Abstract:
This report describes the assessment of three specific safety-related specifications in the consideration of an alternate oxygenator; first the grip strength relationship between various oxygenator connectors and SMARxT® tubing, second, the grip strength of various biopassive tubings and an isolated SMARxT® connector, and finally, the accuracy of the arterial outlet temperature measurement. Grip strength experiments for the connections between the SMARxT® tubing and the venous reservoir outlet and the oxygenator venous inlet and oxygenator arterial outlet of the Medtronic Affinity®, Sorin Synthesis®, Sorin Primox®, and Terumo Capiox® RX25 oxygenators were performed. In addition we compared the grip strength of polyvinyl chloride, Physio®, Trillium®, Carmeda®, X-Coating®, and SMARxT® tubing. The accuracy of the integrated arterial outlet temperature probes was determined by comparing the temperatures measured by the integrated probe with a precision reference thermometer. Connector grip strength comparisons for the evaluation oxygenators with SMARxT® tubing showed significant variation between oxygenators and connections (p = .02). Evaluation of the arterial outlet showed significant variation between evaluation oxygenators, while at the venous reservoir outlet and oxygenator inlet, there were no significant differences. Grip strength comparison data for the various tubing types demonstrated a main effect for tubing type F(5, 18) = 8.01, p = .002, ηp2 = .77. Temperature accuracy measurements demonstrated that all oxygenators overread the arterial outlet temperature at 15°C, whilst at temperatures ≥25°C, all oxygenators underread the arterial outlet temperature. The integrity of SMARxT® tubing connection is influenced by the connector type, and may decline over time, highlighting the importance to not consider interchanging components of the bypass circuit as inconsequential.
Keywords: cardiopulmonary bypass, tubing, temperature accuracy, rewarming, safety, cardiac surgery
The evaluation and selection of an oxygenator for clinical use is an important process for the perfusionist in the practice of cardiopulmonary bypass (CPB). Within the products available there exists a spectrum of features that may influence the assessment of a particular oxygenator for routine clinical use, including gas transfer capability, priming volume, membrane surface area, biocompatibility, heat exchanger performance, and blood flow dynamics. Various sources of information are available when evaluating alternative devices for this purpose, including the manufacturer’s specifications and published, peer-reviewed literature. Typically manufacturers focus on physical (size, flow rate, etc) and performance-related characteristics including gas transfer and heat exchanger capabilities. Clinical studies will often focus on a narrow spectrum of features of interest to the clinical group conducting the evaluation; these have recently included studies evaluating oxygen exchange (1–3), trans-oxygenator pressure drop (1,3,4), heat exchanger performance (1), blood trauma (1,2), and biocompatibility (5). Both clinical and in vitro evaluations have comparatively assessed the air separation performance of membrane oxygenators (6–9). Two safety-related specifications of particular interest to our group to evaluate when considering an alternate oxygenator are the strength of the connections between the oxygenator and the circuit tubing and the accuracy of the arterial temperature measurement.
Newling and Morris (10) demonstrated a decrease in tension required to disconnect SMARxT® tubing (Sorin, Milano, Italy) compared to polyvinyl chloride (PVC) tubing using an isolated tubing connector. However, a comparative evaluation of the connections between SMARxT® tubing with various oxygenator connectors has not been reported, nor has a comparison using different types of biopassive tubing. We previously reported on the integrity of oxygenator-tubing connections prompted by two clinical incidents in which disconnection of SMARxT® tubing at the venous reservoir outlet of the Capiox SX25RX® oxygenator (Terumo Corporation, Japan) occurred during CPB (11). Investigation revealed that in the case of the Capiox SX25RX® oxygenator, displacement of SMARxT® tubing occurs over time when connected to the venous reservoir outlet connector. The integrity of this venous reservoir outlet connector/tubing connection was influenced by the physical characteristics of the connector, in particular the relationship between the distances between barbs and the width of the applied cable tie and the tapered nature of the neck of the connector, leading to a decline in integrity over time (11).
We have previously reported the accuracy of the oxygenator arterial temperature measurement used in our routine practice (12), however limited data exist for comparison (13,14). The impact of hyperthermia on the brain has been acknowledged as pivotal to any discussion on the consequences of temperature management during CPB; the pivotal factors involved include the rate of rewarming and the temperature of the arterial blood being delivered to the patient (12). Underreading of the arterial outlet temperature at 37 °C has been previously reported (12–14), therefore the evaluation of the accuracy of this measurement prior to clinical use is important in the context of avoidance of cerebral hyperthermia.
This report describes the assessment of three specific safety-related specifications of interest to our group in the consideration of an alternate oxygenator. First, we investigated the grip strength relationship between various oxygenator connectors and SMARxT® tubing. Second, we looked at grip strength of various biopassive tubings and an isolated SMARxT® connector. Finally, we assessed the accuracy of the arterial outlet temperature measurement.
METHODS
Four oxygenators from three manufacturers were chosen to evaluate for routine use: Affinity® (Medtronic, Minneapolis, MN), Synthesis® and Primox® (Sorin), and the Capiox RX25® (Terumo Corporation).
Part A: Determination of a Baseline Criterion for Grip Strength
To define a baseline grip strength, we measured the change in the force required to disconnect SMARxT® tubing from the venous outlet connector of the Capiox SX25R03® oxygenator after 24 hours. This oxygenator was chosen as we had used it routinely in over 6000 cases without incident (11). A 10 cm length of SMARxT® 3/8″ × 3/32″ tubing was pushed on to the maximal limit of the venous reservoir connector for three separate Capiox SX25R03s®. A 2.6 mm cable tie was positioned between the middle barbs of the connector and secured with a Panduit GS2B cable tie gun (Panduit Corporation, Tinley Park, IL) set at tension position 8. The oxygenator reservoirs were secured into a purpose built housing and secured into a vice attached to the bed of an MDV4–5 milling machine (Eumega, Hsin Chuang City, Taiwan) with adjustable height. A Mecmesin AFG1000N electronic force gauge (Mecmesin, Slinfold, UK) was attached to the body of the milling machine and set to record maximum force. A 6.1 mm hole was drilled at 20 mm from the end of the tubing for insertion of a hook attached to the force gauge, with vice grip pliers securing the hook to the tubing to reduce tearing. The height of the milling machine bed was adjusted continuously via the electronic height adjustment to create increasing force on the tubing. The height adjustment was continued until tubing disconnection occurred. To create the influence of weight over time the process was repeated with a 500 g mass attached to the end of the tubing for 24 hours prior to measurement of the force required for disconnection. To allow quantification and comparison with our previously reported incident (11), this method was repeated on the venous outlet connector of the Capiox SX25RX® oxygenator.
Part B: Evaluation Oxygenator Connector Physical Measurements
The physical characteristics of the arterial outlet, venous inlet, and venous reservoir outlet connectors were measured in three of each evaluation oxygenator using a digital micrometer (Mitutoyo, Japan) to identify any variation in barb diameter or overall length of the connectors. Where the end of the connector was bevelled, the connector length was measured from the bottom of the neck to the shortest part of the bevelled end. The first barb diameter was measured as the external diameter of the barb closest to the neck of the connector, and the second barb diameter, the external diameter of the barb closest to the end of the connector.
Part C: Evaluation Oxygenator Connector Grip Strength
To measure the tubing/connector grip strength interaction for the venous reservoir outlet, the oxygenator venous inlet, and oxygenator arterial outlet in each of the evaluation oxygenators, the experimental protocol in Part A was repeated for each combination three times.
Part D: Comparison of Different Tubing Types
To compare the grip strength of different biopassive tubings, we compared each against PVC tubing. Five different tubing types were evaluated: Physio® (Sorin, Milano, Italy), Trillium® (Medtronic, Minneapolis, MN), Carmeda® (Medtronic, Minneapolis, MN), X-Coating® (Terumo Corp., Tokyo, Japan), and SMARxT® tubing. A 10 cm length of 3/8″ × 3/32″ PVC tubing (Sorin) was pushed on to the maximal limit of a 3/8″–1/2″ SMARxT® connector (Sorin). A 2.6 mm cable tie positioned between the middle barbs of the connector was secured with a Panduit GS2B cable tie gun set at tension position 8. A snug-fitted steel rod was inserted into the 1/2″ end of the connectors, and secured into a vice attached to the bed of the milling machine. A 6.1 mm hole was drilled at 20 mm from the end of the tubing for insertion of a hook attached to the force gauge, with vice grip pliers securing the hook to the tubing to reduce tearing. The height of the milling machine bed was adjusted continuously via the electronic height adjustment to create increasing force on the tubing. The height adjustment was continued until tubing disconnection occurred. Measurements were repeated three times using a new connector and length of tubing.
Part E: Evaluation Oxygenator Arterial Temperature Measurement Accuracy
To determine the accuracy of the arterial outlet temperature measurement, experiments were performed in vitro. Each experimental circuit consisted of an evaluation oxygenator and an arterial outlet line that connected to the venous inlet of the reservoir. The line consisted of 80 cm of SMARxT® tubing with an internal diameter of 3/8″, a 3/8–1/2″ SMARxT® connector, and 20 cm of SMARxT® tubing with an internal diameter of 1/2″. After being CO2 flushed for 3 minutes, circuits were primed and debubbled according to the manufacturer’s instructions. The circuits were primed with expired donor blood and diluted with .9% saline solution to a hematocrit of 21–24%. Flow through the circuits was maintained at 4 L/min using a COBE roller pump (COBE Cardiovascular, Arvada, CO) with appropriate calibration and occlusion. Line pressure was maintained at 150 ± 10 mmHg by the use of a gate clamp. Room air gas flow through the test oxygenators was maintained at 500 mL/min. Oxygenator sampling manifolds remained open, whilst the oxygenator purge lines were closed. Temperature probe cables for each oxygenator were obtained from the manufacturers. The accuracy of the integrated arterial outlet temperature probes were determined by comparing the temperatures measured by the integrated probe and the temperature of the blood inside the tubing adjacent to the probe. The temperature of the blood was measured using an Instrulab 4601–40–01–03–07 precision reference thermometer (Instrulab, Dayton, OH) inserted into the tubing through a tightly fitted hole, as previously reported (11). Calibration of the thermometer was reported as 99% occurrence of ±.05°C (Commonwealth Scientific and Industrial Research Organisation, Adelaide, Australia). The tip of the reference probe was fully inserted and directed across the internal diameter of the tubing to minimize influence on the measurement as a result of immersion stem effects or boundary layer effects. The blood temperature was regulated using a Hemotherm® (Cincinnati Sub-Zero, Cincinnati, OH) heater cooler unit and recirculated through the circuits at temperatures of 15, 20, 25, 30, 35, 37, and 38°C. Arterial outlet measurements were taken once the target reference temperature was stabilized and maintained for 3 minutes. Once the target temperature was obtained, measurements were repeated three times at 1-minute intervals using one of each of the test oxygenators. Experiments were performed a total of three times using new oxygenators and circuits.
STATISTICAL ANALYSIS
The general linear model was used for between group comparisons while post-hoc tests specified Fisher’s Least Significant Difference. Repeated measures data were analyzed by calculating a change score, that is, subtracting Time 2 observations from Time 1 observations. In all analyses p < .05 was considered as statistically significant and no adjustment was made for multiple comparisons. All data were analyzed using SPSS V18.0.0 (SPSS Inc., Chicago, IL).
RESULTS
Part A: Quantification of a Baseline Criteria
The average grip strength of SMARxT® tubing connected to the venous reservoir of the Capiox SX25R03® venous reservoir outlet at initial connection was found to be 27.9 ± 1.6 kg. No decline over 24 hours was found (Table 1). A 14.3% decline was observed after 24 hours with the Capiox SX25RX® (not significant).
Table 1.
Oxygenator connector grip strength.
| Disconnection Weight (kg) |
||||
|---|---|---|---|---|
| Oxygenator (n = 3) | Tubing Type | Time = 0 hours | Time = 24 hours | Percent Change |
| Baseline | ||||
| Capiox SX25R03 | SMARxT | 27.9 ± 1.6 | 29.3 ± 3.4 | 5.0 |
| Incident (13) | ||||
| Capiox SX25RX | SMARxT | 27.5 ± 1.0 | 23.5 ± 2.4 | −14.3 |
F(1, 4) = 5.79, p = .07, ηp2 = .59
Part B: Evaluation Oxygenator Connector Physical Measurements
Measurements obtained of the connector physical dimensions are shown in Table 2. All of the connectors were found to have a tapered profile, as demonstrated by the average diameter of the second barb in relation to the first. The Primox® and Synthesis® reservoir outlet connectors had bevelled outlets. There was considerable variation between the lengths of the connectors; however we were unable to identify a particular oxygenator as consistently different in its various connector lengths.
Table 2.
Oxygenator connector physical measurements.
| Oxygenator (n = 3) | First Barb Diameter (mm) | Second Barb Diameter (mm) | Minimum Length (mm) |
|---|---|---|---|
| Venous reservoir outlet | |||
| Synthesis | 12.0 ± 0 | 11.1 ± .01 | 18.1 ± .04 |
| Affinity | 12.2 ± .01 | 11.7 ± .01 | 19.1 ± .05 |
| Capiox RX25 | 11.9 ± .02 | 11.4 ± .01 | 16.4 ± .09 |
| Primox | 12.0 ± .02 | 11.2 ± .02 | 18.1 ± .06 |
| Oxygenator venous inlet | |||
| Synthesis | 12.2 ± .02 | 11.3 ± .01 | 17.4 ± .09 |
| Affinity | 12.0 ± 0 | 11.6 ± .01 | 29.8 ± .17 |
| Capiox RX25 | 12.5 ± .03 | 12.0 ± 0 | 23.2 ± .12 |
| Primox | 12.2 ± .01 | 11.3 ± .02 | 17.5 ± .03 |
| Oxygenator arterial outlet | |||
| Synthesis | 12.2 ± .02 | 11.9 ± .01 | 19.6 ± .04 |
| Affinity | 12.0 ± .01 | 11.6 ± 0 | 19.1 ± .07 |
| Capiox RX25 | 12.5 ± .01 | 12.0 ± 0 | 24.6 ± .10 |
| Primox | 11.8 ± .02 | 11.6 ± .01 | 20.2 ± .01 |
Part C: Evaluation Oxygenator Connector Grip Strength
Connector grip strength comparisons for the evaluation oxygenators with SMARxT® tubing are summarized in Table 3. SMARxT® tubing connected to the four test oxygenators showed significant variation in grip strength (p = .02), with Affinity®, Capiox RX25®, and Primox® oxygenators each demonstrating a decrease in grip strength on average over 24 hours at each connection site.
Table 3.
Oxygenator connector grip strength – evaluation oxygenators.
| Oxygenator (n = 3) | Disconnection Weight (kg) | Percent Change | F(3, 8)* | p | ηp2 | ||
|---|---|---|---|---|---|---|---|
| Venous reservoir outlet | Time = 0 hours | Time = 24 hours | 3.38 | .08 | .56 | ||
| Affinity | 33.8 ± 4.5 | 28.4 ± 4.2 | −15.9 | ||||
| Capiox RX25 | 31.9 ± 2.3 | 27.5 ± 1.9 | −14.1 | ||||
| Primox | 22.6 ± 2.3 | 21.5 ± .8 | −4.9 | ||||
| Synthesis | 24.2 ± 1.6 | 24.4 ± .2 | 1.0 | ||||
| Oxygenator venous inlet | 1.17 | .38 | .31 | ||||
| Affinity | 33.2 ± 3.3 | 31.1 ± .5 | −6.3 | ||||
| Capiox RX25 | 31.2 ± 7.0 | 30.6 ± 4.1 | −1.9 | ||||
| Primox | 22.8 ± 4.2 | 22.3 ± 7.6 | −2.2 | ||||
| Synthesis | 11.7 ± 1.3 | 18.6 ± 1.9 | 58.7 | ||||
| Oxygenator arterial outlet | 4.74 | .04 | .64 | ||||
| Affinity | 37.6 ± 1.7 | 32.7 ± 2.5 | −13.2 | ||||
| Capiox RX25 | 42.2 ± 3.8 | 41.3 ± 2.1 | −2.1 | ||||
| Primox | 40.9 ± 6.4 | 27.9 ± 2.9 | −32.0 | ||||
| Synthesis | 33.7 ± 7.9 | 36.8 ± 2.0 | 9.2 | ||||
| Overall | 3.87 | .02 | .27 | ||||
DF = (3, 32) for overall comparisons.
Evaluation of the arterial outlet observations showed significant differences between the different oxygenators, with the Primox® and Affinity® demonstrating most change. At the venous reservoir outlet and oxygenator venous inlet, there were no significant differences between oxygenators.
Part D: Biopassive Tubing Grip Strength
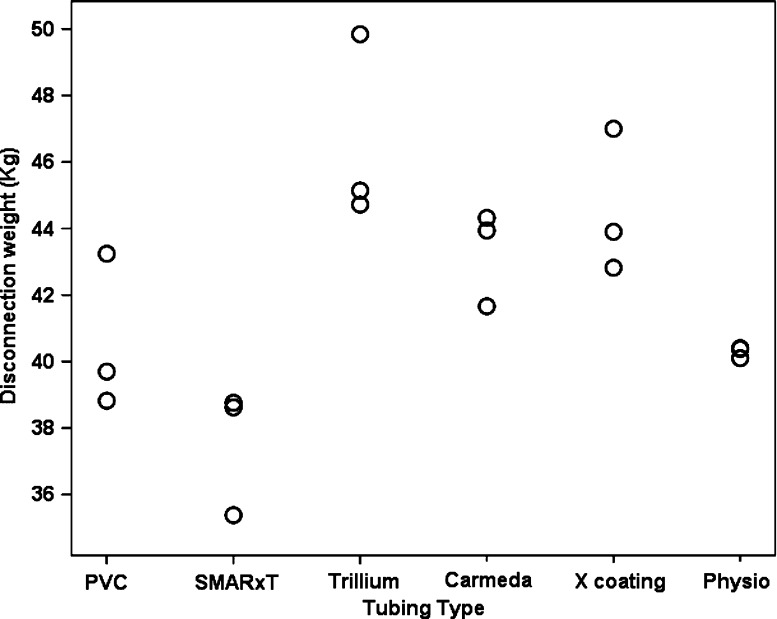
Figure 1 summarizes the grip strength comparison data for the various tubing types. There was a main effect for tubing type F(5, 18) = 8.01, p = .002, ηp2 = .77. Post-hoc tests found that Trillium® had significantly greater grip strength than PVC (p = .003), SMARxT® (p < .001), and Physio® (p = .002). X-coating® also had significantly greater grip strength than PVC (p = .031), SMARxT® (p = .001), and Physio® (p = .022).
Figure 1.
Disconnection weight measurements for different biopassive tubing types. There was a main effect for tubing type (F(5, 18) = 8.01, p = .002, ηp2 = .77); Trillium®, and X-coating® were significantly greater than PVC, SMARxT®, and Physio®.
Part E: Evaluation Oxygenator Arterial Temperature Measurement Accuracy
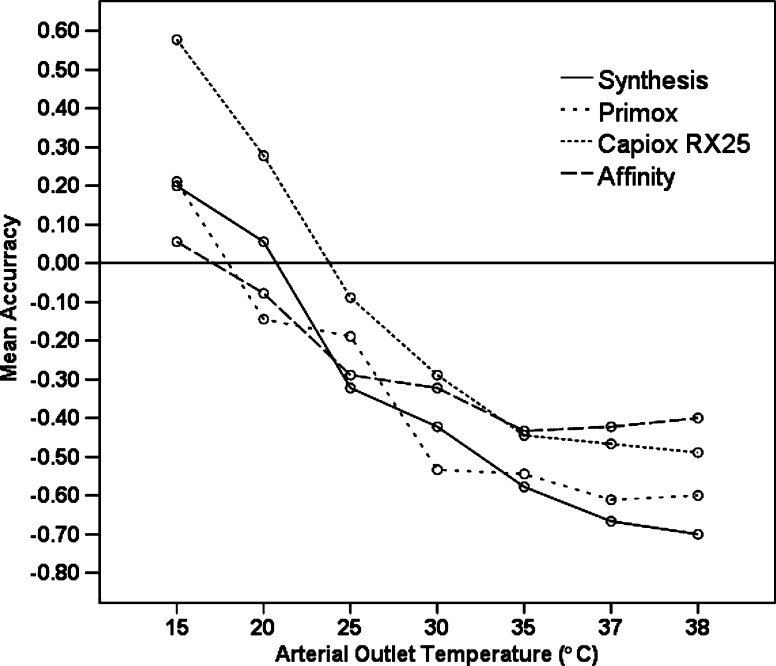
The average difference between arterial outlet and reference thermometer temperatures are illustrated in Figure 2. All oxygenators overread the arterial outlet temperature at 15°C. At 15°C the Capiox RX25® oxygenator arterial outlet temperature probe was found to have the least accuracy (over reading .58 ± .02°C). At temperatures ≥25°C, all oxygenators underread the arterial outlet temperature. At 37°C the Affinity® was found to underread by .33 ± .03°C, the Capiox® by .47 ± .06, the Primox® by .61 ± .05, and the Synthesis® by .67 ± .09. Comparison according to the accuracy of the arterial outlet temperatures overall, found there was a significant between-oxygenator difference F(3, 248) = 6.23, ηp2 =.07, p < .001.
Figure 2.
Accuracy of the arterial outlet blood temperature measurement for each of the evaluation oxygenators at temperatures between 15°C and 38°C. Values >0 indicate overreading, values <0 indicate underreading. All oxygenators underread the arterial temperature at 37°C.
DISCUSSION
Three important clinical considerations are highlighted in this report. First, connectors on the evaluation oxygenators interact differently with SMARxT® tubing. Second, different biopassive tubings interact differently with connectors, and finally, none of the commercially available devices we evaluated had accurate arterial outlet temperature measurements. Each of these factors is clinically significant when considering changes to hardware or arterial outlet temperature management.
The variation we report in grip strength in relation to both oxygenator connectors and various biopassive tubings is important in the current environment where we are being encouraged to tailor specific combinations of hardware for individual patients, and where components of the bypass circuit may be promoted to be relatively “plug and play” in nature. We have shown this not to be the case, demonstrating large variation between both different oxygenator connections and SMARxT® tubing and with different tubing types.
Our interest to address the safety concerns in relation to connector grip strength and SMARxT® tubing arose from disconnection incidents that occurred during CPB. The root cause analysis investigation that followed these incidents identified both the SMARxT® tubing and the shape of the venous reservoir outlet connector on the Terumo Capiox SX25RX® oxygenator as the major contributors, resulting in degradation in integrity of the connection over time. Hence, our objective in the evaluation of an alternative oxygenator for routine use was to determine whether a decrease in grip strength with the use of SMARxT® tubing could be observed over time. A limiting factor prior to undertaking this study was that the quantification of a safe tubing connection strength in CPB circuits has not been defined, therefore we examined as a baseline the grip strength of the connection between SMARxT® tubing and the venous reservoir outlet of the Capiox SX25R03® oxygenator, a device which we had used without incident for over 6000 cases. There was no decline in the force required to achieve disconnection using SMARxT® tubing and this outlet after 24 hours. There was a decline after 24 hours with the Capiox SX25RX®; the difference between devices was not found to be statistically significant. Since our observations were limited to one time point (24 hour), we are unable to report what may occur under these conditions if they were extended. The decrease in grip strength observed after 24 hours with the Capiox SX25RX®, combined with the physical characteristics of the connector, in particular the taper, supports the root cause for the disconnection previously reported (11).
Our grip strength findings for the evaluation oxygenators indicate that overall for the group there was not a significant change after 24 hours using SMARxT tubing on the venous reservoir and venous inlet connectors, however there was significant change after 24 hours using SMARxT tubing on the arterial outlet connectors. These results suggest that in respect to safety, clinicians should be aware that the strength of all connections will vary when a new device is used. It is not possible for us to make a clear safety recommendation with respect to the magnitude of the reported grip strength. Our baseline values of 27.9 ± 1.6 at time 0 and 29.3 ± 3.4 at 24 hours provide some guidance for our own clinical environment, however we cannot relate this absolute value to a safe value for any given clinical situation.
We found no major variation in the barb diameters of the connectors between the different oxygenators. We did find variations in the length of the different connectors, although we were unable to identify a particular connector as an obvious safety concern given the overall dimensions and taper.
An important finding with respect to the different biopasssive tubing types was that overall there was a significant difference between the different types of biopassive tubing, suggesting that it is not reasonable to assume that interchanging different types of tubing into a clinical circuit is inconsequential. In our comparison only SMARxT® tubing had a grip strength less than PVC, however this difference was not statistically significant. We observed only a 7.4% decrease in grip strength with the use of SMARxT® tubing versus PVC, compared with a 28% decrease reported by Newling and Morris (10). This is likely to be attributed to differences in experimental method, in particular the tubing was positioned just after the first barb on the connector and a cable tie was not reported to be applied in the Newling and Morris technique, possibly explaining the differences in the amount of force required to dislodge the tubing between the studies (83.3 ± 7.3 N (Newling and Morris) compared with 368.7 ± 27.4 N (37.6 ± 1.9 kg) in our study).
In terms of clinical outcomes for our patients, potentially our most important finding is reporting the inaccuracy of the arterial outlet temperature measurements of all the oxygenators that we studied. The temperature measurements of all oxygenators overread at 15°C and underread at temperatures ≥25°C. These results are similar to those observed by Salah et al. (13) and Potger and McMillan (14), and to what we have found previously with the Capiox SX25® oxygenator (12). The rationale of avoiding cerebral hyperthermia has been summarized by Shann et al. (15), and in the recommendation made by those authors they added a caveat that “Coupled temperature ports for all oxygenators should be checked for accuracy and calibrated”. Our findings reinforce the need to continue this practice. Despite this recommendation and the ongoing interest in arterial outlet temperature measurement, manufacturers have not been able to provide clinicians with an accurate temperature measurement. To avoid exceeding an arterial blood temperature of 37°C the maximum arterial outlet temperature should vary depending on the oxygenator being used. From our study to maintain an arterial outlet temperature <37°C a target temperature of <36.7°C should be targeted with the Affinity®, <36.5°C with the Capiox RX25®, <36.4°C with the Primox®, and <36.3°C with the Synthesis® oxygenator.
LIMITATIONS
This report must be considered in light of its in vitro nature and the implicit limitations imposed by this design. Since the report is focused on the devices in consideration by our unit, further investigations of other devices are warranted, particularly in regard to the evaluation of arterial outlet temperature measurement accuracy. The results from the grip strength comparisons are limited by the variation observed in repeated measures; therefore we have focused our assessment to whether a decline over time was observed as opposed to relative comparisons between devices. The variation in measurements of grip strength under experimental conditions based on consistency of connection and securing of the tubing to the connector, and the method of measuring the force required for disconnection highlight the importance of maintaining consistency in making circuit connections in clinical practice. Standardization of connection technique and checking of the visual assessment of the integrity of connections prior to the initiation of CPB, through inclusion on the pre-CPB checklist, may be important (11).
CONCLUSIONS
The integrity of SMARxT® tubing connection is influenced by the connector type, and may decline over time, highlighting the importance to not consider interchanging components of the bypass circuit as inconsequential. All of the oxygenators temperature measurement overread at 15°C and underread at temperatures ≥25°C, demonstrating the need for clinicians to recognize the variation between devices.
REFERENCES
- 1.Segers P, Heida JF, de Vries I, Maas C, Boogaart AJ, Eilander S.. Clinical evaluation of nine hollow-fibre membrane oxygenators. Perfusion. 2001;16:95–106. [DOI] [PubMed] [Google Scholar]
- 2.Chukwuemeka AO, Turtle MRJ, Trivedi UH, Venn GE, Chambers DJ.. A clinical evaluation of platelet function, haemolysis and oxygen transfer during cardiopulmonary bypass comparing the Quantum HF-6700 to the HF-5700 hollow fibre membrane oxygenator. Perfusion. 2000;15:479–484. [DOI] [PubMed] [Google Scholar]
- 3.Noora J, Lamy A, Smith KM, et al. . The effect of oxygenator membranes on blood: A comparison of two oxygenators in open-heart surgery. Perfusion. 2003;18:313–320. [DOI] [PubMed] [Google Scholar]
- 4.Ündar A, Owens WR, McGarry MC, et al. . Comparison of hollow-fiber membrane oxygenators in terms of pressure drop of the membranes during normothermic and hypothermic cardiopulmonary bypass in neonates. Perfusion. 2005;20:135–138. [DOI] [PubMed] [Google Scholar]
- 5.Hoel TN, Videm V, Baksaas ST, Mollnes TE, Brosstad F, Svennevig JL.. Comparison of a Duraflo II-coated cardiopulmonary bypass circuit and a trillium-coated oxygenator during open-heart surgery. Perfusion. 2004;19:177–184. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson TA, Riley JB, Crowley JC, Zabetakis PM.. In vitro evaluation of the air separation ability of four cardiovascular manufacturer extra-corporeal circuit designs. J Extra Corpor Technol. 2006;38:206–213. [PMC free article] [PubMed] [Google Scholar]
- 7.Myers GJ, Voorhees C, Haynes R, Eke B.. Post-arterial filter gaseous microemboli activity of five integral cardiotomy reservoirs during venting: An in vitro study. J Extra Corpor Technol. 2009;41:20–27. [PMC free article] [PubMed] [Google Scholar]
- 8.Preston TJ, Gomez D, Olshove VF, Phillips A, Galantowicz M.. Clinical gaseous microemboli assessment of an oxygenator with integral arterial filter in the pediatric population. J Extra Corpor Technol. 2009;41:226–230. [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Newland RF, Tully PJ, Tuble SC, Baker RA.. In vitro evaluation of gaseous microemboli handling of cardiopulmonary bypass circuits with and without integrated arterial line filters. J Extra Corpor Technol. 2011;43:107–114. [PMC free article] [PubMed] [Google Scholar]
- 10.Newling R, Morris R.. SMARxT tubing presents an increased risk of disconnection during extracorporeal circulation. J Extra Corpor Technol. 2005;37:400–401. [PMC free article] [PubMed] [Google Scholar]
- 11.Ottens J, Baker RA, Sanderson AJ, Newland RF.. Disconnection of Cobe SMARxT® tubing from the venous outlet of the Terumo Capiox® SX25RX oxygenator during cardiopulmonary bypass. J Extra Corpor Technol. 2010;42:153–157. [PMC free article] [PubMed] [Google Scholar]
- 12.Newland RF, Sanderson AJ, Baker RA.. Accuracy of temperature measurement in the cardiopulmonary bypass circuit. J Extra Corpor Technol. 2005;37:32–37. [PMC free article] [PubMed] [Google Scholar]
- 13.Salah M, Sutton R, Tsarovsky G, Djuric M.. Temperature inaccuracies during cardiopulmonary bypass. J Extra Corpor Technol. 2005;37:38–42. [PMC free article] [PubMed] [Google Scholar]
- 14.Potger KC, McMillan D.. In vitro validation of the Affinity NT oxygenator arterial outlet temperatures. J Extra Corpor Technol. 2005;37:207–212. [PMC free article] [PubMed] [Google Scholar]
- 15.Shann KG, Likosky DS, Murkin JM, et al. . An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132: 283–290. [DOI] [PubMed] [Google Scholar]