasmapheresis process but the dissolved oxygen concentration in plasma is reduced. Proponents of whole blood perfusion systems argue that plasma separation complicates liver support, increases cost, and increases the extra-corporeal priming space. On the other hand, the use of whole blood would require an anticoagulant like heparin to prevent thrombosis within the circuit, greatly increasing the risk of bleeding in patients with coagulopathy from liver failure (62).
Cryogel Bioreactor: Future Design for the BAL Device
Technologies for efficient cell support system for various biomedical applications are poorly developed today. It is not surprising because the cell supportive matrices entail great difficulties during surgery. Our group has designed and developed smart polymeric supermacroporous matrices, i.e., “Cryogel,” for different applications in biomedical research. Cryogels are gel matrices that are formed in moderately frozen solution of monomer or polymeric precursors (63,64). Cryogel has been established as a potential cell support matrix (65–69). A system of large interconnected pores (up to 200 μm) is a main characteristic feature of Cryogels. The interconnected porous architecture in Cryogel provides an internal capillary network through which flow of media can easily occur that result in using the cells that are being cultured on the porous surfaces of the Cryogel bioreactor. These supermacroporous polymeric matrices have been successfully developed to culture hybridoma cells for long-term continuous production of monoclonal antibodies (70). Essentially the continuous pore structure in Cryogel helps to mimic currently most widely used bioreactors for hepatocyte culture in BALs, i.e., hollow fiber bioreactor. This provides cell growth conditions that are very similar to in vivo conditions. Generally the cells are grown as a monolayer in hollow fiber BAL, which does not provide efficient liver functions, although in case of Cryogel bioreactors, the cells can grow as spheroid leading to efficient functioning of cells. The unhindered transport of nutrients and a three-dimensional homogenous environment for growth may help the attached cells to grow and perform better. The high porosity of Cryogels minimizes a mass transfer barrier by practically providing unrestricted nutritional transport both by convection and diffusion, which is also a constrain in presently developed hollow fiber bioreactors in which convectional transport is limited. Cryogel matrices also offer higher surface area than the conventional hollow fiber bioreactors. Moreover, cartridge clogging is a frequent occurrence in case of hollow fibers. To circumvent these limitations, Cryogels can be used as an efficient alternative. Use of Cryogels for designing bioreactors will have dual functionality resembling a packed bed bioreactor and also mimic hollow fibers, which provide advantages of both of the designs. Moreover, Cryogel-based bioreactor designs can be very much cost-effective, more so than both the hollow fiber and packed bed bioreactor designs. These findings suggest the potential use of Cryogel as an extracorporeal bioartificial liver support system. Different composite Cryogel matrices have been developed using biocompatible synthetic and natural polymers like polyacrylamide, polyacrylonitrile, chitosan, etc. The selection is based on the polymer characteristics and the hydrophilic and hydrophobic characteristics. These polymeric Cryogels are further screened for growth of HepG2 (Figure 3) and examined for differentiated liver function. The Cryogel bioreactor can be designed in different formats like monolithic column, discs and beads, etc. The different formats may affect the growth of cells by the difference in oxygen and nutrition supply to cells. Immobilizing and maintaining functional hepatocytes in a three-dimensional monolithic Cryogel bioreactor brings together the multidisciplinary expertise of polymer chemistry, cell biology, bioengineering, and biomedical technology with an aim of developing efficient means of treatment of ALF.
Figure 3.

Scanning electron micrographs of HepG2 cells cultured for 10 days on polyacrylonitrile Cryogels (unpublished data from authors).
Current Status of BALs
From the last 20 years, almost nine BAL systems have been clinically tested and most of them use a hollow fiber technology. A large number of BAL systems in preclinical tests has been analyzed and it is felt that they show increased functional performance (71–73). Some of them are the HepatAssist, extracorporeal liver assist device (ELAD), the bioartificial liver support system, the modular extracorporeal liver support (MELS), the Academic Medical Center bioartificial liver, the liver support system, and the radial flow bioreactor (74–77). Most of these systems are based on hollow fiber technology and are in Phase I/II clinical trials. Others like UCLA-BAL and PUC-BAL are based on packed bed technology, flat membrane technology, or encapsulation technology and are in preclinical stages. Here we have discussed in detail a few of the BALs, which are in various stages of clinical trials. Also, comparisons of various available BAL devices in brief have been described in Table 1.
Table 1.
Comparison of the available bioartificial liver approaches.
| BAL Designs | Features | Cell Source | Cell Growth Time | Merit/Demerits | Success |
|---|---|---|---|---|---|
| Flat membrane | Sandwiched cultivation of cell in flat configuration on membranes | Pig | <1 month | Advantages: | Preclinical trials going on in rats and pigs |
| Rat | 1. Uniform cell distribution | ||||
| Humans | 2. Ease of scale up | ||||
| 3. In vivo like microenvironment | |||||
| Disadvantages: | |||||
| 1. Large dead volume | |||||
| 2. Low surface area/volume ratio | |||||
| 3. Susceptible to viral infection | |||||
| Hollow fiber system | Cell cultivation inside or outside the tubes in tube shell configuration | Rat | <25 days | Advantages: | Clinical trials conducting on ELAD, HepatAssist LSS, BLSS, RFB-BAL, AMC-BAL |
| Pig | 1. large surface area/volume ratio | ||||
| 2. Efficient exchange of nutrients and waste material | |||||
| 3. Porous system for cell support | |||||
| 4. High product recovery | |||||
| 5. Immunoisolation | |||||
| 6. Ease of scale up | |||||
| 7. In vivo like microenvironment | |||||
| Disadvantages: | |||||
| 1. Expensive | |||||
| 2. Nonuniform cell distribution | |||||
| 3. Ineffective convective mass transfer | |||||
| Packed bed | Beads used to entrap cells and then packing them in a column | Rat | <16 days | Advantages: | Preclinical trials of UCLA-BAL |
| Pig | 1. High surface area/volume | ||||
| 2. In vivo-like microenvironment | |||||
| 3. Ease of scale up | |||||
| 4. Immunoisolation | |||||
| 5. High capacity for cell mass | |||||
| Disadvantages: | |||||
| 1. Risk of release of cells from beads | |||||
| Spheroids culture technology | Culturing of cell aggregates called spheroids on substratum | Rat | <21 days | Advantages: | Preclinical trials on PUF-HALSS |
| Pig | 1. Cell-to-cell contact | ||||
| 2. Ease of scale up | |||||
| Disadvantages: | |||||
| 1. Limited oxygen and nutrient supply | |||||
| 2. Chances of viral infiltration |
ELAD, extracorporeal liver assist device; LSS, liver support system; BLSS, bioartificial liver support system; RFB, radial flow bioreactor; AMC-BAL, Academic Medical Center bioartificial liver.
HepatAssist 2000 Device
The HepatAssist (Circe Biomedical Inc., Lexington, MA) extracorporeal device is composed of a polysulphone-based hollow fiber bioreactor with a cellulose-coated activated charcoal column that is functional to remove inorganic toxins and an oxygenator used to oxygenate the hepatocytes (78). Demetriou’s laboratory performed HepatAssist therapy on the patients with liver failure using frozen porcine hepatocytes (79). In general, sodium citrate, an anticoagulant, is used to prevent thrombosis within the system. Porcine hepatocytes are contained within the extrafiber space of a hollow fiber module and attached to collagen-coated Dextran microcarriers. The patient’s plasma is allowed to circulate through these hollow fibers. After this, the clean plasma is reconstituted with the blood stored in the plasmapheresis and the whole blood is reinfused into the patient (Figure 4).
Figure 4.

Schematic representation of HepatAssist device. The device basically consists of A) plasmapheresis device, B) pump, C) plasma reservoir, D) charcoal column, E) oxygenerator, F) bioartificial liver (hollow fibre cartridge), G) poricine hepatocytes, H) plasma and I) hollow fibre. [Figure reproduced with permission from Kobayashi et al. J Artif Organs 2003;6:236–44].
Extracorporeal Liver Assist Device
ELAD (Vitagen Inc., formerly Hepatix Inc., La Jolla, CA) has created the first medical device to incorporate “immortalized” human liver cells, i.e., “C3A” cell line. This cell line is a highly differentiated clonal population isolated from a human hepatoblastoma cell line (HepG2). Cells are originally seeded and grown in the extracapillary space of a hemodialysis cartridge containing approximately 10,000 cellulose acetate-based hollow fibers (80). The device is then perfused with the patient’s plasma through the cartridge containing hepatocytes. A hemodialysis-type catheter carries blood to an ultrafiltration device with the ultrafiltrate (plasma) flowing through the ELAD being exposed to the metabolic activities of the immortalized liver cells, which execute some activities like gluconeogenesis, ureagenesis, and P450 activity but not all of the normal liver’s metabolic function. VitaGen C3A cells in the ELAD cartridge produce several human liver-specific proteins, metabolize drugs, use galactose, and reproduce very well in culture (50). Finally, the treated plasma is then filtered and returned back to the patient body (Figure 5).
Figure 5.
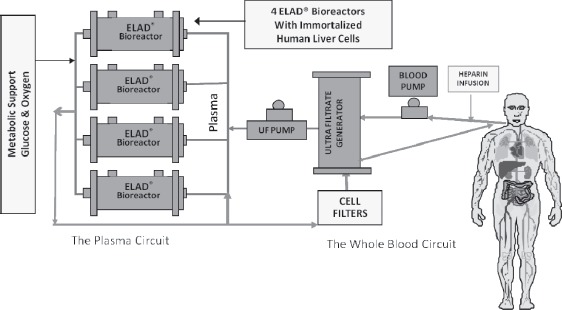
Schematic illustration of extracorporeal liver assist device (ELAD) designed by Vitagen Inc. ELAD uses immortalized human liver cells seeded cellulose acetate based hollow fiber bioreactor. [Figure courtesy of Vital Therapies Inc. TM, CA].
Modular Extracorporeal Liver Support
MELS is designed by combining the different extra-corporeal medical devices together. The cell module is composed of a bioreactor loaded with primary human liver cells isolated from discarded donor livers, which are unsuitable for transplantation. On the other hand, removal of albumin-bound toxins is governed by the detoxification module through single-pass albumin dialysis. In this device, an additional dialysis module performing continuous venovenous hemodiafiltration is also used (Figure 6) (81). The bioreactor consists of hollow fiber membranes and hydrophobic membranes interwoven to four independent compartments. Of these four compartments, two allow perfusion of the liver cells with the patient’s plasma and one is used for integrated oxygenation as well as carbon dioxide elimination. The fourth compartment used for the seeding of liver cells between the hollow fibers. In between the void space of fibers, cells grow, adhere, and form active cell aggregates. This leads to the hollow fibers getting interwoven into a three-dimensional capillary network with numerous subunits enabling exchange of decentralized mass with an adequate amount of nutrient supply and effective metabolite removal (82).
Figure 6.

Schematic representation of Modular Extracorporeal Liver Support (MELS). The MELS consists of a multi-compartment hollow fiber bioreactor (CellModule), a toxin removal albumin dialyzer (DetoxModule) and a kidney dialyzer (DialysisModule). The system uses multi-interwoven independent compartments, where cells are present in separate compartment with hollow fibers. [Figure reproduced with permission from Kobayashi et al. J Artif Organs 2003;6:236–44].
Future Aspects of the BAL
Nevertheless, with advances in biomedical engineering, successive treatment of liver failure is still a challenge, which causes considerable morbidity and mortality in the developed world. The introduction of liver transplantation has been the major melioration in the treatment of patients with acute liver diseases. As a result of the lack of donor availability and long waiting lists, unluckily, many patients die. During the last decade, scientists have developed keen interest in the development of an extracorporeal liver support device using isolated liver cells. The development of nonbiological systems to support critically ill patients with ALF that incorporate plasmapheresis, charcoal hemoperfusion, and ultrafiltration techniques has not had a significant impact on survival. Therefore, the liver cells incorporating biological approaches have attracted intense attention worldwide but have also some concern. A commonly used HepG2 cell line in most of the currently available bioartificial liver systems have been found not to be very effective in performing fully differentiated metabolic functions. The better alternative choice is the use of primary hepatocytes, but they are extremely difficult to handle and tend to lose their differentiated functions in a short time. Research is going on to potentially use human hepatic progenitor cells, embryonic stem cells, or fetal stem cells as the continuous source of metabolically active hepatocytes. In the near future, it is also possible that the genetically engineered metabolic pathway, useful in treating liver failure, can be applied into immortalized hepatocyte cell lines. The extensive research is also moving on to the development of advance extracorporeal devices by altering or engineered matrix properties for effective cell support together with amended mass transport ability. These approaches would be a major advancement, eliminating the time-and resource-intensive processes for obtaining primary hepatocytes. With all this research going on throughout the scientific community, it is possible to develop next-generation bioartificial liver devices that will be used extracorporeally and thus will have potential clinical implications.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from Department of Biotechnology (DBT), Ministry of Science and Technology, Govt. of India, and India-Japan (DST-JSPS) project. AT acknowledges the Council of Scientific and Industrial Research (CSIR) for providing the senior research fellowship.
REFERENCES
- 1.Matsumura KN, Guevara GR, Huston H, et al. Hybrid bioartificial liver in hepatic failure: Preliminary clinical report. Surgery. 1987;101:99–103. [PubMed] [Google Scholar]
- 2.Jones RS, ed. Atlas of Liver and Biliary Surgery. Chicago: Year Book Medical Publ.; 1990:307–312. [Google Scholar]
- 3.Sasse D.. Regulation of hepatic metabolism, intra and intercellular compartmentalization. New York: Plenum; 1986:43–53. [Google Scholar]
- 4.Bioulac-Sage P, Le Bail B, Balabaud C.. Liver and biliary-tract histology. In: Bircher J, Benhamou JP, McIntyre N, Rizzeto M, Rodes J, eds. Oxford Textbook of Clinical Hepatology. Oxford/New York: Oxford University Press; 1999:13–22. [Google Scholar]
- 5.Rappaport AM, Borowy ZJ, Lougheed WM.. Subdivision of hexagonal liver lobules into a structural and functional unit. Anat Rec. 1996;199:11–33. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz CM, Guibert E, Bilir B, Gumucio JJ.. Functional compartmentalization of hepatocytes. In: LeBouton AV, ed. Molecular and Cell Biology of the Liver. Oxford/New York: Oxford University Press; 1993;346–367. [Google Scholar]
- 7.Leeuw AM, Brouwer A, Knook DL.. Sinusoidal endothelial cells of the liver: Fine structure and function in relation to age. J Electron Microsc Tech. 1990;14:218–236. [DOI] [PubMed] [Google Scholar]
- 8.Gumucio JJ, Chianale J.. Liver Cell Heterogeneity and Liver Function. In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA, eds. The Liver: Biology and Pathobiology. New York: Raven Press; 1988:931–947. [Google Scholar]
- 9.Vander AJ.. Human Physiology. The Mechanism of Body Function, 7th edition New York: McGraw-Hill; 1999:123–143. [Google Scholar]
- 10.Ponfick VA.. Ueber leberresection und leberreaction. Germany: Verhandl DeutschGessellsch Chir; 1890:19–28. [Google Scholar]
- 11.Starzl TE, Porter KA, Francavilla JA.. A hundred years of the hepatotrophic controversy. Ciba Found Symp. 1977:111–129. [DOI] [PubMed] [Google Scholar]
- 12.Fausto N, Webber EM.. Liver regeneration. In: Arias M, Boyer JL, Fausto N, Jakoby WB, Schachter D, Shafritz DA, eds. The Liver Biology and Pathobiology. New York: Raven Press; 1994:1059–1084. [Google Scholar]
- 13.Diehl AM, Rai R.. Regulation of liver regeneration by pro-inflammatory cytokines. J Gastroenterol Hepatol. 1996;11:466–470. [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos GK, DeFrances MC.. Liver regeneration. Science. 1997;276:60–66. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Williams R, Jalan R.. The pathophysiological basis of acuteon-chronic liver failure. Liver. 2002;22(Suppl 2):5–13. [DOI] [PubMed] [Google Scholar]
- 16.Jauregui HO, Mullon CJ-P, Solomon B.. Extracorporeal artificial liver support. In: Lanza RP, Langer R, Chick WL, eds. Principles of Tissue Engineering. San Diego, CA: Academic Press & Austin, TX: Landes; 1997:568–612. [Google Scholar]
- 17.Hoofnagle JH, Carithers RL, Shapiro C, Ascher N.. Fulminant hepatic failure: Summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 18.Carpentier B, Gautier A, Legallais C.. Artificial and bioartificial liver devices: Present and future. Gut. 2009;58:1690–1702. [DOI] [PubMed] [Google Scholar]
- 19.Powers KA, Ribeiro RM, Patel K, et al. Kinetics of hepatitis C virus reinfection after liver transplantation. Liver Transpl. 2006; 12:207–216. [DOI] [PubMed] [Google Scholar]
- 20.Stickel F, Hoehn B, Schuppan D, Seitz HK.. Review article: Nutritional therapy in alcoholic liver disease. Aliment Pharmacol Ther. 2003;18:357–373. [DOI] [PubMed] [Google Scholar]
- 21.Hoofnagle J, Carithers R, Shapiro C, Ascher N.. Fulminant hepatic failure, summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 22.Bernuau J, Rueff B, Benhamou JP.. Fulminant and subfulminant liver failure definitions and causes. Semin Liver Dis. 1996;6:97–106. [DOI] [PubMed] [Google Scholar]
- 23.Hughes RD, Nicolaou N, Langley PG, Ellis AJ, Wendon JA, Williams R.. Plasma cytokine levels and coagulation and complement activation during use of the extracorporeal liver assist device in acute liver failure. Artif Organs. 1998;22:854–858. [DOI] [PubMed] [Google Scholar]
- 24.Rave SD, Tilanus HW, Linden JVD, et al. The importance of orthotopic liver transplantation in acute hepatic failure. Transpl Int. 2002;15:29–33. [DOI] [PubMed] [Google Scholar]
- 25.Bernuau J, Rueff B, Benhamou JP.. Fulminant and subfulminant liver failure, definitions and causes. Semin Liver Dis. 1986;6:97–106. [DOI] [PubMed] [Google Scholar]
- 26.Bernal W, Wendon J.. Liver transplantation in adults with acute liver failure. J Hepatol. 2004;40:192–197. [DOI] [PubMed] [Google Scholar]
- 27.Rivera PT, Moreno J, Skaff C, McDiarmid S, Vargas J, Ament ME.. Delayed encephalopathy in fulminant hepatic failure in the pediatric population and the role of liver transplantation. J Pediatr Gastroenterol Nutr. 1997;24:128–134. [DOI] [PubMed] [Google Scholar]
- 28.Pauwels A, Mostefa N, Florent C, Levy VG.. Emergency liver transplantation for acute liver failure. J Hepatol. 1993;17:124–127. [DOI] [PubMed] [Google Scholar]
- 29.PHS Guideline on Infectious Disease Issues in Xenotransplantation, Food and Drug Administration, January 19, 2001. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/xenotransplantation/ucm074727.htm. Accessed December 29, 2011.
- 30.Jalan R, Sen S, Williams R.. Prospects for extracorporeal liver support. Gut. 2004;53:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opolon P.. High permeability membrane hemodialysis and hemofiltration in acute hepatic coma. Artif Organs. 1979;3:354–360. [DOI] [PubMed] [Google Scholar]
- 32.Knell AJ, Dukes DC.. Dialysis procedures in acute liver failure. Lancet. 1976;2:402–403. [DOI] [PubMed] [Google Scholar]
- 33.Denis J, Opolon P, Delmore M.. Long-term extracorporeal assistance by continuous hemofiltration during fulminant hepatic failure. Gastroenterol Clin Biol. 1992;3:337–348. [PubMed] [Google Scholar]
- 34.Gleissner M, Bornemann R, Stemerowicz R, Meissler M, Neuhaus P, Gerlach JC.. Immunoisolation of hybrid liver support systems by semipermeable membranes. Int J Artif Organs. 1997;20:644–649. [PubMed] [Google Scholar]
- 35.Nyberg SL, Misra SP.. Hepatocyte liver-assist systems—A clinical update. Mayo Clin Proc. 1998;73:765–771. [DOI] [PubMed] [Google Scholar]
- 36.Ellis AJ, Hughes RD, Nicholl D.. Temporary extracorporeal liver support for severe acute alcoholic hepatitis using the BioLogic-DT. Int J Artif Organs. 1999;22:27–34. [PubMed] [Google Scholar]
- 37.Arkadopoulos N, Detry O, Rozga J, Demetriou AA.. Liver assist systems, state of the art. Int J Artif Organs. 1998;21:781–787. [PubMed] [Google Scholar]
- 38.Chari RS, Collins BH, Magee JC, et al. Treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. N Engl J Med. 1994;331:234–237. [DOI] [PubMed] [Google Scholar]
- 39.Strain AJ, Neuberger JM.. A bioartificial liver—State of the art. Science. 2002;295:1005–1009. [DOI] [PubMed] [Google Scholar]
- 40.Detry O, Arkadopolulos N, Ting P.. Clinical use of a bioartificial liver in the treatment of acetaminophen-induced fulminant hepatic failure. Am Surg. 1999;65:934–938. [PubMed] [Google Scholar]
- 41.Gerlach J.. Development of a hybrid liver support system, a review. Int J Artif Organs. 1996;19:645–654. [PubMed] [Google Scholar]
- 42.Stange J, Mitzner S.. Cell sources for bioartificial liver support. Int J Artif Organs. 1996;19:14–17. [PubMed] [Google Scholar]
- 43.Sielaff TD, Hu MY, Rao S, Groehler K.. A technique for porcine hepatocyte harvest and description of differentiated metabolic functions in static culture. Transplantation. 1995;59:145–163. [DOI] [PubMed] [Google Scholar]
- 44.Nyberg SL, Payne WD, Amiot B, Shirabe K.. Demonstration of biochemical function by extracorporeal xenohepatocytes in an hepatic animal model. Transplant Proc. 1993;25:1944–1945. [PubMed] [Google Scholar]
- 45.Dixit V, Gitnick G.. Artificial liver support, state of the art. Scand J Gastroenterol Suppl. 1995;220:1101–1114. [DOI] [PubMed] [Google Scholar]
- 46.Roberts EA, Letarte M, Squire J, Yang SY.. Characterization of human hepatocyte lines derived from normal liver tissue. Hepatology. 1994;19:1390–1399. [PubMed] [Google Scholar]
- 47.Schumacher IK, Okamoto T, Kim BH, Chowdhury NR, Chowdhury JR, Fox IJ.. Transplantation of conditionally immortalized hepatocytes to treat hepatic encephalopathy. Hepatology. 1996; 24:337–343. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Pan J, Naik S, Santangini H, Trenkler D.. Characterization and evaluation of detoxification functions of a nontumorigenic immortalized porcine hepatocyte cell line. Cell Transplant. 1999;8:219–232. [DOI] [PubMed] [Google Scholar]
- 49.Jauregui HO, Chowdhury NR, Chowdhury JR.. Use of mammalian cells for artificial liver support. Cell Transplant. 1996;5:353–367. [DOI] [PubMed] [Google Scholar]
- 50.Sussman NL, Gislason GT, Conlin CA, Kelly JH.. The hepatic extra-corporeal liver assist device, initial clinical experience. Artif Organs. 1994;18:390–396. [DOI] [PubMed] [Google Scholar]
- 51.Landry J, Bernier D, Ouellet C, Goyette R.. Spheroidal aggregate culture of rat liver cells, histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones EA, Tosh D, Wilson DI, Lindsay S, Forrester LM.. Hepatic differentiation of murine embryonic stem cells. Exp Cell Res. 2002;272:15–22. [DOI] [PubMed] [Google Scholar]
- 53.Court FG, Wemyss-Holden SA, Dennison AR, Maddern GJ.. Bioartificial liver support devices, historical perspectives. ANZ J Surg. 2003;73:739–748. [DOI] [PubMed] [Google Scholar]
- 54.Hu WS, Friend JR, Wu FJ, Sielaff T.. Development of a bioartificial liver employing xenogeneic hepatocytes. Cytotechnology. 1997;23:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerlach JC, Schnoy N, Vienken J, Smith M, Neuhaus P.. Comparison of hollow fibre membranes for hepatocyte immobilization in bioreactors. Int J Artif Organs. 1996;19:610–616. [PubMed] [Google Scholar]
- 56.Wu FJ, Friend JR, Lazar A, Mann HJ.. Hollow fiber bioartificial liver utilizing collagen-entrapped porcine hepatocytes spheroids. Biotechnol Bioeng. 1996;52:34–44. [DOI] [PubMed] [Google Scholar]
- 57.Giorgio TD, Moscioni AD, Rozga J, Demetriou AA.. Mass transfer in a hollow fiber device used as a bioartificial liver. ASAIO J. 1993;39:886–892. [PubMed] [Google Scholar]
- 58.Qiang S, Yaoting Y, Hongyin L, Klinkmann H.. Comparative evaluation of different membranes for the construction of an artificial liver support system. Int J Artif Organs. 1997;20:119–124. [PubMed] [Google Scholar]
- 59.Jozwiak A, Karlik W, Wiechetek M, Werynski A.. Attachment and metabolic activity of hepatocytes cultivated on selected polymeric membranes. Int J Artif Organs. 1998;21:460–466. [PubMed] [Google Scholar]
- 60.Yanagi K, Miyoshi H, Fukuda H, Ohshima N.. A packed-bed reactor utilizing porous resin enables high density culture of hepatocytes. Appl Microbiol Biotechnol. 1992;37:316–320. [DOI] [PubMed] [Google Scholar]
- 61.Weston MJ, Mellon PJ, Langley PG.. Resin column perfusion with whole blood or plasma separated by continuous flow centrifuge. Clin Sci Mol Med. 1975;48:187–192. [DOI] [PubMed] [Google Scholar]
- 62.Patzer JF, Mazariegos GV, Lopez R.. Novel bioartificial liver support system-preclinical evaluation. Ann N Y Acad Sci. 1999;875:340–352. [DOI] [PubMed] [Google Scholar]
- 63.Lozinsky V, Galaev IY, Plieva FM, Savina IN, Jungvid H, Mattiasson B.. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003;21:445–451. [DOI] [PubMed] [Google Scholar]
- 64.Lozinsky VI, Plieva FM, Galaev YI, Mattiasson B.. The potential of polymeric cryogels in bioseparation. Bioseparation. 2006;10:163–188. [DOI] [PubMed] [Google Scholar]
- 65.Tripathi A, Kathuria N, Kumar A.. Elastic and macroporous agarose–gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. J Biomed Mater Res A. 2009;90A:680–694. [DOI] [PubMed] [Google Scholar]
- 66.Kathuria N, Tripathi A, Kar KK, Kumar A.. Synthesis and characterization of elastic and macroporous chitosan-gelatin cryogel for tissue engineering. Acta Biomater. 2009;5:406–418. [DOI] [PubMed] [Google Scholar]
- 67.Kumar A, Plieva FM, Galaev IY, Mattiasson B.. Affinity fractionation of lymphocytes using a monolithic cryogel. J Immunol Methods. 2003;283:185–194. [DOI] [PubMed] [Google Scholar]
- 68.Lozinsky VI, Plieva FM.. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. III. Overview of recent research and developments. Enzyme Microb Technol. 1998;23:227–242. [Google Scholar]
- 69.Bloch K, Lozinsky VI, Galaev IY, et al. Functional activity of insulinoma cells (INS-1E) and pancreatic islets cultured in agarose cryogel sponges. J Biomed Mater Res A. 2005;75A:802–809. [DOI] [PubMed] [Google Scholar]
- 70.Nilsang S, Nandakumar KS, Galaev IY, et al. Monoclonal antibody production using a new supermacroporous cryogel bioreactor. Biotechnol Prog. 2008;23:932–939. [DOI] [PubMed] [Google Scholar]
- 71.Xue YL, Zhao SF, Luo Y, et al. A hybrid artificial liver support system in treatment of acute liver failure. World J Gastroenterol. 2001;7:826–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding YT, Qiu YD, Chen Z, et al. The development of a new bioartificial liver and its application in 12 acute liver failure patients. World J Gastroenterol. 2003;9:829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donini A, Baccarani U, Risaliti A, Degrassi A.. Temporary neurological improvement in a patient with acute or chronic liver failure treated with a bioartificial liver device. Am J Gastroenterol. 2002;95:1102–1104. [DOI] [PubMed] [Google Scholar]
- 74.Mizumoto H, Funatsu H.. Liver regeneration using a hybrid artificial liver support system. Artif Organs. 2004;28:53–57. [DOI] [PubMed] [Google Scholar]
- 75.Dixit V, Gitnick G.. The bioartificial liver—State of the art. Eur J Surg. 1998;164:71–76. [DOI] [PubMed] [Google Scholar]
- 76.Bartolo D, Jarosch SL, Haverich G, Bader A.. A novel full-scale flat membrane bioreactor utilizing porcine hepatocytes, cell viability and tissue-specific functions. Biotechnol Prog. 2000;16:102–108. [DOI] [PubMed] [Google Scholar]
- 77.Watanabe FD, Mullon CJ, Hewitt WR, et al. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg. 1997;225:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rozga J, Holzman MD, Ro MS, et al. Development of a hybrid bioartificial liver. Ann Surg. 1993;217:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi N, Okitsu T, Nakaji S, Tanaka N.. Hybrid bioartificial liver: establishing a reversibly immortalized human hepatocyte line and developing a bioartificial liver for practical use. J Artif Organs. 2003;6:236–244. [DOI] [PubMed] [Google Scholar]
- 80.Ellis AJ, Hughes RD, Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446–1451. [DOI] [PubMed] [Google Scholar]
- 81.Sauer IM, Zeilinger K, Obermayer N, et al. Primary human liver cells as source for modular extracorporeal liver support—A preliminary report. Int J Artif Organs. 2002;25:1001–1005. [DOI] [PubMed] [Google Scholar]
- 82.Flendrig LM, La JW, Joming GG.. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound non-woven polyester matrix for hepatocyte culture as small aggregates. J Hepatol. 1997;26:1379–1392. [DOI] [PubMed] [Google Scholar]


