Abstract:
Edema acquired during the perioperative period has long been associated with increased mortality. Edema acquired during cardiopulmonary bypass (CPB) may contribute to this mortality. The intent of this retrospective study was to test the premise that edema in the form of a positive fluid balance change (FBC) acquired during CPB correlated to mortality. If so, FBC from the beginning of CPB (baseline; FBC = 0) to the end of CPB may need to be monitored, measured, and controlled on CPB with the same ardor as blood pressure and pH. This retrospective analysis reviewed the FBC of 1540 pediatric and congenital heart surgery patients at the end of CPB. Additions and subtractions of fluid to the combined patient/CPB circuit were routinely quantified during CPB procedures and during periods of modified ultrafiltration (MUF). The primary outcome assessed was mortality during hospitalization. The overall mortality of the 1540 patients was 5.65%from all causes. Eighty percent (n = 1226, mortality = 4.65%) of the patients had a zero or negative FBC immediately after CPB/MUF. Twenty percent (n = 314, mortality = 9.55%) had a positive FBC. Positive FBC patients tended to be in higher risk categories, weighed more, and had longer pump times (p < .05) with an adjusted odds ratio for mortality of 1.73 (1.01–2.96, 95% confidence interval). There is a correlation between edema acquired during CPB and increased mortality in pediatric and congenital heart surgery patients. The potential exists for the perfusionist to optimize the fluid balance changes while on CPB to reduce mortality rates.
Keywords: cardiopulmonary bypass, congenital, edema, fluid balance, pediatric
The Causes and Risks of Edema
The intent of this clinical review was to test the premise that edema in the form of a positive fluid balance change (FBC) in the patient acquired during cardiopulmonary bypass (CPB) correlated to mortality. Edema acquired during the perioperative period has long been associated with increased mortality (1–3). Hemodilution and systemic inflammatory response syndrome (SIRS) caused by CPB may be a major contributor to this problem (4). A review article by Hirleman and Larson describes the major causes, consequences, and preventative treatments for the edema acquired during the perioperative period (4). These are listed in Table 1. A positive FBC during CPB in adults of as little as 500 mL (∼ +7 mL/kg in a 70 kg patient) is associated with an increased length of stay and the need for blood transfusion in adults (1). Neonates have been known to accumulate 18% of their body weight (∼ +180 mL/kg) in edema during CPB, severely increasing their postoperative morbidity (3). In fact, fluid accumulation in the perioperative period directly relates to mortality in any kind of major surgery patient with accumulations of greater than 20% of dry body weight being uniformly fatal (5). Dry body weight means the patient’s weight without extra fluid (edema). Consequently, edema in small and large amounts can be a serious problem.
Table 1.
Likely causes, frequent consequences, and preventative treatments for edema in cardiopulmonary bypass patients.
| Likely Causes | Frequent Consequences | Preventative Treatments |
|---|---|---|
|
|
|
Information derived from Hirleman and Larson, 2008 (4).
Perioperative Time Periods and Sources of Edema Fluid
The perioperative period of cardiac surgery involves three distinct time periods during which edema may occur. Before CPB the patient will receive intravenous solutions for the dehydration which occurs during fasting before the procedure. Patients may also present in the operating room with significant fluid overload due to chronic congestive heart failure or, less commonly, as a result of fluid resuscitation for acute circulatory failure. After CPB the patient may require fluid due to blood loss or for fluid resuscitation. In addition, pre or post CPB renal failure may further magnify edema formation. During these two time periods the perfusionist has no control over the circumstances involved. However, during the intermediate period, while on CPB, the perfusionist has control over most of the patient’s intake and output (I & O) fluid volumes. Additionally, during CPB the perfusionist may have an impact on the pre or post CPB periods by removing excess fluid and minimizing accumulation on CPB that otherwise would extend into the post operative period (2).
The Need to Monitor and Measure Fluid Balance on CPB
Variances such as low blood pressure or acidosis on CPB are monitored, measured, and corrected by the perfusionist using specific interventions. However, FBC is not routinely monitored or measured during CPB. And most of the treatments for edema are not very specific to individual patients (Table 1). The use of ultrafiltration during CPB and possibly modified ultrafiltration (MUF) afterwards are specific interventions because they allow the perfusionist to add and/or remove selected amounts of fluid at will (6). However, without measuring I & O, the perfusionist cannot control FBC with any certainty during CPB/MUF; too much fluid removed might be just as bad as too little fluid removed.
The most accurate way to measure FBC is to weigh the patient. This is not possible during CPB. The only practical alternative is to monitor I & O during CPB to measure the FBC. The FBC can then be addressed by adding or removing fluid to achieve a specific goal at the end of CPB/MUF.
Hypothesis and Purpose
The hypothesis is that from the beginning of CPB (zero base line for FBC) to the end of CPB/MUF, fluid balance changes will correlate to changes in mortality. The purpose of this review was to determine the magnitude of FBC on CPB, whether positive or negative, and how this correlated to mortality by a retrospective analysis of the fluid balance changes of 1540 pediatric and congenital heart surgery patients.
MATERIALS AND METHODS
Institutional Review Board Authorization
The authorization to collect data was approved by the Children’s Mercy Hospitals & Clinics of Kansas City, MO, Pediatric Institutional Review Board protocol # 03–06–067X entitled “A Descriptive, Retrospective Review of all Patients with Congenital Heart Disease Presenting to the Children’s Mercy Hospital from 1980 to Present”. It was approved in June 2003 and reviewed and renewed annually. This specific study protocol # 10 10–196X was approved for exempt status on October 29, 2010. Patient consent was not required by the institutional review board for this retrospective review under section 45 code of federal regulations 46.101(b) (4) by expedited review.
Data Collection and Classification
Data were collected from 1540 consecutive patients operated on from August 2003 through December 2009 who had complete data sets (Table 2). Data collected included preoperative weight in kilograms, FBC in milliliters per kilogram (mL/kg), the Risk Adjustment for Congenital Heart Surgery-1 (RACHS-1) risk score, CPB time in minutes, aortic cross clamp time in minutes, post CPB hematocrit (%), length of stay (LOS; days stayed inclusive of the day of admission and day of discharge), and mortality (%) during hospitalization. The RACHS-1 risk score correlates to mortality after surgical repair or palliation in patients with congenital heart disease; a score of 1 having the lowest risk and a score of 6 having the highest risk (7). Only 87% of the patients underwent aortic cross clamping (n = 1337). Patients were further categorized into negative and positive FBC categories.
Table 2.
Summary of demographics and data collection.
| All Patients (100%) | Negative FBC Patients (80%) | Positive FBC Patients (20%) | |||
|---|---|---|---|---|---|
| n = | 1540 | 1226 | 314 | p (power) | |
| Weight kilograms | Average | 13.9 ± 18.2 | 12.7 ± 16.3 | 18.5 ± 23.7 | <.05 (>99%) |
| Median | 7.3 (2.0–134) | 6.8 (2.0–120) | 9.7 (2.5–134) | ||
| Fluid balance mL/kg | Average | −32 ± 55 | −47 ± 50 | 27 ± 27 | <.05 (>99%) |
| Median | −24 (−888–160) | −33 (−888–0) | 18 (1–160) | ||
| RACHS-1 score | Average | 2.84 ± 1.3 | 2.77 ± 1.3 | 3.11 ± 1.4 | <.05 (>95%) |
| Median | 3 (1–6) | 3 (1–6) | 3 (1–6) | ||
| CPB time minutes | Average | 93 ± 59 | 87 ± 57 | 114 ± 60 | <.05 (>99%) |
| Median | 77 (11–632) | 72 (11–632) | 103 (19–426) | ||
| CPB time minutes | Average | 93 ± 59 | 87 ± 57 | 114 ± 60 | <.05 (>99%) |
| Median | 77 (11–632) | 72 (11–632) | 103 (19–426) | ||
| Aortic cross clamp time minutes | Average | 38 ± 30 | 36 ± 28 | 44 ± 35 | <.05 (>95%) |
| Median | 31 (0–208) | 30 (0–208) | 37 (0–189) | ||
| n = | 1337 | 1075 | 262 | ||
| Hematocrit % post-CPB | Average | 36 ± 6 | 36 ± 6 | 36 ± 7 | NS (>80%) |
| Median | 36 (18–62) | 36 (18–62) | 35 (19–58) | ||
| Length of stay in days | Average | 13 ± 18 | 13 ± 18 | 13 ± 18 | .02 (>20%) |
| Median | 7 (1–278) | 7 (1–278) | 8 (1–203) | ||
| Hospital mortality | Percentage | 5.65 | 4.65 | 9.55 | <.05 (>90%) |
Summary of demographics and data collection in 1540 cardiopulmonary bypass patients from August 2003 through December 2009. Averages are expressed with standard deviations and medians with ranges. Only 87% of the patients received cardiac cross clamping. The hospital mortality percentage represents patient deaths after cardiac surgery from all causes and co-morbidities at any time before discharge.
For further analysis, patients were divided into three weight categories, three combined risk categories, three CPB time categories, and six FBC categories.
Circuit Components and Prime Volume
Each CPB circuit contained an arteriovenous loop, hard shell cardiotomy/venous reservoir, pump raceway, oxygenator, bubble trap, a 1:4 cardioplegia to blood set, and associated circuit tubing. The cardioplegia set contained a pump raceway, heat exchanger/bubble trap, and hemoconcentrator with associated tubing. Each circuit also included the ventricular vent and field sucker tubing. Specific types of circuit components (Medtronic, Minneapolis, MN, Sorin Group USA, Arvada, CO, and Terumo Cardiovascular Systems, Ann Arbor, MI) depended on the size of the patient. The smallest circuit (used with neonates) had a static prime volume of 380 mL including a manufacturer’s venous reservoir safe operating volume of 15 mL. The largest circuit (used with large adults) had a static volume of 1800 mL including a manufacturer’s venous reservoir safe operating volume of 200 mL. Presented for purposes of description only, the static circuit prime volume was not a factor in calculating the FBC from the beginning of CPB (zero base line for FBC) to the end of CPB/MUF for any size patient.
Surface Coatings
The arteriovenous loops were Trillium Biosurface® coated (Medtronic, Minneapolis, MN 55,432). The oxygenators were either PHISIO® coated (Sorin Group USA, Arvada, CO) or Xcoated® (Terumo Cardiovascular Systems, Ann Arbor, MI). All other components were SmartX® coated (Sorin Group USA, Arvada, CO) with the exception of the bubble traps which were not coated.
Crystalloid Prime
All circuits received an initial crystalloid prime using a balanced bicarbonate and electrolyte solution. After de-bubbling, clear primed circuits received 100–200 mL of 25% albumin.
Blood Prime
Circuits for patients weighing less than 13 kg most often received a reconstituted blood prime after clear priming. The reconstituted blood was made by combining a unit of packed red blood cells with a unit of fresh frozen plasma (8). The packed red blood cells (approximately 320 mL/unit) and fresh frozen plasma (approximately 280 mL/unit) were matched from a single blood donor in 90% of the cases. The reconstituted blood was washed through a hemoconcentrator with a bicarbonate adjusted balanced electrolyte solution. This removed excess glucose, preservatives, and unwanted metabolites. The electrolyte content, osmolarity, and pH were physiologically normalized in the washing process (9). After washing, the circuit blood prime hematocrit value ranged from 32–48%, depending on the circuit size. Before heparinization, excess washed reconstituted blood (120–240 mL) was removed from the circuit for administration to the patient in the post CPB period. The actual amount of reconstituted blood remaining in the prime was 360–480 mL.
Prime Medications
Prime medications added to the circuit volume (after washing and removal of excess reconstituted blood in the case of blood primed circuits) included two units of heparin per milliliter of circuit volume, 30 mg/kg (up to 1 g) of methylprednisolone, and 25 mg/kg (up to 1 g) of cephazolin. Patients sensitive to cephazolin received an alternate antibiotic parenterally. Due to the increased potential for anaphylaxis, these alternate antibiotics were not used in the pump prime. Circuit primes of patients who were not undergoing aortic cross clamping with cardioplegia received 500–1000 mg of calcium gluconate. Otherwise, patients received the calcium while on CPB after removal of the aortic cross clamp later in the procedure. Patients undergoing repeat sternotomy or weighing <6 kg received 100 mg/kg (up to 5 g) of aminocaproic acid in the circuit prime. During the time period of October 28, 2003 through December 30, 2005, 263 patients received 30,000 KIU/kg of aprotinin in the pump prime instead of aminocaproic acid.
FBC Monitoring and Measuring
The patient’s fluid balance status prior to CPB was irrelevant because only the FBC as controlled by the perfusionist during CPB/MUF was being measured. Fluid administered after CPB/MUF was also not included for the same reason. The authors acknowledge that fluid status prior to CPB and fluid administered after CPB/MUF may have an impact on mortality. However, this review only seeks to determine what role, if any, FBC as controlled by the perfusionist during CPB/MUF has on mortality.
Additions and subtractions of fluid to the combined patient/CPB circuit were routinely quantified during CPB procedures and during periods of MUF after CPB. A spreadsheet incorporating the necessary categories and calculations was incorporated directly into the computerized chart used by perfusionists during CPB/MUF. Real time fluid balance changes could be calculated by entering current data during CPB or MUF. This is illustrated in Figure 1. Under the CPB section, items in the left CPB column represent fluid additions to the patient/circuit during CPB. Items in the right CPB column represent fluid removed from the patient/circuit during CPB. The totaled CPB deductions are subtracted from the totaled CPB additions to arrive at the FBC during CPB. Under the MUF section, items in the left MUF column represent fluid additions to the patient/circuit during MUF. Items in the right MUF column represent fluid removed from the patient/circuit during MUF. The totaled MUF deductions are subtracted from the totaled MUF additions to arrive at the FBC during MUF. The fluid balance changes during CPB and MUF are then combined to determine the FBC (perfusion intake and output) after CPB/MUF. No significant amounts of fluid or blood products were administered independently by the anesthesiologist during CPB/MUF. However, if an anesthesiologist administers fluid or blood during CPB/MUF as a part of the institutional practice, this volume should be added to the FBC calculation.
Figure 1.
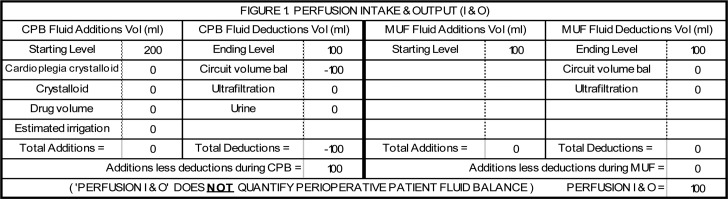
This is a hypothetical example of the simplest fluid balance change calculation. In this example, no fluids were added to the circuit after the initiation of CPB. There was no significant blood loss or urine output during CPB. There was no ultrafiltration during CPB and MUF was not performed. The venous reservoir starting level of 200 mL dropped to 100 mL upon weaning from CPB. The patient’s fluid gain was 100 mL. The fluid gain can be divided by the patient’s weight to calculate the FBC in mL/kg.
Figure 1 illustrates a simple, hypothetical calculation on the spreadsheet. Figures 2 and 3 illustrate actual cases with more complex calculations. The total FBC was converted to the FBC in milliliters per kilogram of body weight (mL/kg) for data collection.
Figure 2.
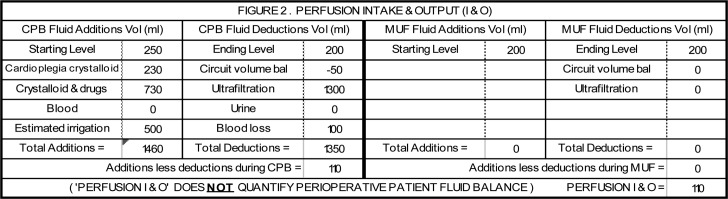
This was a 92 kg patient undergoing a primum atrial septal defect and mitral valve cleft repair. CPB time was 68 minutes. Aortic cross clamp time was 35 minutes with 1150 mL of 1:4 cardioplegia/blood solution administered (230 mL crystalloid). During CPB, 730 mL of crystalloid and drugs and 500 mL of irrigation were added to the circuit. Fluid removed included 1300 mL of ultrafiltration and 100 mL of actual blood loss. The initial hematocrit on CPB of 26% was the same at the termination of CPB. The FBC at the end of CPB was +110 mL (+1.2 mL/kg). MUF was not performed. After CPB, the remaining 1270 mL of circuit volume was processed through cell salvage equipment with 330 mL of red cells being returned to the patient in the post CPB period, but this was not included in this FBC calculation because it did not occur during CPB.
Figure 3.
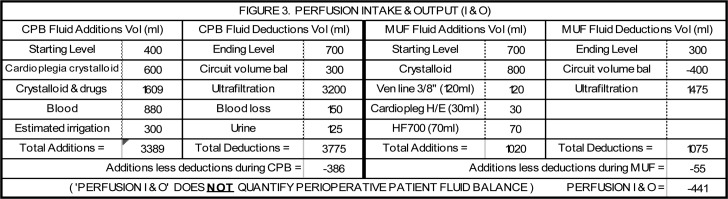
This was a 47 kg patient undergoing an aortic valve repair and aortoplasty. CPB time was 150 minutes. Aortic cross clamp time was 103 minutes with 3000 mL of 1:4 cardioplegia/blood solution administered (600 mL crystalloid). During CPB, 1609 mL of crystalloid and drugs, 880 mL of blood in the form of packed red blood cells, and 300 mL of irrigation were added to the circuit. Fluid removed included 3200 mL of ultrafiltration, 150 mL of actual blood loss and 125 mL of urine. The initial hematocrit on CPB of 17% was increased to 27% by ultrafiltration and the addition of the packed red blood cells. The FBC at the end of CPB was −386 mL (−8.2 mL/kg). During MUF, 800 mL of crystalloid was added to the circuit to chase the blood being infused into the patient and to keep the circuit primed. In addition, the venous blood line (120 mL), the cardioplegia heat exchanger (30 mL) and the hemoconcentrator (70 mL) were drained into the circuit and infused into the patient for a total of 1020 mL added to the circuit. Ultrafiltration during MUF removed 1475 mL. During MUF the hematocrit increased from 27–32%. The FBC at the end of MUF was −55 mL (−1.2 mL/kg). The net combined FBC was −441 mL (−9.4 mL/kg). After MUF the circuit volume was primarily crystalloid so it was not processed through cell salvage equipment.
Conduct of Ultrafiltration
During the period under review, perfusionists did not strive to achieve any specific FBC goal. Based on their past experiences, the perfusionists and surgeons thought that patients would benefit from a slightly positive FBC to help wean from CPB followed by a slightly negative FBC after MUF to benefit pulmonary function. Excessive positive or negative FBCs were to be avoided if possible. However, there was no specific quantity assigned to “slightly” or “excessive”; terms which were, at best, subjective. Ultrafiltration was used primarily to optimize the hematocrit without a specific concern for FBC because surgical protocol assigned specific hematocrit goals (35% for biventricular procedures and 45% for univentricular procedures) to be achieved in neonates, infants, and many other pediatric patients by the termination of CPB/MUF. Specific FBCs were not similarly designated.
Data Analysis
All data were recorded on a Microsoft Excel® spreadsheet (Microsoft Corporation, Redmond, WA) for creation of tables and figures. Data were then transferred for analysis to the GraphPad Instat® statistical package (version 3.01 for Windows 95/NT, GraphPad Software, San Diego, CA). Continuous variables were expressed as averages and standard deviations, medians, and ranges, using the t test or Wilcoxon Rank Sum tests for comparisons as appropriate. Categorical variables were compared side-by-side on a scale and by Fisher’s Exact tests where appropriate. The primary outcome evaluated was hospital mortality. Hospital mortality was defined as death after cardiac surgery from any cause, including non-cardiac co-morbidities, during the admission. The power analysis for the 1540 patient comparison is summarized in Table 2. Multiple logistic regression analysis compared FBC to RACHS-1, weight, and pump time. Statistical results were independently reviewed and validated using the SAS statistical package (version 9.2, SAS Institute Inc., Cary,NC).
RESULTS
Table 2 Summary
Table 2 summarizes the data. Twenty percent of the 1540 patients had positive FBCs at the end of CPB/MUF. Patients with a positive FBC had higher risk scores, weighed more, and had longer CPB and aortic cross clamp times than negative FBC patients. There was no difference in the post CPB hematocrit. The LOS difference was statistically significant (Wilcoxon Rank Sums) due to the greater number of outliers in the much larger negative FBC group. However, the difference in medians was one day which was not considered clinically significant. The primary outcome showed that positive FBC patients had a mortality rate twice as high as that of negative FBC patients.
Positive balance patients tended to be in higher risk categories, weighed more, and had longer pump times (p < .05). Multiple logistic regression analysis showed that a positive FBC had an odds ratio for mortality of 1.73 (95% confidence interval: 1.01–2.96) after adjusting for RACHS-1, weight, and pump time.
Fluid Balance by Weight and Correlation to Mortality
Figure 4 compares FBC by weight categories. Patients weighing less than 6 kg had an overall mortality rate twice that of the combined population. However, positive FBC patients weighing less than 6 kg had a mortality rate twice that of patients weighing less than 6 kg with negative FBCs. Patients weighing 6–12 kg had a mortality rate of only 2.0%, but the positive FBC patient mortality in this weight category was three times that of the negative FBC patients. Positive FBC patients weighing over 12 kg had a mortality rate about six times greater than negative FBC patients weighing over 12 kg, even though the combined mortality in this weight category was only 1.5%.
Figure 4.
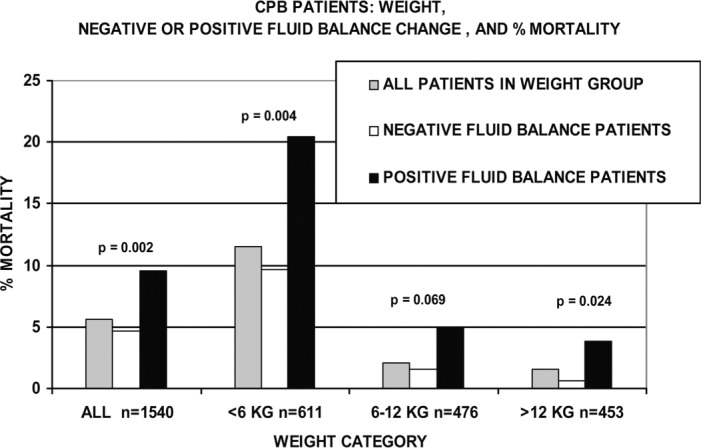
CPB patients are compared by weight category, negative or positive FBC, and mortality. Within the three weight groups, the mortality of positive FBC patients was 2, 3, and 6 times greater than the negative FBC patients in the same groups. Significant differences between negative and positive sub-groups are indicated. The total number of patients in each category is indicated by n =.
Fluid Balance by Risk and Correlation to Mortality
Figure 5 compares FBC by risk categories (RC). RC 1 and 2 was compiled from all the 1 and 2 risk level RACHS-1 score patients. RC 3 and 4 was compiled from all the 3 and 4 risk level RACHS-1 score patients. RC 5 and 6 was compiled from all the 5 and 6 risk level RACHS-1 score patients.
Figure 5.
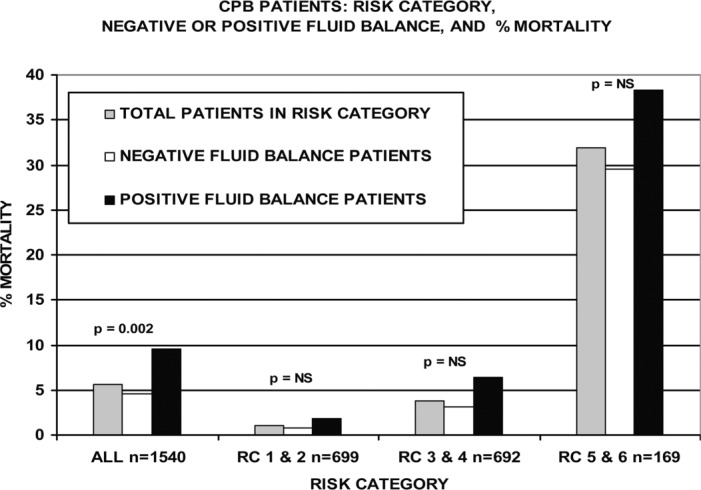
CPB patients are compared by risk category, negative or positive FBC, and mortality. Within the three risk groups, the mortality of positive FBC patients was greater than the negative FBC patients in the same groups. The total number of patients in each category is indicated by n =.
Categories RC 1 and 2 and RC 3 and 4 had lower than average mortality rates. However, within both of these groups the mortality rate for positive FBC patients was approximately twice that of negative FBC patients. Positive FBC patients in RC 5 and 6 also had a greater mortality rate than the negative FBC patients, although not to the same degree as in the other two groups. Unlike the weight categories (Figure 4) the mortality rate differences between negative FBC and positive FBC patients did not approach statistical significance when comparing risk categories.
Fluid Balance by CPB Time and Correlation to Mortality
Figure 6 compares FBC by CPB time categories. Patients on CPB 2 hours or less comprised 75% of the population and had lower than average mortality. And the mortality did not correlate with positive FBCs if the CPB time was ≤120 minutes. However, as the CPB time increased to >120 minutes, mortality increased as well. Patients with a positive FBC after 2 hours of CPB had a mortality 51% higher than zero or negative FBC patients, although this was not statistically significant perhaps due to the small population in this category.
Figure 6.
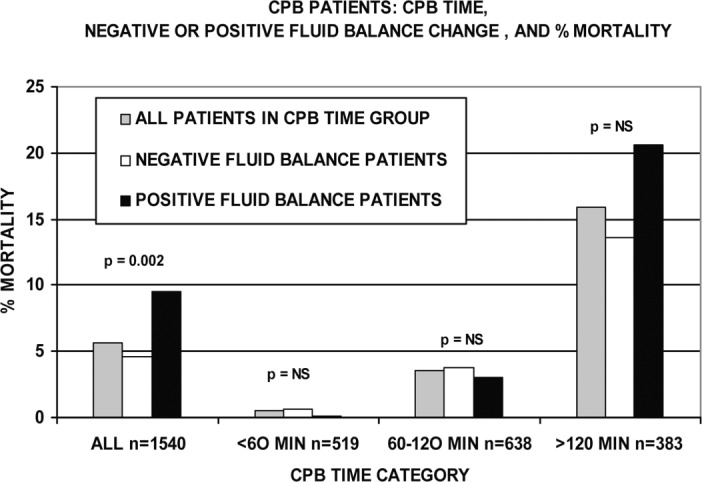
CPB patients are compared to CPB time category, negative or positive FBC, and mortality. Within the three CPB time categories, the patients on CPB longer had higher mortality. However, within each time group, there was no significant mortality difference between positive and negative FBC patients. Significant differences between negative and positive sub-groups are indicated. The total number of patients in each category is indicated by n =.
CPB Time Correlation to Fluid Balance Magnitude
Figure 7 categorizes patients by CPB time and the median magnitude of the FBC. During the first 2 hours of CPB, negative and positive FBC patients tended to have stable fluid balances: −32 mL/kg for the negative FBC patients and +15 mL/kg for the positive FBC patients. For CPB lasting over 2 hours, the positive FBC doubled and the negative FBC became 45% more negative.
Figure 7.
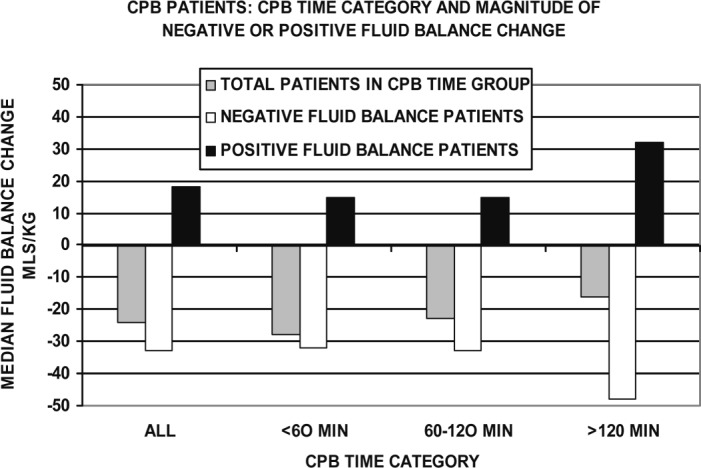
CPB patients are categorized by CPB time category and magnitude of negative or positive FBC. During the first 2 hours of CPB, negative and positive FBC patients tended to have stable fluid balances. Over 2 hours, the positive FBC doubled and the negative FBC became more negative by 45%.
Fluid Balance by Magnitude and Correlation to Mortality
Figure 8 lists patients by the magnitude of the FBC into six categories. The lowest mortality occurred when the FBC was 0 to −40 mL/kg. Only 14 patients of the 1540 patients reviewed had a FBC of zero. These patients had zero mortality as well. Patients with an FBC of +1 to +40 mL/kg had two to three times the mortality and tended to have higher RACHS-1 scores than the 0 to −40 mL/kg groups.
Figure 8.
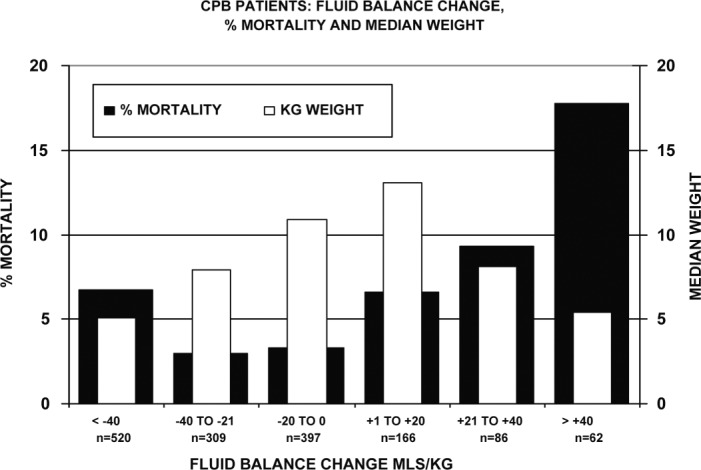
CPB patients are listed by FBC category, mortality, and weight. The median weights are shown to avoid distortion of their averages by extreme outliers. Within the three negative FBC groups, patients with the greatest negative FBC had the highest mortality. Within the three positive FBC groups, patients with the greatest positive FBC had the highest mortality of any group. The total number of patients in each category is indicated by n =.
Negative FBCs Larger than −40 mL/kg
Patients with a negative FBC larger than −40 mL/kg tended to have higher RACHS-1 scores, weigh less and stay longer than the 0 to −40 mL/kg groups. They also had the highest mortality of the three negative FBC groups at 6.7%, compared with the 0 to −20 mL/kg group (3.3%) and the −21 to −40 mL/kg group (2.9%).
Positive Balances Larger than +40 mL/kg
Patients with a positive FBC larger than +40 mL/kg tended to have higher RACHS-1 scores, weigh less, and stay longer than the +1 to +40 mL/kg groups. They also had the highest mortality at 17.7%, compared with the +1 to +20 mL/kg group (6.6% mortality) and the +21 to +40 mL/kg group (9.3% mortality).
DISCUSSION
A Positive FBC Caused by the Perfusionist
A positive FBC on CPB may have been caused by the perfusionist overloading the patient prior to termination of CPB/MUF resulting in an iatrogenic correlation to the increased mortality rate. However, there is no direct evidence of this in the data collected. From Table 2, the negative FBC and positive FBC patients had the same post CPB hematocrit, minimizing the possibility of excessive fluid administration by the perfusionist during CPB. The length of stay between the two groups was similar indicating that post CPB morbidity was not excessive in the positive FBC patients, in general. Furthermore, only 20% of the patients had a positive FBC. If the perfusionists were randomly and unintentionally fluid-overloading patients on CPB, a distribution closer to 50/50 of negative and positive FBCs would be expected.
A Positive FBC and Circulatory Failure
If the positive FBCs on CPB were caused by the need to add fluid during weaning, this may have been due to circulatory shock of some kind caused by myocardial failure or a change in pulmonary or systemic vascular resistance (10). There is evidence that this may be the case. In Table 2, positive FBC patients tended to have higher RACHS-1 scores and have longer CPB and aortic cross clamp times. This implies a more complex repair with the potential for greater damage to the heart along with a greater risk of pulmonary hypertension.
In patients with biventricular heart anatomy and poor contractility, the need for a positive FBC at weaning may be indicative of a myocardial function incapable of sustaining life after surgery. In other biventricular patients, a positive FBC at weaning may be indicative of an increased pulmonary vascular resistance. This can result in right ventricular failure due to excessive pressure load and subsequent left ventricular failure due to reduced preload even though the myocardium itself is not failing.
A Positive FBC and Increased Mortality
The presence of circulatory failure will become obvious if, during the weaning process, the patient requires substantial fluid resuscitation to wean from CPB, resulting in a positive FBC. In this review, larger positive FBCs correlated to an increased mortality rate (Figure 8). In addition, progressive increases in the positive FBC correlated directly to increases in the risk scores, CPB time, and LOS and inversely to decreases in patient weight.
A Positive FBC and Circulatory Enhancement
In certain situations, patients may benefit from a positive FBC at weaning. Patients with stiff, hypertrophied ventricles, such as seen in tetralogy of Fallot or left ventricular outflow tract obstruction, may require a positive FBC to enhance ventricular preload (11,12). Univentricular patients with increased pulmonary vascular resistance will not necessarily need a positive FBC to wean from CPB due to myocardial failure. The failure to wean may be primarily related to failure to oxygenate. In this situation, a surgeon may elect to infuse fluid into the patient during weaning to increase the blood pressure to the pulmonary circulation (13). In this review these specific situations were not evaluated separately. However, positive FBC patients who were on CPB ≤ 120 minutes had slightly less mortality than negative FBC patients in the same time categories suggesting that the positive FBC was beneficial in these instances and not otherwise detrimental (Figure 6).
A Zero or Negative FBC and Decreased Mortality
A zero or negative FBC was associated with decreased mortality in this review (Figure 8). This also implies that there was no need for fluid resuscitation at the termination of CPB/MUF. Even relatively large negative FBCs (−21 to −40 mL/kg), which it seems would partially deplete the circulating blood volume, were associated with low mortality.
The Ready Reservoir
About 33% of all pediatric patients with congenital and acquired heart disease have congestive heart failure (CHF), 78%of them in the first year of life (14,15). Consequently, a substantial number of patients coming to heart surgery will be carrying some amount of excess fluid. In addition, all patients coming to the operating room will receive intravenous rehydration with crystalloid solution in varying amounts to compensate for the prior period of fasting. A few patients will have received intravenous fluid resuscitation prior to surgery. These three factors make up a “ready reservoir” of fluid that can be removed during CPB/MUF by ultrafiltration. Evidently, as much as 40 mL/kg can be removed from the ready reservoir while maintaining a low mortality rate (Figure 8). If this fluid is not removed by ultrafiltration or urination during CPB/MUF, it may contribute to any post CPB edema that occurs. Moreover, patients receiving additional fluid during CPB that is not removed by ultrafiltration, MUF, or urination will have a positive FBC at the end of CPB/MUF. Depending on the circumstances, this may correlate with a higher than average mortality.
Patients coming to surgery with a gross fluid overload (a large ready reservoir) may be in desperate need of fluid removal. But, the perfusionist has no way of knowing how much fluid is removed during CPB/MUF unless it is measured as suggested in the text. If the surgery corrects the heart failure, the patient will wean from CPB/MUF with a negative fluid balance change. If the heart failure is not corrected or if the patient develops an increased pulmonary vascular resistance, the patient will require additional fluid resuscitation to wean from CPB/MUF. This will cause a positive FBC which will further aggravate the already overloaded physiology, increasing the mortality risk.
Excessive Fluid Removal and Increased Mortality
The largest volumes of fluid were removed from smaller patients during CPB/MUF (Figure 8). Patients with more than 40 mL/kg removed during CPB/MUF had higher risk scores than those patients with smaller negative FBCs. A high risk score implies a greater potential for more severe CHF and its accompanying fluid retention. Theoretically, this would make the ready reservoir larger, enabling the removal of larger volumes of fluid. However, in the <−40 mL/kg patient group the mortality rate was higher compared with patients with a smaller negative FBC, the smaller weight and higher risk score notwithstanding. These patients with a large negative FBC were weaned from CPB without the obvious need for fluid resuscitation. This and the fact that CHF is cured in 78% of congenital heart disease patients after surgery leads to the conclusion that circulatory failure was not the primary cause for the increased mortality rate in this particular group of patients (15,16).
The Native Reservoir
Adults have a total body water weight (TBW) of 58%, of which 19% is extracellular water (ECW) with approximately 4% intravascular (plasma) and 15% interstitial. By comparison, neonates have a TBW of 79%, of which 44%is ECW with 6% being intravascular and 38% being interstitial (17). These relatively large volumes of interstitial fluid may act as protection against dehydration or acute blood loss (5,18). Part of this interstitial fluid can be removed without depleting the intravascular volume by using a slow, continuous ultrafiltration as is used during CPB/MUF. Since smaller patients have more ECW than larger patients, this fluid is more easily accessed for removal by ultrafiltration, particularly when the CPB time is lengthy (Figure 7). The authors believe that the normal volume of ECW constitutes a “native reservoir” of fluid that can be partially removed by ultrafiltration techniques, but only at the risk of homeostatic imbalance (19,20).
Native Reservoir Encroachment, Increased Mortality, and Fluid Balance Target
Children have a greater percent of TBW and ECW than adults; the ECW acting to protect against shock. However, this native reservoir may be partially depleted by ultrafiltration during CPB/MUF. Other factors such as a higher pediatric metabolic rate, increased insensible fluid loss, and reduced ability to concentrate urine in the stressful post operative period may further deplete the ECW volume (10). This may compromise organ perfusion even though blood volume seems adequate. The resultant morbidity can become crucial. The initial signs and symptoms of a depleted native reservoir may be subtle because blood pressure is an unreliable indicator of cardiac homeostasis in children. As the post operative period progresses, the physical findings may become more impressive, comparable to overt hypovolemia in adults (10). Patients with a negative FBC larger than −40 mL/kg from ultrafiltration during CPB/MUF may have had all of the ready reservoir fluid removed with subsequent intrusion upon the native reservoir fluid, thereby disrupting the normal homeostasis, precipitating shock and creating the need for copious fluid administration in the post operative period to maintain a sagging cardiac output. This may explain the higher mortality rate seen in this category compared to patients with a smaller negative FBC. Therefore, prudence suggests the perfusionist target a post CPB/MUF fluid balance of −20 mL/kg (±20) to remove most of the ready reservoir fluid while avoiding encroachment upon the native reservoir.
Third Spacing
In addition to the intravascular and interstitial compartments, a third extracellular compartment is thought to develop during times of physiologic stress such as sepsis, trauma, or major surgery (21). In the classic third space scenario, fluid migrates from the intravascular space into the third space where it becomes trapped; refractory to efforts to remove it. The effect is a reduced circulatory fluid volume and hypotension in the presence of severe tissue edema. The treatment is the continuous infusion of intravascular crystalloid or colloid fluids to maintain hemodynamics. The patient may develop either a gross tissue edema and expire of organ failure or return to homeostasis which is signaled by a spontaneous diuresis and recovery (21).
None of the negative FBC patients seemed to exhibit any third space type of scenario while on CPB. Positive FBC patients as a group had a relatively stable fluid load (median of +15 mL/kg) for the first 2 hours of CPB (Figure 7). This probably represented the positive FBC needed for preload to wean from CPB rather than a continuous infusion to maintain a normal circulating volume. After 2 hours of CPB, the positive fluid load increased to +32 mL/kg, which may have been caused by some degree of third spacing. However, the need for even a small but constant infusion of crystalloid fluid to maintain CPB flow and which was not recoverable before the termination of CPB/MUF was uncommon in this patient population. This supports a recently published conclusion that fluid migrating to an actively consuming third space may only be a fictional entity (21). The detrimental impact may be more related to an inadvertent iatrogenic fluid overload or confused with SIRS (21).
SIRS Mediated Fluid Retention
SIRS mediated capillary leak is thought to be a major contributor to edema associated with heart surgery (4). During CPB, SIRS is triggered by blood contact with the synthetic surfaces of the pump circuit. However, 80% of the patients in this review achieved a negative FBC at the end of CPB/MUF. So, the characteristic capillary leak associated with SIRS did not seem to be a significant factor causing edema during CPB in negative FBC patients. The coated circuits, albumin surface pacification, methylprednisolone, and colloid enhanced prime may have effectively stifled much of the SIRS related capillary leak.
In addition, ultrafiltration on CPB has been shown to remove some inflammatory mediators associated with capillary leak (22,23). Virtually, all of the review patients received some amount of ultrafiltration during CPB/MUF. So, this may be another reason why these patients did not seem to manifest SIRS related capillary leak. Theoretically, larger volumes of ultrafiltration would remove larger quantities of inflammatory mediators. It would be incorrect, however, to assume that negative FBC patients underwent more ultrafiltration than the positive FBC patients. Positive FBC patients may have undergone substantial ultrafiltration for the removal of cardioplegia crystalloid, entrained irrigation, excess venous reservoir volume, and ready reservoir fluids, but ultimately needed fluid resuscitation to wean from bypass (Figure 2).
The magnitude of SIRS (and its associated capillary leak and edema) is also related to such things as large incisions in relation to body surface area, long CPB times, long aortic cross clamp times, and low temperatures on CPB (24–27). In this review, positive FBC patients were on CPB for about 40% longer and the aortic cross clamp time was 23% longer (Table 2). Only 10% of the patients on CPB < 60 minutes and 21% of the patients on CPB 60–120 minutes had a positive FBC. However, the magnitude of the positive FBC did not significantly increase until CPB exceeded 2 hours duration and even then, only 33% of the patients had a positive FBC (Figure 7). The longer CPB and aortic cross clamp times indicated a more severe cardiac pathology, meaning that the positive FBC was probably related more to greater cardiac dysfunction necessitating a greater need for fluid resuscitation to wean from CPB than to SIRS-related capillary leak and edema.
SIRS mediated capillary leak can play a pivotal role in morbidity and mortality in CPB patients (28). However, in these study patients, it may have manifested in the post CPB period, which was not quantified in this review. Quite possibly, any positive FBC patients who experienced SIRS in the post-CPB period may have been at a distinct physiological disadvantage due to the acquired perioperative edema compared with the negative FBC patients. This may have contributed to the comparatively large mortality rates seen in positive FBC patients.
Lessons Learned
A zero or negative FBC was achieved in 80% of these patients. Many, but not all, of the 20% of patients with a positive FBC post CPB/MUF probably needed fluid resuscitation to wean from CPB due to myocardial failure or increased pulmonary vascular resistance. However, there is no doubt that some positive FBC patients were left with an unnecessary iatrogenic fluid load. Simple mechanical complications such as undersized or malpositioned venous cannulae requiring additional fluid to assist with venous return might contribute to a positive FBC (29,30). The ignorance of the perfusionist to the magnitude of the FBC coupled with failure to remove the excess fluid during CPB would also contribute (4). This positive FBC, possibly aggravated by post CPB SIRS, may have unnecessarily contributed to a mortality rate as high as 20.6% (Figure 6). Perfusionists should strive to achieve a zero or negative FBC prior to weaning from CPB/MUF to minimize any perfusion related cause of a positive FBC that could contribute to any generalized edema in the post CPB period.
The use of ultrafiltration during CPB was done quickly if there was excess venous reservoir volume. When the amount of fluid available for removal was limited, the use of ultrafiltration was slower or more intermittent during CPB to prevent the loss of circulating volume. Slow continuous ultrafiltration was a common practice during CPB to optimize the hematocrit. This may have encroached upon the native reservoir, because 34% of all the patients (520/1540) had a negative FBC larger than −40 mL/kg. Along with smaller weights and higher RACHS-1 scores, this was associated with a mortality of 6.7% (Figure 8). To prevent this, an ongoing cognizance of the FBC would allow for the removal of ready reservoir fluid and optimization of the hematocrit without excessive fluid removal from the native reservoir.
Ultrafiltration (or patient urination) can assist in keeping the ready reservoir relatively dry. For example, fluid may need to be added temporarily during CPB to assist with variations in venous return, but then it should be removed at a later time during CPB. An accurate I & O during CPB/MUF would assist in accomplishing this and alert the perfusionist when a zero or negative FBC is achieved. At that point, if the patient needs fluid resuscitation to wean from CPB, the fluid can be infused from the venous reservoir.
The gravest danger occurs when CPB lasts longer than 2 hours. Patients on CPB longer than 120 minutes had a mortality rate over seven times greater than patients with shorter times (p < .0001). Up to that point sloppy control of FBC poses little risk to the patient, the mortality being only 2.3% for the combined groups of positive (2.1% mortality) and negative (2.3% mortality) patients. However, in the third hour of CPB the combined groups of positive and negative FBC patients had a mortality of 15.9% (Figure 6) with the positive FBC patients having a mortality rate 51% greater than negative FBC patients (13.6% vs 20.6% mortality). Diligence in maintaining the desired fluid balance for the first 2 hours may make it easier to achieve a zero or negative FBC in the third hour.
Summary
The hypothesis that FBCs at the end of CPB/MUF will correlate to changes in mortality is supported by this review. The FBC on CPB should be monitored and measured and the perfusionist should strive to achieve a zero or negative FBC without encroaching on the native reservoir fluid. This is possible in the majority of patients and, when optimized, correlates directly to a decreased mortality. A positive FBC should be avoided if possible unless it is beneficial in specific situations. Otherwise, a positive FBC may signal a decreased cardiac function leading to an increased mortality. This is supported by a published report that approximately 50% of deaths after congenital heart surgery are due to low cardiac output causes (16). Most of the remaining deaths are caused by sudden cardiac arrest, sepsis, and procedural complications (16).
The limitations of this review are related to its retrospective design, covering 7 years, and changes in personnel and management during that time period. These may alter outcomes and are difficult to evaluate in a retrospective analysis. The open nature of the operative field and absorption of fluids by sterile drapes and other spillage make the quantification of irrigation and blood loss difficult. Nonetheless, attentive and quantifiable I & O, including open field estimates of irrigation and blood loss, may help the perfusionist control edema in the perioperative period and ultimately reduce mortality.
ACKNOWLEDGMENT
The authors wish to thank Ashley K. Sherman, Associate Research Biostatistician, Research and Grants Department at The Children’s Mercy Hospitals and Clinics for her assistance.
REFERENCES
- 1.Toraman F, Evrenkaya S, Yuce M, et al. Highly positive intraoperative fluid balance during cardiac surgery is associated with adverse outcome. Perfusion. 2004;19:85–91. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Raman J, Ronco C.. Intensive care unit management of the critically ill patient with fluid overload after open heart surgery. Cardiology. 2001;96:169–176. [DOI] [PubMed] [Google Scholar]
- 3.Castaneda A, Jonas R, Mayer J, Hanley F.. Cardiac surgery of the neonate and infant: Chapter 2 cardiopulmonary bypass, hypothermia, and circulatory arrest. In: Morbidity of Cardiopulmonary Bypass, 1st Ed. Philadelphia: W.B. Saunders Company, 1994:25. [Google Scholar]
- 4.Hirleman E, Larson DF.. Cardiopulmonary bypass and edema: Physiology and pathophysiology. Perfusion. 2008;23:311–322. [DOI] [PubMed] [Google Scholar]
- 5.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M.. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–740. [DOI] [PubMed] [Google Scholar]
- 6.Maluf MA.. Modified ultrafiltration in surgical correction of congenital heart disease with cardiopulmonary bypass. Perfusion. 2003; 18(Suppl 1):61–68. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins KJ.. Risk adjustment for congenital heart surgery: The RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:180–184. [DOI] [PubMed] [Google Scholar]
- 8.Mou SS, Giroir BP, Molitor-Kirsch EA, et al. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351:1635–1644. [DOI] [PubMed] [Google Scholar]
- 9.Osthaus WA, Sievers J, Breymann T, Suempelmann R.. Bicarbonate buffered ultrafiltration leads to a physiologic priming solution in pediatric cardiac surgery. Interact Cardiovasc Thorac Surg. 2008;7:969–972. [DOI] [PubMed] [Google Scholar]
- 10.McKiernan CA, Lieberman SA.. Circulatory shock in children: An overview. Pediatr Rev. 2005;26:451–460. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Maron MS, Wigle ED, Braunwald E.. The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy: From idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:191–200. [DOI] [PubMed] [Google Scholar]
- 12.Starr JP.. Tetralogy of fallot: Yesterday and today. World J Surg. 2010;34:658–668. [DOI] [PubMed] [Google Scholar]
- 13.Bando K, Turrentine MW, Sharp TG, et al. Pulmonary hypertension after operations for congenital heart disease: Analysis of risk factors and management. J Thorac Cardiovasc Surg. 1996;112:1600–1607, discussion 1607–1609. [DOI] [PubMed] [Google Scholar]
- 14.Massin MM, Astadicko I, Dessy H.. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol. 2008;31:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommers C, Nagel BH, Neudorf U, Schmaltz AA.. Congestive heart failure in childhood. An epidemiologic study. Herz. 2005;30:652–662 [in German]. [DOI] [PubMed] [Google Scholar]
- 16.Ma M, Gauvreau K, Allan CK, Mayer JE Jr, Jenkins KJ.. Causes of death after congenital heart surgery. Ann Thorac Surg. 2007;83: 1438–1445. [DOI] [PubMed] [Google Scholar]
- 17.Robinson M, Roberton D.. The child who needs fluid replaced. In: Practical Pediatrics, 5th Ed. New York: Churchill Livingston, Elsevier, 2003:190. [Google Scholar]
- 18.Brooks ER, Fatallah-Shaykh SA, Langman CB, Wolf KM, Price HE.. Bioelectric impedance predicts total body water, blood pressure, and heart rate during hemodialysis in children and adolescents. J Ren Nutr. 2008;18:304–311. [DOI] [PubMed] [Google Scholar]
- 19.Yashiro M, Kamata T, Segawa H, Murakami T, Kadoya Y, Muso E.. How does higher ultrafiltration within the conventional clinical range impact the volume status of hemodialysis patients? Blood Purif. 2009;27:253–260. [DOI] [PubMed] [Google Scholar]
- 20.Ishibe S, Peixoto AJ.. Methods of assessment of volume status and intercompartmental fluid shifts in hemodialysis patients: Implications in clinical practice. Semin Dial. 2004;17:37–43. [DOI] [PubMed] [Google Scholar]
- 21.Jacob M, Chappell D, Rehm M.. The ‘third space’–fact or fiction? Best Pract Res Clin Anaesthesiol. 2009;23:145–157. [DOI] [PubMed] [Google Scholar]
- 22.Osthaus WA, Gorler H, Sievers J, et al. Bicarbonate-buffered ultrafiltration during pediatric cardiac surgery prevents electrolyte and acidbase balance disturbances. Perfusion. 2009;24:19–25. [DOI] [PubMed] [Google Scholar]
- 23.Berdat PA, Eichenberger E, Ebell J, et al. Elimination of proinflammatory cytokines in pediatric cardiac surgery: Analysis of ultrafiltration method and filter type. J Thorac Cardiovasc Surg. 2004;127:1688–1696. [DOI] [PubMed] [Google Scholar]
- 24.Hammer S, Fuchs AT, Rinker C, Daebritz S, Kozlik-Feldmann R, Netz H.. Interleukin-6 and procalcitonin in serum of children undergoing cardiac surgery with cardiopulmonary bypass. Acta Cardiol. 2004;59:624–629. [DOI] [PubMed] [Google Scholar]
- 25.Prat C, Ricart P, Ruyra X, et al. Serum concentrations of procalcitonin after cardiac surgery. J Card Surg. 2008;23:627–632. [DOI] [PubMed] [Google Scholar]
- 26.Schupp M, Swanevelder JL, Peek GJ, Sosnowski AW, Spyt TJ.. Postoperative extracorporeal membrane oxygenation for severe intraoperative SIRS 10 h after multiple trauma. Br J Anaesth. 2003; 90:91–94. [PubMed] [Google Scholar]
- 27.Peek GJ, Firmin RK.. The inflammatory and coagulative response to prolonged extracorporeal membrane oxygenation. ASAIO J. 1999;45:250–263. [DOI] [PubMed] [Google Scholar]
- 28.Mei YQ, Ji Q, Liu H, et al. Study on the relationship of APACHE III and levels of cytokines in patients with systemic inflammatory response syndrome after coronary artery bypass grafting. Biol Pharm Bull. 2007;30:410–414. [DOI] [PubMed] [Google Scholar]
- 29.Manzer R, Sutton RG, Ploessl J, Niles S, Behrendt D.. Cardiopulmonary bypass venous cannulation challenges in a paediatric patient with complex congenital heart disease: A case report. Perfusion. 1997;12:203–206. [DOI] [PubMed] [Google Scholar]
- 30.Jegger D, Chassot PG, Bernath MA, et al. A novel technique using echocardiography to evaluate venous cannula performance perioperatively in CPB cardiac surgery. Eur J Cardiothorac Surg. 2006;29:525–529. [DOI] [PubMed] [Google Scholar]


