Abstract:
We report a case of a 9-year-old female with acute pulmonary hemorrhage and refractory hypoxemic respiratory failure secondary to Goodpasture syndrome (GS). After failing treatment with high frequency oscillatory ventilation and inhaled nitric oxide, she was successfully managed with venovenous extracorporeal membrane oxygenation (VV ECMO). The patient’s weight at the time of cannulation was 31 kg. A 19 French 18 cm (arterial) Biomedicus cannula was inserted in the right internal jugular vein and used as the drain. A 17 French 50 cm (venous) Biomedicus cannula was inserted in the right femoral vein and used as the return. Then the patient was anticoagulated with 100 units/kg of intravenous heparin and the circuit was primed with one unit of packed red blood cells. VV ECMO was performed with an SIII Sorin roller head pump with integrated servo regulator and a Quadrox D Bioline coated oxygenator. Despite systemic anticoagulation with heparin, the patient’s pulmonary hemorrhage resolved. Extracorporeal membrane oxygenation served as a platform through which we were able to provide renal replacement therapy and plasmapheresis. The patient was successfully discharged home with normal pulmonary function.
Keywords: Goodpasture syndrome, extracorporeal membrane oxygenation, pulmonary hemorrhage
Massive pulmonary hemorrhage from Goodpasture syndrome (GS) is a rare, but life threatening, occurrence in children (1). Even advanced forms of mechanical ventilation may not provide adequate oxygenation for such patients. Due to the risk of bleeding with anticoagulation therapy, pulmonary hemorrhage was once considered a contraindication for extracorporeal membrane oxygenation (ECMO). We describe a case of the successful use of venovenous extracorporeal membrane oxygenation (VV ECMO) for a previously healthy 9-year-old female with massive pulmonary hemorrhage and refractory hypoxemic respiratory failure from new-onset GS.
DESCRIPTION
A 9-year-old female with no significant past medical history presented to our emergency department with respiratory distress, edema, and bloody urine. Four days prior to admission, the patient was prescribed amoxicillin for pharyngitis.
In the emergency department, she was found to be tachypneic and to have generalized edema. Her initial blood pressure was 145/109 mmHg, oxygen saturation was 92% on room air, and her heart rate was 140 beats per minute. On physical exam she was awake, alert, and had no focal deficits. She had normal heart sounds and a capillary refill of less than 2 seconds. She was noted to have hepatomegaly with a liver edge palpable 3 cm below her right costal margin. Her breath sounds were equal but coarse bilaterally. Initial laboratory tests are shown in Table 1. The urinalysis showed numerous red blood cells and red blood cell and granular casts.
Table 1.
Patient weight and height and relative first day laboratory data.
| Weight | 31 kg |
| Height White blood cell count | 132 cm 15.4 103/uL |
| Hemoglobin Platelet count | 5.3 g/dL 167 103/uL |
| Creatinine | 7.35 mg/dL |
| Blood urea nitrogen | 85 mg/dL |
| Protein in urine | 300 mg/dL |
In the pediatric critical care unit (PCCU), the patient gradually developed worsening respiratory distress requiring escalation of non-invasive, bilevel positive airway pressure. A kidney biopsy done 1 day after admission showed anti-glomerular basement membrane antibody-mediated glomerulonephritis with crescents in all glomeruli sampled. An 11.5 French dialysis catheter was inserted in the left femoral vein to start intermittent hemodialysis. Shortly after the procedure, the patient developed increased tachypnea, worsening hypoxia, and significant hemoptysis. She was intubated and placed on mechanical ventilation. Figures 1 and 2 demonstrate the patient’s significant lung disease. An emergent bronchoscopy showed normal anatomy, an edematous airway throughout, and a friable mucosa with no active bleeding. Due to severe hypoxia, the patient was placed on high frequency oscillatory ventilation and inhaled nitric oxide. However, within 8 hours of intubation, the patient had progressive hypoxemia with an oxygenation index that peaked at 91. An arterial blood gas done at that time showed a pH of 7.09, a partial pressure of carbon dioxide of 64 mmHg, a partial pressure of oxygen of 44 mmHg, and an oxygen saturation of 81% on a fraction of inspired oxygen of 1.0.
Figure 1.
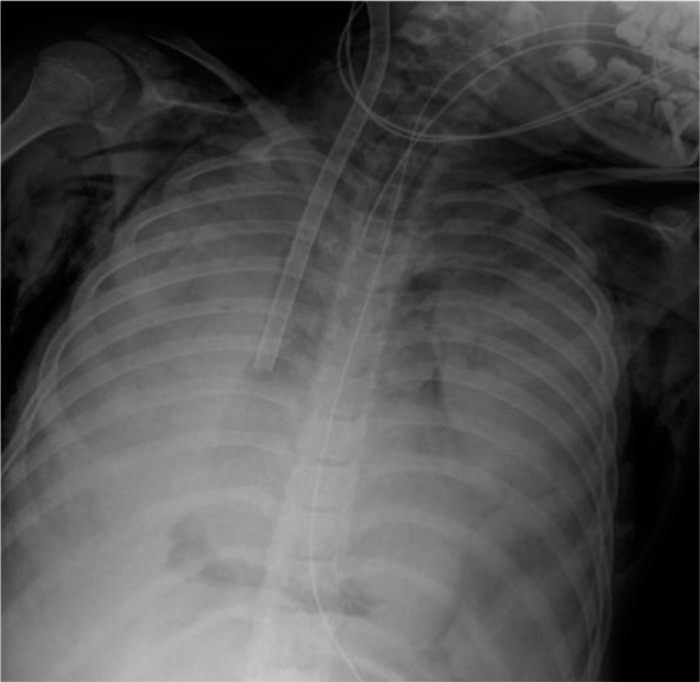
A chest x-ray taken immediately after cannulation showed bilateral pulmonary whiteout, pneumomediastinum, and subcutaneous emphysema.
Figure 2.
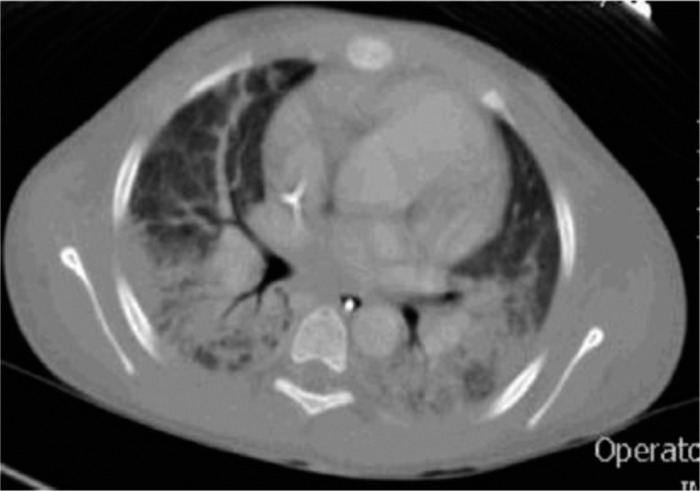
A computed tomography scan of the chest done a few hours before ECMO cannulation showed that the lungs contained bilateral interstitial and air space densities. Small foci in the non-dependent locations were seen in the right middle lobe and the lung apices representing areas of pulmonary edema and hemorrhage.
The patient was urgently placed on VV ECMO. Her weight at the time of cannulation was 31 kg. A 19 French 18 cm (arterial) Biomedicus cannula (Medtronic Incorporated, Minneapolis, MN) was inserted in the right internal jugular vein and used as the drain. A 17 French 50 cm (venous) Biomedicus cannula was inserted in the right femoral vein and used as the return. Then the patient was anticoagulated with 100 units/kg of intravenous heparin and the circuit was primed with one unit of packed red blood cells, 50 mL of 25% albumin, 50 mL of fresh frozen plasma, 10 mEq of sodium bicarbonate, and 400 mg of calcium chloride. We used a SIII roller head pump with integrated servo regulator (Sorin Group USA, Incorporated, Arvada, CO), SMARxT integrated 3/8″ tubing (Cobe Cardiovascular Incorporated USA, Arvada, CO), and a Quadrox D Bioline coated oxygenator (MAQUET Medical Systems USA, Wayne, NJ). VV ECMO flow was slowly advanced to 100 mL/kg/min with marked improvement in the patient’s arterial oxygen saturation.
A heparin infusion was begun 4 hours after cannulation. Thereafter, the patient continued on heparin, targeting an activated clotting time of 200–220 seconds. Due to a low initial hemoglobin level of 5.3 g/dL and active pulmonary hemorrhage, the patient required a total of 2663 mL of packed red blood cells in the first 48 hours after admission to the PCCU. She was supported with VV ECMO for a total of 6 days, during which she received daily plasmapheresis, methylprednisolone, cyclophosphamide, and continuous renal replacement therapy. The bleeding from her airway resolved and the patient remained on mechanical ventilation for an additional 8 days before being successfully extubated. She spent a total of 21 days in the PCCU and 47 days in the hospital before she was successfully discharged home. Currently, she requires peritoneal dialysis, but pulmonary function testing shows that she has no residual lung disease.
COMMENT
This is an example of a child with GS that presented as acute glomerulonephritis and pulmonary alveolar hemorrhage. The glomerulonephritis of GS is caused by the formation of autoantibodies against the α3 chain of type IV collagen in the glomerular basement membrane. These same antibodies also react to the alveolar basement membrane to cause pulmonary hemorrhage (1). GS is rare in children (2). According to the 2011 United States Renal Data System data report from 2005–2009, GS was the underlying cause of end-stage renal disease in only 28 pediatric patients (.4%). Of those pediatric patients with end-stage renal disease secondary to GS, 89.3% were white, 67.9% were female, and 21.4% required renal transplant in the first year (3).
Our patient presented with profuse pulmonary hemorrhage and refractory hypoxemic respiratory failure secondary to new onset GS. Although systemic anticoagulation carries considerable risk in the patient with pulmonary hemorrhage, the heparinized ECMO circuit was used successfully as a platform through which we provided continuous renal replacement therapy and plasmapheresis until the patient’s underlying disease was controlled.
ECMO has been used in past cases of pulmonary hemorrhage with success (4–7). Critical care practitioners should consider ECMO as a treatment modality for profuse pulmonary hemorrhage due to GS if adequate oxygenation cannot be provided by mechanical ventilation. This patient did not suffer from any complications related to ECMO or to the use of anticoagulation therapy. To the authors’ knowledge, this is the first report in the medical literature of the successful use of ECMO in a child with acute pulmonary hemorrhage secondary to GS.
REFERENCES
- 1.Williamson SR, Phillips CL, Andreoli SP, Nailescu C.. A 25-year experience with pediatric anti-glomerular basement membrane disease. Pediatr Nephrol. 2011;26:85–91. [DOI] [PubMed] [Google Scholar]
- 2.Poddar B, Singhal S, Azim A, Gulati S, Baronia A.. Goodpasture’s syndrome in children. Saudi J Kidney Dis Transpl. 2010;21:935–939. [PubMed] [Google Scholar]
- 3.United States Renal Data System. Annual Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. In: Pediatric End-Stage Renal Disease, 2011. Available at: http://www.usrds.org/atlas.aspx. [DOI] [PubMed]
- 4.Agarwal HS, Taylor MB, Grzeszczak MJ, et al. Extra corporeal membrane oxygenation and plasmapheresis for pulmonary hemorrhage in microscopic polyangiitis. Pediatr Nephrol. 2005;20:526–528. [DOI] [PubMed] [Google Scholar]
- 5.Daimon S, Umeda T, Michishita I, Wakasugi H, Genda A, Koni I.. Goodpasture’s-like syndrome and effect of extracorporeal membrane oxygenator support. Intern Med. 1994;33:569–573. [DOI] [PubMed] [Google Scholar]
- 6.Kolovos NS, Schuerer DJ, Moler FW, et al. Extracorporal life support for pulmonary hemorrhage in children: A case series. Crit Care Med. 2002;30:577–580. [DOI] [PubMed] [Google Scholar]
- 7.Locsey L, Kakuk G, Worum I, et al. Goodpasture’s syndrome: Haemodialysis, pacing and extrapulmonary oxygenation. Acta Med Acad Sci Hung. 1980;37:289–298. [PubMed] [Google Scholar]


