Abstract:
Patients with heparin-induced thrombocytopenia (HIT) that require anticoagulation for cardiovascular procedures represent a challenging and high-risk group. Bivalirudin and argatroban have been successfully used as alternative anticoagulants in adult patients with HIT. There have been few experiences published involving the pediatric population and controversy exists regarding the properties and optimal dosing of these drugs. This report describes the experience of managing two pediatric patients with HIT that underwent cardiovascular procedures requiring anticoagulation. Bivalirudin was used in both cases for anticoagulation during cardiopulmonary bypass, while argatroban was used without complications during cardiac catheterization. A description of perfusion and anticoagulation protocols is included.
Keywords: cardiopulmonary bypass, pediatric, heparin-induced thrombocytopenia, bivalirudin, argatroban
Heparin is the standard therapy in adults and children undergoing cardiovascular procedures that require anticoagulation; however 17% of patients will develop immunoglobulin antibodies to the complex of heparin and platelet factor-4 (anti-heparin-platelet actor 4 antibody). Furthermore 1–3% will manifest heparin-induced thrombocytopenia (HIT) (1). HIT is a serious complication associated with high morbidity and mortality.
Patients with HIT represent a challenging and high-risk group for anticoagulation management. A comprehensive approach to anticoagulation management involving anesthesia, surgical, and perfusion personnel should be used to safely treat patients with these alternative drugs. These drugs include the direct thrombin inhibitors (bivalirudin, argatroban, lepirudin, and dabigatran). Successful use of bivalirudin for anticoagulation of adult patients with HIT has been previously reported (2). Experience in the pediatric population is limited to several case reports (3,4) and controversy exists regarding the properties and optimal dosing of the drug (5).
This report describes one institution’s experience in managing two patients with HIT that underwent cardiovascular procedures requiring anticoagulation. The first case required cardiopulmonary bypass and bivalirudin was used. In the second case, argatroban was used during cardiac catheterization and subsequently, bivalirudin was used during cardiopulmonary bypass. A description of the perfusion and anticoagulation protocols used is included.
DESCRIPTION
Case Report 1
A baby born with transposition of the great arteries, ventricular septal defect, and severe pulmonary stenosis underwent initial palliation with a modified Blalock-Taussig shunt at 6 days of life. At 6 months of age a bidirectional cavo-pulmonary connection was performed on cardiopulmonary bypass (CPB) with heparin being used for anticoagulation. Cardiac catheterization at 3 years of age demonstrated low pulmonary artery pressures, good biventricular function and anatomy suitable for a bi-ventricular repair. The patient therefore underwent takedown of the bidirectional cavo-pulmonary connection and a Rastelli operation (intracardiac baffle from the inlet ventricular septal defect to the aorta and a valved conduit from the right ventricle to the pulmonary artery). Heparin was used for anticoagulation during CPB. The early postoperative course required invasive monitoring. Heparin was used to flush the monitoring lines to prevent clot formation. On postoperative day 2, the platelet count decreased from a preoperative count of 312,000/mm3 to 63,000/mm3 and it remained low despite platelet transfusions. On postoperative day 4, the platelet count was 51,000/mm3 and antiheparin antibodies were positive, therefore heparin was discontinued and substituted with bivalirudin. During this time, the patient remained intubated and there was no clinical evidence of infection. A serotonin releasing assay was negative. Over the course of the following weeks the patient developed increasing aortic valve insufficiency and congestive heart failure and an aortic valve repair was planned. After consultation with hematology it was agreed that the patient’s clinical course and positive heparin antibodies made the diagnosis of HIT likely and that a nonheparin anticoagulation strategy was warranted during CPB.
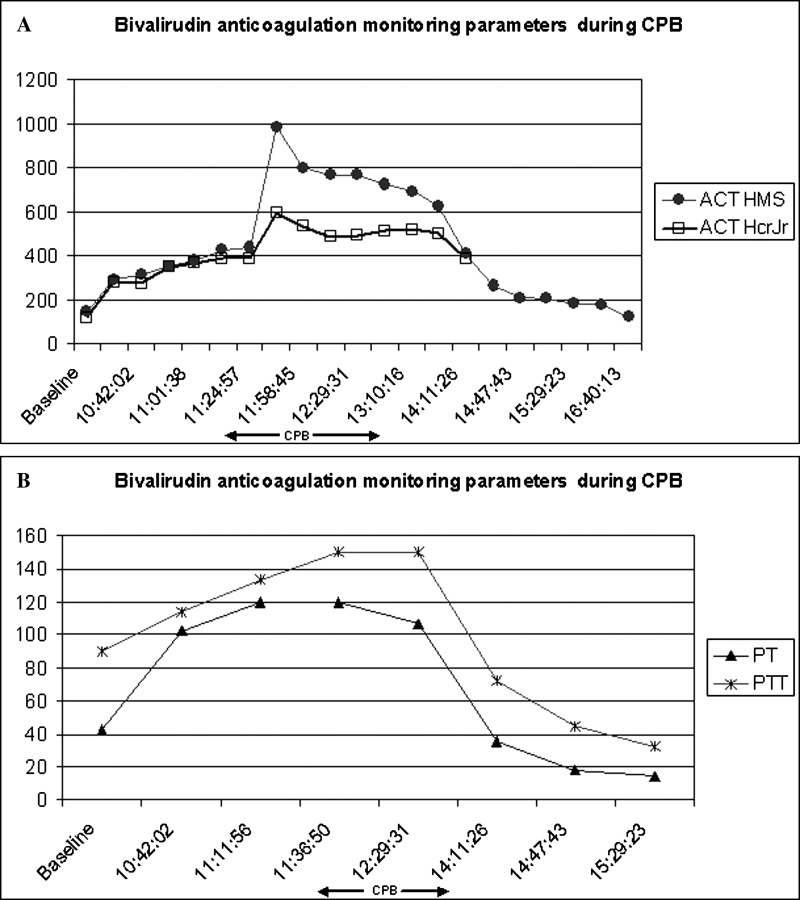
CPB protocols were as follows: immediately prior to bypass, a bolus of 1 mg/kg of bivalirudin (Angiomax®, The Medicines Company, Parsippany, NJ) was administered intravenously followed by an infusion at 2.5 mg/kg/h. In addition, bivalirudin 50 mg was added to the pump prime. Activated clotting time (ACT) was measured every 15 minutes using two different systems, the HMS Plus™ (Hemostasis Management System; Medtronic Inc., Minneapolis, MN) and the Hemochron® Jr (ACT + cuvette; International Technidyne Corp., Edison, NJ) (Figure 1A). Over the course of the next hour the infusion was increased to 4.5 mg/kg/h and four additional boluses of bivalirudin were given (total 1.8 mg/kg) to achieve a target ACT >400 seconds which was reached after 28 minutes of initial bolus allowing the initiation of CPB. The CPB circuit details used were as follows: Medtronic centrifugal pump BP-50 Biopump®, Terumo Capiox® RX15 baby oxygenator (Terumo, Ann Arbor, MI), and Capiox® CXAF02X arterial filter (Terumo) utilizing a venous-pull circuit. The oxygenator recirculation and manifold lines were open at all times with a distally placed transonic flow probe. The system was coated with XCoating® with a pump prime of 420 mL. The patient was cooled to 28°C and the cardiotomy reservoir was kept below 200 mL achieved by intermittent ultrafiltration. The venous line and CPB circuit volume were chased to the cell saver 10 minutes after separation from CPB. The surgical staff was reminded every 15 minutes to suction any pooled blood from the pleural cavity. Over the course of CPB the bivalirudin infusion rate was decreased 2 mg/kg/h as the ACTs (measured by HMS Plus™) were approaching 1000 seconds. The bivalirudin infusion was continued until CPB was terminated and an intra-operative transesophageal echocardiogram confirmed the adequacy of the aortic valve repair. Total time of CPB was 305 minutes with a cross-clamp time of 140 minutes. Following discontinuation of the bivalirudin infusion, the coagulation parameters returned to normal over the next 2 hours. Figure 1B shows the post bypass prothrombin and partial thromboplastin times. Following CPB the patient received 38 mL/kg of fresh frozen plasma, 60 mL/kg of washed red blood cell saver, 28 mL/kg of platelets, and 8 mL/kg of cryoprecipitate. Once coagulation parameters were normal and surgical field bleeding was minimal, the chest was closed and the patient was taken to the pediatric cardiovascular intensive care unit (CICU). Postoperative course was uncomplicated, the patient was extubated 24 hours later and was discharged home on post operative day 8.
Figure 1.
Case 1, bivalirudin anticoagulation monitoring parameters during CPB. (A) ACT measured values. (B) Prothrombin time and partial thromboplastin time values. Arrows indicate the CPB period.
Case Report 2
A male baby with the diagnosis of hypoplastic left heart syndrome underwent stage 1 palliation at 3 days of life. After surgery, the patient remained oxygen dependent (25%) and at 10 days of age a cardiac catheterization was performed which revealed stenosis of the right pulmonary artery and mild stenosis of the distal Blalock-Taussig (BT) shunt. Both the right pulmonary artery and BT shunt were stented uneventfully but with minimal improvement in oxygenation. Therefore, at 14 days of age the patient underwent shunt revision, right pulmonary arterioplasty, and removal of both stents on CPB. The patient tolerated the procedure well and was transferred to CICU. Heparin was used for anticoagulation during these procedures and as a prophylactic infusion postoperatively to prevent BT shunt thrombosis.
During a prolonged hospitalization the patient developed generalized petechiae associated with severe thrombocytopenia (platelet count of 35,000/mm3) that was not responsive to multiple platelet transfusions. Although anti-heparin antibodies were negative, a hematology consultation made a presumptive diagnosis of HIT based on the clinical presentation. Heparin was discontinued and the platelet count started recovering. With increasing oxygen requirements, an echocardiogram reported recurrent narrowing of the distal BT shunt. Prophylactic anticoagulation therapy with a continuous infusion of argatroban at 1.45 mcg/kg/min was initiated with a target partial thromboplastin time (PTT) in the range of 55–80 seconds. PTT was measured every 4 hours and the range was achieved with this dose. A repeat cardiac catheterization with BT shunt stent implantation was performed utilizing the continuous argatroban infusion. The patient was transferred back to the CICU in stable condition and was discharged home 11 days later.
At 4 months of age, the patient was admitted to CICU for observation due to inconsolable irritability and increasing oxygen requirements. A CT-angiogram revealed mild stenosis of the mid portion of stented BT shunt with preferential flow to the left lung, a likely consequence of intimal hyperplasia. Anticoagulation was achieved for the catheterization procedure with bivalirudin using an initial bolus of 4.3 mg and a continuous infusion at 1.99 mL/h, which was discontinued at the end of the procedure. A second stent was implanted within the existing stent distally without complications.
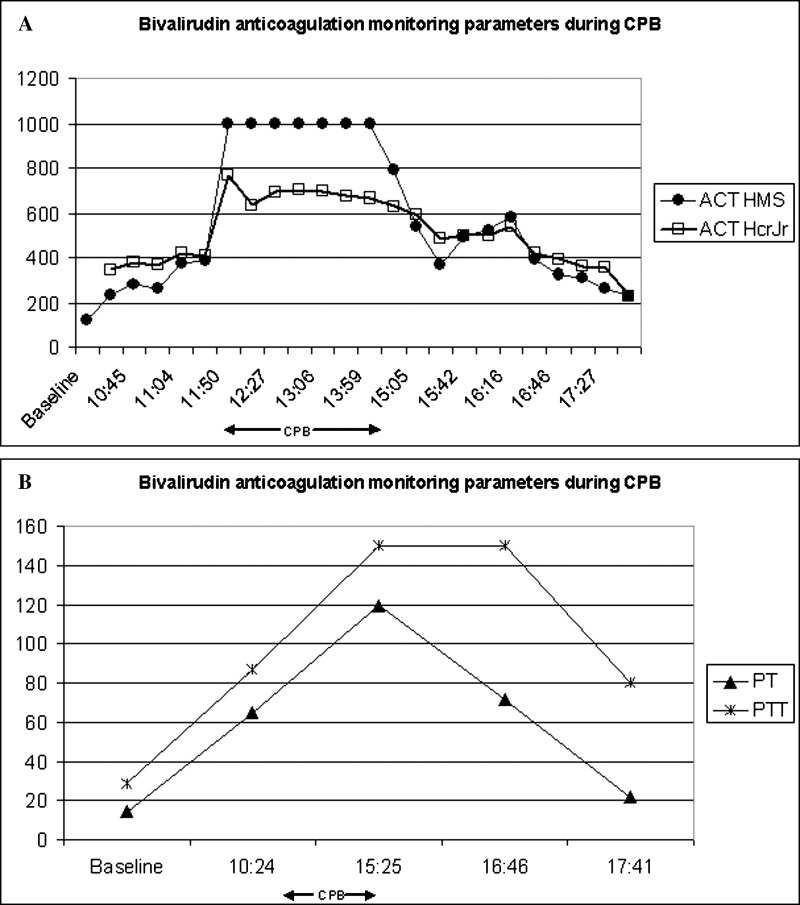
The patient had a prolonged hospital stay complicated by feeding intolerance and irritability. Serial echocardiograms over the following weeks demonstrated increasing tricuspid regurgitation. At 5 months of age, he underwent tricuspid valvuloplasty on CPB. Anticoagulation was achieved using bivalirudin. The following protocol was used: initial intravenous bolus of 1.0 mg/kg, followed by a continuous infusion of 2.5 mg/kg/h, and 50 mg of bivalirudin was added to the CPB circuit. ACT monitoring was performed every 15 minutes (Figure 2A) with a target of >400 seconds, which was reached after 65 minutes of initial bolus. Sub-therapeutic ACT was treated with an additional bolus of .1–.5 mg/kg. CPB circuit configuration was similar to that used in Case 1 except for a prime volume of 320 mL and Terumo Capiox® RX05 oxygenator. The patient was cooled to 25°C and crystalloid cardioplegia was used to avoid thrombus formation in the cardioplegia circuit and/or in the coronary arteries. The surgical staff was reminded every 15 minutes to suction any pooled blood from the pleural cavity. Cross clamp time was 168 minutes and total CPB time was 272 minutes. The continuous infusion of bivalirudin was stopped 15 minutes prior to separation from bypass. Figure 2B shows the post bypass prothrombin and partial thromboplastin times. The patient tolerated the procedure well, without complications and was transferred to the CICU for monitoring. This patient continued to struggle with tricuspid valve insufficiency and developed severe congestive heart failure with gross hepatomegaly, ventilatory dependence, and feeding intolerance. The case was discussed in cardiac conference and the team agreed that the patient needed to be evaluated for heart transplantation. One week later, the patient was transferred to a heart transplantation center.
Figure 2.
Case 2, bivalirudin anticoagulation monitoring parameters during CPB. (A) ACT measured values. (B) Prothrombin time and partial thromboplastin time values. Arrows indicate the CPB period.
COMMENTS
This report included two patients with a clinical diagnosis of HIT who underwent cardiovascular procedures requiring anticoagulation. Argatroban was used during cardiac catheterization in one patient without complications and bivalirudin was successfully used in both patients during cardiopulmonary bypass. To our knowledge, these two cases in addition to those described by Almond et al. (3) are the only reports of pediatric patients undergoing surgical repair requiring cardiac arrest with the aid of bivalirudin anticoagulation during CPB. Bivalirudin (Angiomax®) and argatroban are specific and reversible direct thrombin inhibitors. They inhibit circulating and clot-bound thrombin, thus preventing the conversion of fibrinogen to fibrin. In addition, they inhibit thrombin-mediated platelet activation and aggregation. Bivalirudin has a quick onset of action and a short half-life of 25 minutes, while argatroban has a longer half-life of 60 minutes. As they do not require the binding cofactor anti-thrombin (as heparin does), and do not activate platelets, they are suitable alternatives to heparin in the setting of HIT.
HIT increases the risk of adverse outcomes after cardiac surgery and it can be difficult to diagnose. HIT should be suspected in patients that acutely develop thrombocytopenia who have been exposed to heparin or are receiving prolonged infusions of the drug through solutions used to flush monitoring lines. The diagnosis of HIT is based primarily on clinical findings and heparin antibodies are used to corroborate the diagnosis and to monitor its presence (6); however, it has been suggested that the cut off level for the anti-heparin antibodies assay may not be applicable to children (7,8). This reiterates the importance of clinical findings in making the diagnosis, as anti-heparin antibodies were not found in one of our patients but the platelet count returned to normal after discontinuing heparin. HIT requires specialized perioperative multidisciplinary management involving anesthesiology, surgery or interventional cardiology, perfusion, hematology, and intensive care.
Patients with HIT that require cardiac surgery should receive a non-heparin based anticoagulation strategy. Alternative anticoagulants include direct thrombin inhibitors (argatroban, lepirudin, bivalirudin) and factor Xa inhibitors (danaparoid, fondaparinux) (5), however, none of these agents are ideal. Problems include inadequacy of anticoagulation, difficulty in monitoring concentration or efficacy, long half-lifes, variable metabolism and elimination, lack of a reversal agent, inability to dialyze, and the potential for post-procedure bleeding (4). Perioperative techniques need to be adapted to fit the characteristics of the selected drug. Bivalirudin has become an attractive alternative anticoagulant for patients with HIT because it has an organ dependent, short half life (25 minutes) and it can be ultrafiltrated (4). Furthermore, The American College of Chest Physicians recommends anticoagulation with bivalirudin for patients with HIT in whom a cardiovascular procedure can not be delayed until heparin antibodies are negative (9).
The literature concerning the use of bivalirudin in pediatric patients with HIT is limited. The successful use of bivalirudin for an orthotopic pediatric heart transplant (3), its’ short half life and proteolytic elimination, as well as our experience with the drug during the patient’s cardiac catheterization led us to use it during CPB.
Our protocol for anticoagulation with bivalirudin was evidence-based. Anticoagulation was monitored using two systems, the HMS plus (Hemostasis Management System) and the Hemochron Jr (ACT+ cuvette). The operative course was prolonged due to an extended time to achieve an ACT value >400 seconds, requiring repeated boluses of bivalirudin. This phenomenon has been reported by Almond et al. (3). We observed discrepancies between the HMS plus and the Hemochron Jr values. In case 2, the pre-bypass ACT values were significantly different between the two measuring devices with the Hemochron® Jr giving lower ACT values suggesting additional boluses of bivalirudin were required. Similarly, the ACT values during CPB with the Hemochron® Jr were consistently lower than the HMS Plus™ values in both cases. The correlation between ACT values and bivalirudin concentration is linear and the Hemochron® Jr was designed to monitor the nonlinear concentration response of heparin (10). In addition, the ACT sensitivity decreases at levels exceeding 10 μg/mL and this might increase the variability of ACT measurement in children when compared to adults (11). We decided to proceed with using the HMS Plus™ values without any modification to our protocol. ACT trends correlated to infusion manipulation for both devices. Pre-CPB ACT measures were not different between devices. However, on CPB, ACT were significantly different between devices. The HMS Plus™ values suggested a larger decrease of the infusion rate than the Hemochron® Jr values. This observed ACT difference between devices disappears later post CPB. On CPB, mainly hypothermia and hemodilution might be the factor affecting the ACT values.
Our second case received argatroban during a cardiac catheterization. Argatroban has a halflife of 60 minutes and although it has been successfully used in the catheterization laboratory, significant bleeding after cardiopulmonary bypass has been reported (6). The risk of hemorrhage is minimal for most catheterization making the prolonged half life of argatroban of less clinical importance. The successful experience with the first patient and the fact that a protocol had been already developed were determining factors for the use of bivalirudin for anticoagulation during CPB in the second patient. It has been described that Factor VII and modified ultra filtration (MUF) help improve the time to achieve hemostasis (3,4). In this case we incorporated MUF to the perfusion protocol. MUF decreased the ACT from 580–328 seconds but it did not reduce the volume of blood products transfused (case 1 = 104 mL/kg versus case 2 = 129 mL/kg). After CPB, ACT returned to near normal values at 96 minutes in case 1 and 89 minutes in case 2. In the future, if anticoagulation with bivalirudin is necessary we would consider initiating bypass at 2.5 times ACT baseline. Also, we would estimate more accurately, stopping the bivalirudin infusion 20 minutes before CPB is ending rather than continue the infusion due to the risk of going back on bypass.
Adequate anticoagulation of pediatric patients with HIT is challenging as experience is limited. Bivalirudin and argatroban can be successfully used in children when heparin is not an option. Monitoring of anticoagulation during CPB is inconsistent resulting in prolonged times to achieve appropriate ACT values and an increase in postoperative bleeding with greater use of blood products to achieve hemostasis. A standardized protocol for the use of alternative anticoagulants and reliable equipment to monitor their effects are needed to better manage HIT in pediatric patients requiring cardiac surgery. When comparing our experience with reports in adult patients it seems like blood transfusion after CPB with bivalirudin is common practice in this population also but the volume is much greater in pediatrics (12).
ACKNOWLEDGMENTS
The authors would like to thank Dr. David Nykanen and Dr. Don Eslin for the expert advice provided in taking care of these patients and for their input to this manuscript.
REFERENCES
- 1.Grubb KJ, Salehi P, Chedrawy EG.. Bivalirudin: Alternative anticoagulation during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia. Recent Patents Cardiovasc Drug Discov. 2010;5:20–24. [DOI] [PubMed] [Google Scholar]
- 2.Simsir SA, Schwarz ER, Czer LS, Hamburg SI.. Heart transplantation using bivalirudin as anticoagulant. Interact Cardiovasc Thorac Surg. 2010;10:150–151. [DOI] [PubMed] [Google Scholar]
- 3.Almond CS, Harrington J, Thiagarajan R, et al. Successful use of bivalirudin for cardiac transplantation in a child with heparin-induced thrombocytopenia. J Heart Lung Transplant. 2006;25:1376–1379. [DOI] [PubMed] [Google Scholar]
- 4.Gates R, Yost P, Parker B.. The use of bivalirudin for cardiopulmonary bypass anticoagulation in pediatric heparin-induced thrombocytopenia patients. Artif Organs. 2010;34:667–669. [DOI] [PubMed] [Google Scholar]
- 5.Chan VH, Monagle P, Massicotte P, Chan AK.. Novel paediatric anticoagulants: A review of the current literature. Blood Coagul Fibrinolysis. 2010;21:144–151. [DOI] [PubMed] [Google Scholar]
- 6.Potter KE, Raj A, Sullivan JE.. Argatroban for anticoagulation in pediatric patients with heparin-induced thrombocytopenia requiring extracorporeal life support. J Pediatr Hematol Oncol. 2007;29:265–268. [DOI] [PubMed] [Google Scholar]
- 7.Risch L, Huber AR, Schmugge M.. Diagnosis and treatment of heparin-induced thrombocytopenia in neonates and children. Thromb Res. 2006;118:123–135. [DOI] [PubMed] [Google Scholar]
- 8.Boshkov LK, Kirby A, Shen I, Ungerleider RM.. Recognition and management of heparin-induced thrombocytopenia in pediatric cardiopulmonary bypass patients. Ann Thorac Surg. 2006;81:S2355–S2359. [DOI] [PubMed] [Google Scholar]
- 9.Augoustides JG.. Update in hematology: Heparin-induced thrombocytopenia and bivalirudin. J Cardiothorac Vasc Anesth. 2011;25:371–375. [DOI] [PubMed] [Google Scholar]
- 10.Forbes TJ, Hijazi ZM, Young G, et al. Pediatric catheterization laboratory anticoagulation with bivalirudin. Catheter Cardiovasc Interv. 2011;77:671–679. [DOI] [PubMed] [Google Scholar]
- 11.Jones PM, Bainbridge D, Dobkowski W, et al. Comparison of MAX-ACT and K-ACT values when using bivalirudin anticoagulation during minimally invasive hybrid off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25:415–418. [DOI] [PubMed] [Google Scholar]
- 12.Koster A, Dyke CM, Aldea G, et al. Bivalirudin during cardiopulmonary bypass in patients with previous or acute heparin-induced thrombocytopenia and heparin antibodies: Results of the CHOOSE-ON trial. Ann Thorac Surg. 2007;83:572–577. [DOI] [PubMed] [Google Scholar]