Abstract:
Open repair of aneurysms and dissections involving the aortic arch has traditionally been associated with high rates of morbidity and mortality, primarily because of the complications related to the need to interrupt normal blood flow to the cerebral circulation. Over the past several years, our approach to these operations has gradually changed largely through the introduction of various techniques aimed at reducing the risk of neurologic complications. Key technical changes have included the shift from using retrograde cerebral perfusion to using antegrade cerebral perfusion, the introduction of axillary artery perfusion, and the change from using the patch technique to using the Y-graft technique to reattach the brachiocephalic branches. By using this combination of techniques, surgeons can perform aortic arch replacement with excellent early outcomes. In this update, we summarize the evolution of our surgical techniques and perfusion strategies for performing open repair of the aortic arch.
Keywords: aneurysm, aortic, aortic arch, aortic operation, cerebral protection, perfusion
Aortic arch replacement remains one of the most formidable operations in cardiovascular surgery. The aortic arch is defined anatomically as that segment of the aorta from which the innominate artery, left common carotid artery (LCCA), and left subclavian artery (LSCA) arise. Open replacement of the arch segment entails interrupting circulation to these brachiocephalic vessels during the repair, necessitating the use of strategies to protect the brain in the process. Recent advances in cerebral protection strategies and in surgical techniques have been developed with the goal of improving postoperative outcomes, and over the past several years, we have gradually introduced several of these techniques in our practice. Key technical changes have included the shift from using retrograde cerebral perfusion (RCP) to using antegrade cerebral perfusion (ACP), the introduction of axillary artery perfusion, and the change from using the island technique to using the Y-graft technique to reattach the brachiocephalic branches (1–3). In this update, we summarize the evolution of our surgical techniques and perfusion strategies for performing open repair of the aortic arch.
AORTIC ARCH REPAIR, CIRCA 2001
Ten years ago, our typical approach to aortic arch repairs featured many of the techniques developed by the pioneers of aortic arch surgery during the preceding four and a half decades (4–6). In all patients with acute aortic dissection and in most with degenerative aortic arch aneurysms without dissection, the arterial cannula for cardiopulmonary bypass (CPB) was inserted into a femoral artery. In suitable patients with nondissection arch aneurysms, we occasionally inserted the cannula directly into the aneurysmal ascending aorta to carry out the cooling phase of CPB. In most cases, bicaval venous drainage cannulas were placed. Under the guidance of electroencephalographic (EEG) monitoring, cooling to profound levels of hypothermia was then carried out until EEG silence was achieved; this generally occurred at temperatures between 15°C to 20°C. After the patient was adequately cooled, we initiated hypothermic circulatory arrest (HCA). To enhance brain protection, we generally used RCP during the HCA period (7,8). This technique directed oxygenated blood from the CPB circuit into the head through the snared superior vena caval cannula. The RCP flow was delivered through a connection between the arterial inflow line and the superior vena caval cannula line.
During HCA and RCP, the ascending aorta and transverse aortic arch were opened. In cases of aortic dissection, the dissecting membrane was excised proximally and the false lumen was obliterated at the level of the planned distal anastomosis. In patients who needed total arch replacement, we first performed a distal anastomosis between the graft and the proximal descending thoracic aorta. This anastomosis was created either at the distal end of the graft or, in patients with an aneurysmal descending thoracic aorta, at the folded edge of an invaginated elephant trunk graft (9). After completing the distal anastomosis, we cut an oval opening in the side of the graft adjacent to the origins of the three brachiocephalic branches. An island of aortic tissue surrounding these vessels was then sutured to the opening in the graft (Figure 1). After the brachiocephalic anastomosis was completed, RCP was stopped and arterial inflow was resumed through either a femoral cannula or a cannula inserted directly into the side of the graft. The distal aorta and the arch were then deaired before we clamped the graft, restored full systemic CPB, and proceeded with the proximal portion of the repair.
Figure 1.
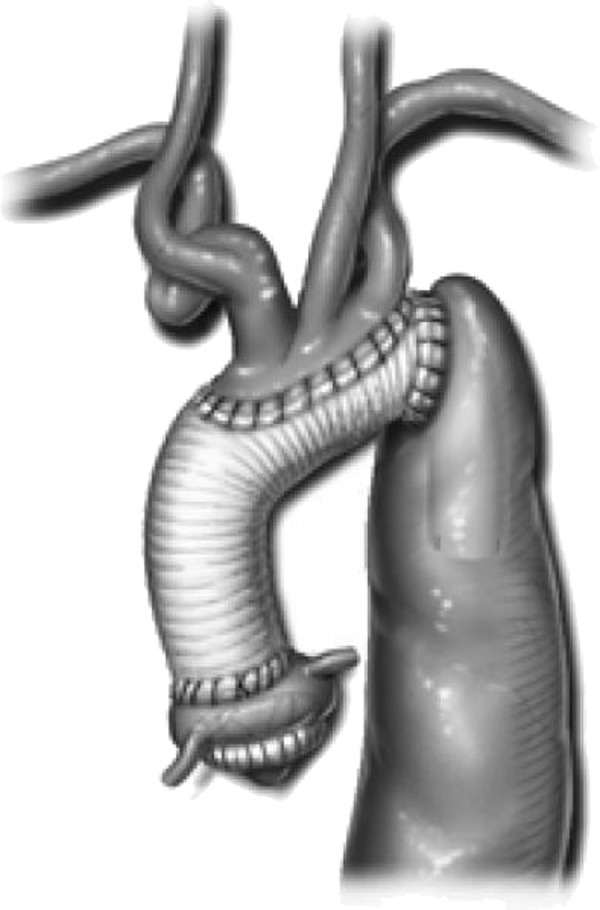
A completed total aortic arch replacement by the traditional elephant trunk approach. At the distal anastomosis, the tubular aortic graft has been sewn directly to the aneurysmal descending thoracic aorta. The brachiocephalic branches have been reattached by using the island technique. The “trunk” of graft is left suspended in the descending thoracic aorta to be used during the subsequent second-stage repair of the remaining distal aneurysm. Reproduced with permission from Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, Mathews JB, Pollock RE, eds. Schwartz’s Principles of Surgery, 9th ed. New York: McGraw-Hill; 2010; Chap. 22, Figure 22-6A.
TECHNICAL ADVANCES
Over the past 10 years, we have incorporated several technical advances in an effort to reduce complications and improve outcomes (10–12). First, we began increasing our use of ACP to provide better cerebral protection than that afforded by HCA or HCA plus RCP. The limitations of using HCA alone (i.e., without perfusion adjuncts) are well recognized. During arch repairs that only necessitate short periods of HCA (i.e., less than 30 minutes, like in elective hemiarch replacement), HCA alone provides satisfactory brain protection. However, during more complex repairs (e.g., total arch replacement) that necessitate substantially longer HCA times, the use of HCA alone is associated with a high risk of stroke and death (13). To address the limitations of HCA, RCP was developed; it gained popularity as a perfusion adjunct and appeared to provide better cerebral protection than HCA alone (7,8). However, experimental data increasingly suggested that RCP was not effectively delivering blood to the brain, and the benefits of RCP seemed to be chiefly related to maintaining regional hypothermia and to flushing air and debris out of the cerebral circulation. Consequently, we began placing balloon-tipped catheters into the origins of the innominate and LCCA to deliver ACP during the HCA period.
Our change from using RCP to using ACP was facilitated by our second major change in technique: the use of right axillary artery perfusion (Figure 2) (12). Delivering CBP inflow from the femoral artery has several disadvantages in patients with extensive aortic disease (1). Most notably, retrograde flow through the diseased atheromatous distal aorta can potentially cause stroke and other complications by plaque embolization. Moreover, in cases of aortic dissection, blood flow may preferentially pressurize the false lumen, causing cerebral malperfusion. These problems can be avoided by using the right axillary artery for CPB inflow. The axillary artery is rarely involved in aneurysmal disease or dissection, which makes it an excellent site for establishing inflow. Furthermore, the right axillary artery provides a direct route to the right common carotid artery for ACP; once systemic HCA is begun, ACP is initiated by reducing axillary inflow to 10 mL/kg/min and occluding the innominate artery by using either a Rumel tourniquet or a vascular clamp (Figure 3). Because most patients have a complete circle of Willis, right-sided ACP generally provides adequate left-sided cerebral protection. When direct left-sided ACP is indicated, it can be delivered through the LCCA by inserting a balloon-tipped catheter that has been connected to a Y-limb from the arterial CBP line.
Figure 2.
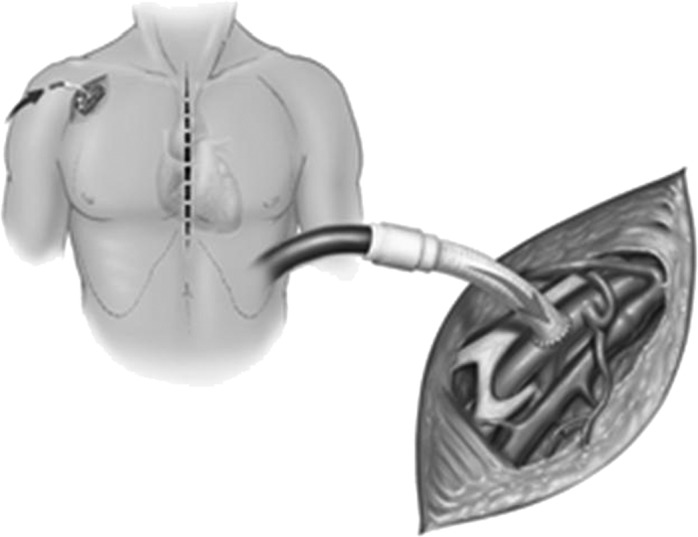
The right axillary artery is exposed through the deltopectoral groove; care is taken to avoid injuring the adjacent vein and brachial plexus. An 8-mm vascular graft is sutured end-to-side to the right axillary artery and then connected to the cardiopulmonary bypass inflow tubing. Reproduced with permission from Coselli JS, LeMaire SA, eds. Aortic Arch Surgery: Principles, Strategies and Outcomes. West Sussex, UK: Blackwell Publishing Ltd; 2008; Chap. 28, Figure 28.1.
Figure 3.
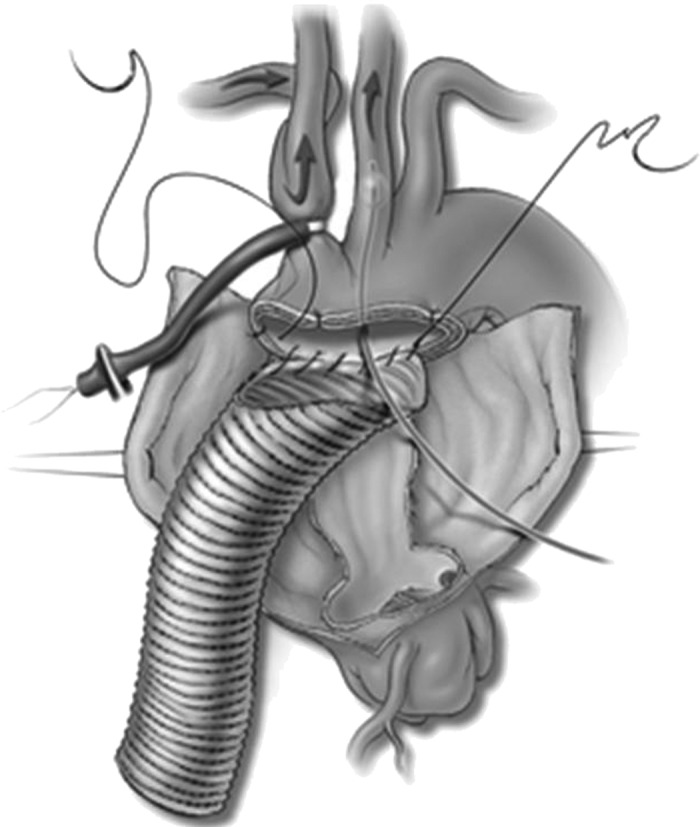
Hemiarch replacement in a patient with acute aortic dissection. The beveled distal anastomosis is performed while the patient receives antegrade cerebral perfusion on the right side through right axillary artery inflow while the innominate artery is occluded with a tourniquet and on the left side through a balloon-tipped catheter placed in the left common carotid artery. Reproduced with permission from Creager MA, Dzau VS, Loscalzo J, eds. Vascular Medicine. Philadelphia, PA: WB Saunders; 2006. Copyright © Saunders/Elsevier; 2006; Figure 354-3F.
The third change in technique involved our approach to reattaching the brachiocephalic branches to the aortic graft during total arch replacement procedures. The traditional “island technique” has two major disadvantages. First, using this technique sometimes results in difficult-to-control bleeding from the posterolateral aspect of the anastomosis. Second, the aortic tissue left behind in the island patch sometimes becomes aneurysmal, necessitating reoperation; this problem is particularly prevalent in patients with connective tissue disorders (14). To simplify the management of the brachiocephalic vessels and to provide safe access for providing continuous ACP, the trifurcated graft technique was developed by Spielvogel and colleagues (3). In this approach, each brachiocephalic branch vessel is sutured end-to-end to one of the three distal ends of a trifurcated graft (Figure 4). This double-Y-graft is then used to provide ACP while the ascending aorta and aortic arch are replaced with an aortic graft. The proximal end of the Y-graft is then anastomosed to an opening in the aortic graft. Encouraged by the excellent results Spielvogel and colleagues (2) achieved with this approach, we adopted a modified version of the trifurcated graft technique (11). This technique allows the surgeon versatility in configuring the arch reconstruction, enabling the site of the distal aortic anastomosis and the lengths and lay of the reconstructed brachiocephalic vessels to be tailored specifically to the patient’s anatomy. The flexibility this technique affords also makes it easier to access any bleeding anastomotic sites for repair.
Figure 4.
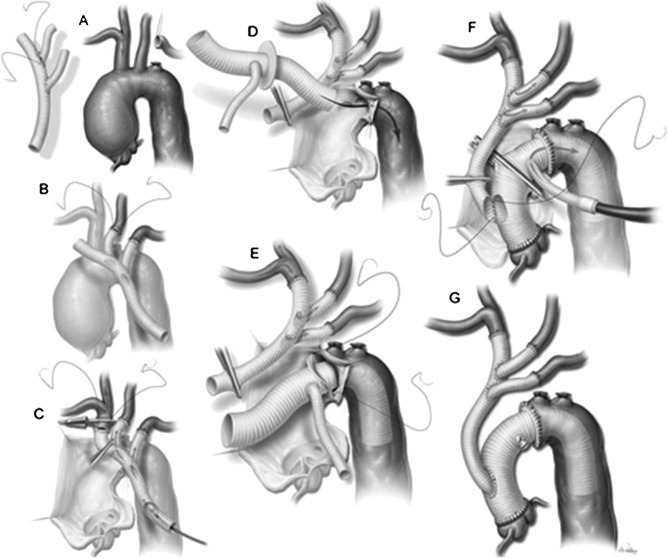
Our current approach to performing total aortic arch replacement, featuring right axillary artery cardiopulmonary bypass inflow, antegrade cerebral perfusion, the Y-graft technique, and a collared elephant trunk graft. See text for details. Reproduced with permission from LeMaire SA, Price MD, Parenti JL, Johnson ML, Lay AD, Preventza O, Huh J, Coselli JS. Early outcomes after aortic arch replacement by using the Y-Graft technique. Ann Thorac Surg 2011;91:700–8. Figure 2. Copyright Elsevier.
Another important change related to the grafts used to reconstruct the arch was the introduction of the collared arch graft. Traditionally, elephant trunk repairs of the aortic arch were done by invaginating the proximal portion of a typical tube graft into its lumen and then sewing the folded edge of the graft to the proximal portion of the descending thoracic aorta (6,9). However, when this technique is used, a substantial size discrepancy between the graft and the aorta is not uncommon; this discrepancy creates undue tension on the anastomosis, causing bleeding or, later, the formation of pseudoaneurysms. The collared elephant trunk graft was developed to simplify the repair and allow a tension-free distal anastomosis (15). The collar is easily cut to match the size of the native aorta and then used for the distal aortic anastomosis (Figure 4).
Finally, the mentioned changes have enabled us to reconsider our approach to temperature management during arch repair. The use of the right axillary artery for CPB and ACP inflow, combined with the Y-graft technique for reattaching the brachiocephalic branches, enables continuous perfusion of the brain during the aortic arch repair, reducing the cerebral circulatory arrest time to zero (16). This has allowed surgeons to use higher temperatures than those necessary when full HCA is required. During most arch replacement operations, we now use moderate hypothermia (i.e., 22–24°C); we have observed that, compared with the use of colder temperatures during HCA, the use of moderate hypothermia is associated with shorter CPB time, shorter length of hospital stay, and lower early mortality. An additional potential benefit of avoiding profound HCA is reduced coagulopathy, which should decrease the amount of blood products transfused and prevent the development of acute lung injury and other transfusionrelated sequelae (16).
CURRENT APPROACH AND EARLY OUTCOMES
Our current routine approach to open repair of the aortic arch features right axillary artery CPB inflow and moderate HCA with ACP; we use this approach for all open arch replacement procedures, including hemiarch replacements (Figure 3), total arch replacements, elephant trunk procedures (Figure 4), aortic dissection repairs, and nondissection aneurysm repairs. When performing total arch replacement, we also use the Y-graft technique (11). The Y-graft can be constructed to specifically suit the patient’s anatomy and the planned reconstruction (Figure 4A). Alternatively, an appropriately sized prefabricated Y-graft can be used. We establish CPB through an 8-mm vascular graft that has been sewn to the right axillary artery (Figure 2) and through a dual-stage right atrial cannula (12). During the cooling phase of CPB, the two side branches of the Y-graft are sequentially sewn end-to-end to the LSCA and LCCA, which have been ligated and transected near their origins from the arch (Figure 4B). Once a satisfactory level of moderate hypothermia (approximately 22–23°C) has been reached, flow is reduced to 10 mL/kg/min and the innominate artery is occluded with a bulldog clamp (Figure 4C); this establishes ACP to the right side of the cerebral circulation. By inserting a balloon-tipped catheter into the proximal end of the Y-graft, ACP can also be delivered through the left-sided branch vessels; this is particularly useful when preoperative imaging or intraoperative monitoring (e.g., cerebral near-infrared spectroscopy) raises concern about cerebral cross-circulation. The ascending aorta and aortic arch are then opened, and the transected innominate artery is anastomosed end-to-end to the distal end of the main body of the Y-graft. After the brachiocephalic anastomoses have been completed, the Y-graft is deaired and clamped proximally to maintain ACP throughout the subsequent insertion of the aortic graft. A flexible suction catheter connected to the cardiotomy suction tubing is placed in the aortic arch to create a bloodless operative field. The distal anastomosis is then performed to the proximal descending thoracic aorta. If a future second-stage repair is anticipated because of disease in the descending thoracic aorta, an elephant trunk repair using a collared graft is performed (Figure 4D–E); because the aortic arch has been debranched, this anastomosis can often be performed within the arch (i.e., proximal to the LSCA or LCCA). After the distal aortic anastomosis is completed, a clamp is applied to the graft, and systemic perfusion is re-established through a side branch off the aortic graft (Figure 4F). After the proximal portion of the aortic repair—which may include replacement of the aortic valve, the aortic root, or just the ascending aorta—has been completed, the beveled proximal end of the Y-graft is anastomosed to an opening in the right anterolateral aspect of the aortic graft in an end-to-side fashion (Figure 4F–G).
The early results of this approach have been very encouraging. We recently published a retrospective analysis of 55 patients in whom we performed total aortic arch replacement by using the mentioned techniques (11). This group included 12 patients (22%) who underwent an urgent or emergent procedure, 27 patients (49%) who underwent a concomitant aortic valve procedure (including root replacement in five), and 33 patients (60%) who had a previous sternotomy. The median lowest nasopharyngeal temperature was 22.0°C, the median systemic circulatory arrest time was 65 minutes, and the median cerebral circulatory arrest time was 0 minutes. For the overall group, the early mortality rate was 2%; stroke occurred in 5% of patients, renal failure necessitating dialysis developed in 5%, and reoperation for bleeding was necessary in 7%. The outcomes in the subset of 46 patients (84%) who underwent elephant trunk repairs were particularly notable; the 2% early mortality rate in the recent cohort compared very favorably to the 12% early mortality rate in our previously reported series of 148 elephant trunk repairs that were performed with the older techniques (9).
SUMMARY
Open repair of aneurysms and dissections involving the aortic arch has traditionally been associated with high rates of morbidity and mortality, largely because of the complications associated with interrupting normal blood flow to the cerebral circulation. Over the past 10 years, our approach to these operations has gradually changed through the introduction of various techniques aimed at reducing the risk of neurologic complications. Key technical changes have included the shift from using RCP to using ACP, the introduction of axillary artery perfusion, and the change from using the patch technique to using the Y-graft technique to reattach the brachiocephalic branches. By using this combination of techniques, surgeons can perform aortic arch replacement with excellent early outcomes.
ACKNOWLEDGMENTS
We thank Scott A. Weldon, MA, CMI, for creating the medical illustrations, and Stephen N. Palmer, PhD, ELS, for contributing to the editing of the manuscript.
REFERENCES
- 1.Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM.. Axillary artery: An alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J Thorac Cardiovasc Surg. 1995;109:885–891. [DOI] [PubMed] [Google Scholar]
- 2.Spielvogel D, Halstead JC, Meier M, et al. . Aortic arch replacement using a trifurcated graft: Simple, versatile, and safe. Ann Thorac Surg. 2005;80:90–95. [DOI] [PubMed] [Google Scholar]
- 3.Spielvogel D, Strauch JT, Minanov OP, Lansman SL, Griepp RB.. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg. 2002;74:S1810–s1814. [DOI] [PubMed] [Google Scholar]
- 4.Coselli JS, Buket S, Djukanovic B.. Aortic arch operation: Current treatment and results. Ann Thorac Surg. 1995;59:19–27. [DOI] [PubMed] [Google Scholar]
- 5.Cooley DA.. Historical perspective: The evolution of aortic arch surgery. In: Coselli JS, LeMaire SA, eds. Aortic Arch Surgery: Principles, Strategies, and Outcomes. Chichester, UK; Hoboken, NJ: Wiley-Blackwell; 2008:3–11. [Google Scholar]
- 6.Crawford ES, Coselli JS.. Replacement of the aortic arch. Semin Thorac Cardiovasc Surg. 1991;3:194–212. [PubMed] [Google Scholar]
- 7.Coselli JS.. Retrograde cerebral perfusion via a superior vena caval cannula for aortic arch aneurysm operations. Ann Thorac Surg. 1994;57:1668–1669. [DOI] [PubMed] [Google Scholar]
- 8.Raskin SA, Fuselier VW, Reeves-Viets JL, Coselli JS.. Deep hypothermic circulatory arrest with and without retrograde cerebral perfusion. Int Anesthesiol Clin. 1996;34:177–193. [DOI] [PubMed] [Google Scholar]
- 9.LeMaire SA, Carter SA, Coselli JS.. The elephant trunk technique for staged repair of complex aneurysms of the entire thoracic aorta. Ann Thorac Surg. 2006;81:1561–1569. [DOI] [PubMed] [Google Scholar]
- 10.Coselli JS, Green SY.. Evolution of aortic arch repair. Tex Heart Inst J. 2009;36:435–437. [PMC free article] [PubMed] [Google Scholar]
- 11.LeMaire SA, Price MD, Parenti JL, et al. . Early outcomes after aortic arch replacement by using the Y-graft technique. Ann Thorac Surg. 2011;91:700–708. [DOI] [PubMed] [Google Scholar]
- 12.Wong DR, Coselli JS, Palmero L, et al. . Axillary artery cannulation in surgery for acute or subacute ascending aortic dissections. Ann Thorac Surg. 2010;90:731–737. [DOI] [PubMed] [Google Scholar]
- 13.Svensson LG, Crawford ES, Hess KR, et al. . Deep hypothermia with circulatory arrest: Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg. 1993;106:19–28. [PubMed] [Google Scholar]
- 14.Svensson LG, Crawford ES.. Cardiovascular and Vascular Disease of the Aorta. Philadelphia, PA: W.B. Saunders Co.; 1997:97– 100. [Google Scholar]
- 15.Neri E, Massetti M, Sani G.. The “elephant trunk” technique made easier. Ann Thorac Surg. 2004;78:e17–e18. [DOI] [PubMed] [Google Scholar]
- 16.Matalanis G, Koirala RS, Shi WY, Hayward PA, McCall PR.. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J Thorac Cardiovasc Surg. 2011;142:809–815. [DOI] [PubMed] [Google Scholar]