Abstract:
Many patient factors have been associated with mortality from extracorporeal membrane oxygenation (ECMO) therapy. Pre-ECMO patient pH and arterial carbon dioxide (paCO2) have been associated with poor outcome and can be significantly altered by ECMO initiation. We hypothesized that the magnitude of change in paCO2 and pH with ECMO initiation could be associated with survival. We designed a retrospective observational study from a single tertiary care center and included all pediatric patients (age younger than 18 years) undergoing ECMO between 2002 and 2010. Electronic records were queried for demographics and clinical characteristics, including the arterial blood gas (ABG) pre- and post-ECMO initiation. Bivariate analysis compared ECMO course characteristics by outcome (survivor vs. nonsurvivor). Multivariable logistic regression was performed on factors associated with the outcome in the bivariate analysis at the significance level of p < .1. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were reported. We identified 201 patients with a median age of 10 days (range, 1 day to 16 years). Indications for ECMO were: respiratory failure (51%), cardiac failure (23%), extracorporeal cardiopulmonary resuscitation (21%), and sepsis (5%). Mortality, defined by death before discharge, was 37% (74 of 201). ABG data pre- and post-ECMO initiations were available in 84% (169 of 201). Age, pH, paCO2, indication, and intracranial hemorrhage were significantly associated with mortality (p < .05). After adjusting for potential confounders (age, use of epinephrine, volume of fluid administered, year of ECMO, ECMO indication, and duration of ECMO) by multivariable logistic regression, the magnitude of paCO2 change (≥25 mmHg) was associated with mortality (adjusted OR, 2.21; 95% CI, 1.06–4.63; p = .036). The decrease in paCO2 with ECMO initiation was associated with mortality. Although this change in paCO2 is multifactorial, it represents a modifiable element of clinical management involving pre-ECMO ventilation, ECMO circuit priming, CO2 administration/removal, and may represent a future therapeutic target that could improve survival in pediatric ECMO.
Keywords: extracorporeal life support, ECLS, extracorporeal membrane oxygenation, ECMO, pediatric, outcome
Extracorporeal membrane oxygenation (ECMO) and other extracorporeal life support (ECLS) technologies have evolved from their original clinical application as a therapy for neonates with respiratory distress and persistent pulmonary hypertension to the contemporary scope of support for patients of all ages who fail maximal medical therapy for respiratory or cardiac failure (1,2). Although survival from neonatal ECMO remains relatively stable (ranges from approximately 48% to 95% for selected neonatal populations), the Extracorporeal Life Support Organization (ELSO) registry shows an increase in use of ECMO in pediatric patients with respiratory failure and cardiac failure with survival ranging from 32% to 50% depending on the age and underlying diagnosis (2,3). This shift in ECMO application from neonates to older infants and children introduces a much broader range of pathophysiologic abnormalities with poorly defined risks and benefits (4,5).
Past clinical studies have focused on electrolyte disturbances and alterations in oxygen-carrying capacity (i.e., differences between patient and ECMO prime values of pH, sodium, calcium, potassium, and hemoglobin) with ECMO initiation in neonates (6). In older children, Mehta et al. found that higher pH (pH > 7.2) before ECMO initiation was associated with increased survival (7). Although pH and other blood gas levels may be the result of illness severity, some of these parameters are influenced by clinical management decisions before and after ECMO initiation. For instance, permissive hypercapnia is a routinely applied ventilator strategy in pediatric and adult patients with respiratory failure, resulting in an intentional pre-ECMO elevation of arterial carbon dioxide (paCO2) and lowering of pH, both factors that have been associated with poor outcome (7,8). The exact relationship between these pre-ECMO values and mortality is unknown. We have observed significant decreases in paCO2 on ECMO initiation. Rapid changes in a patient’s paCO2 and pH could adversely affect cerebral blood flow and perhaps contribute to increased neurologic injury and poor outcome. Therefore, we hypothesized that the magnitude of the acute changes in paCO2 and pH with initiation of ECMO could be associated with survival.
MATERIALS AND METHODS
Given the limitations of the ELSO registry regarding arterial blood gas (ABG) data immediately before and after ECMO initiation, we designed a retrospective observational study of consecutive neonatal and pediatric patients on ECMO at the Johns Hopkins Hospital Children’s Center pediatric intensive care unit from January 1, 2002, to March 31, 2010. The study cohort included all patients younger than 18 years who required ECMO for any indication. To identify all patients receiving ECMO during the study period, we used our institutional ECMO database that keeps track of all ECMO cannulation for administrative purposes. These patients were then cross-referenced with both the ELSO registry and the institutional electronic health records for ECMO initiation orders and billing to make sure no cases went unidentified. During the study period, there was an average of 25 (standard deviation ± 10) ECMO cannulations/courses per year.
Per institutional practice, patients were considered for ECMO if the underlying illness was thought to be a reversible process resulting in cardiovascular and/or pulmonary failure. The primary ECMO indication was determined based on the pediatric intensive care unit (PICU) attending note on the day of cannulation as well as documented echocardiogram reports (it is our institutional policy to obtain an echocardiogram before ECMO cannulation outside of extracorporeal cardiopulmonary resuscitation [ECPR]). Respiratory failure was the primary indication if the PICU attending documented it as such and the echocardiogram pre-ECMO showed normal biventricular function in a structurally normal heart. Cardiac failure was the primary indication if the PICU attending documented it as such and an echocardiogram pre-ECMO showed biventricular dysfunction in an anatomically abnormal heart and/or medical heart disease such as cardiomyopathy or myocarditis. Sepsis was the primary indication if the PICU attending documented it as such and there were also documented positive blood cultures at the time of ECMO cannulation, thought to have led to septic shock refractory to maximal medical therapy. ECPR was defined as cardiac arrest with ongoing chest compressions during ECMO cannulation, in which ECMO was used as part of the initial resuscitation from cardiac arrest. The decision to initiate/terminate ECMO, to adjust the ECMO flows, to manipulate anticoagulation therapy, sedation, and method of cannulation were at the discretion of the clinical care teams after routine institutional protocols. All neurologic imaging (head ultrasound and computed tomography) and neurology consults were obtained at the discretion of the clinical care team.
During the time period of the study, there were no changes in ECMO circuit configuration, management protocols or ABG monitoring. The ECMO circuit consisted of: custom-packed 1/4- or 3/8-inch flexible polyvinylchloride tubing with a silicone reservoir (Medtronic, Minneapolis, MN); bladder box for pressure sensing (Johns Hopkins Hospital, Baltimore, MD); a .8- to 4.5-m2 membrane oxygenator (Medtronic); a heat exchanger (Medtronic); and a roller pump (Sorin Cardiovascular USA, Arvada, CO) (9). Circuit electrolytes were analyzed and corrected as needed per institutional protocol. During the study period, ABG testing was done using a benchtop blood gas analyzer (RadiometerABL800 FLEX analyzer, Bronshoj, Denmark). Per institutional practice, ABGs are obtained before and after ECMO cannulation and then every 4 hours and as needed.
Demographic, clinical, laboratory, and survival data were collected by query of electronic medical records and individual chart review. The primary outcome variable was patient survival to hospital discharge. The Johns Hopkins Hospital Institutional Review Board approved this study and waived the need for individualized consent (NA_00032434).
STATISTICAL METHODS
Exploratory descriptive data analysis was conducted to examine patient and ECMO course characteristics. The last and the first ABG values within 3 hours before and after ECMO initiation, respectively, included: pH, paCO2, and oxygen tension (paO2). The difference in pH and paCO2 obtained pre- and post-ECMO initiation were calculated as: Delta pH = (last pH pre-ECMO — first pH post-ECMO initiation); Delta-paCO2 = (last paCO2 pre-ECMO — first paCO2 post-ECMO initiation). Survivor vs. nonsurvivor groups were created and compared by bivariate analysis with regard to demographics and clinical and ECMO course characteristics. Differences between the two groups were analyzed with the Wilcoxon rank sum test for nonnormally distributed continuous variables, Student’s t test for normally distributed continuous variables and χ2 test for binary variables, as appropriate. Multivariable logistic regression was then performed, controlling for potential confounding risk factors previously described (age, use of epinephrine pre-ECMO, ECMO indication, year of ECMO, volume of intravenous fluids received in the first 24 hours of ECMO, and ECMO duration) (8,10–12). Multivariable logistic regression was performed on factors associated with the outcome in the bivariate analysis at a significance level of p < .1 (13). Odds ratios (ORs) and 95%confidence intervals (95% CIs) were reported for factors that were independently associated with mortality. All statistical analysis was conducted using STATA Statistical Software: Release 11.0 (STATA Corp., College Station, TX).
RESULTS
All consecutive patients over the study duration were analyzed and there were no major changes in our institutional ECMO protocols. There were 201 patients (newborns and children younger than 18 years) who underwent ECMO at our institution during the study period. Median age of the study cohort was 10 days (range, 1 day to 16 years). Indications for ECMO independent of outcome were respiratory failure in 102 of 201 (51%), cardiac failure in 46 of 201 (23%), ECPR in 42 of 201 (21%), and sepsis in 11 of 201 (5%) with a median duration of ECMO of 5 days (range, 1–33 days). Overall mortality was 37% (74 of 201). Adverse neurologic events diagnosed by neuroimaging occurred in 49 of 201 (24%) of patients. Intracranial hemorrhage (ICH) was diagnosed in 34 of 201 (17%) of patients, 15 of 201 (7.5%) had cerebral infarction, and eight of 201 (4%) had a neurologic examination consistent with brain death during ECMO. However, the true prevalence of neurologic injuries in survivors and nonsurvivors was unknown secondary to the elective nature of neuroimaging.
Venoarterial ECMO was performed in 183 of 201 (91%) patients without conversion from venovenous ECMO. Demographic and clinical characteristics by survival are presented in Table 1. The following variables were significantly associated with survival by bivariate analysis (p < .05): age, respiratory failure, pH (both pre- and post-ECMO), paCO2 (both pre- and post-ECMO), epinephrine use pre-ECMO, and ICH.
Table 1.
Comparison of patient characteristics and ECMO courses between survivors and nonsurvivors.
| Variable | Survivors (n = 127) | Nonsurvivors (n = 74) | p Value |
|---|---|---|---|
| Age, no. (%) | |||
| 0 to <1 month | 88 (69.3) | 29 (39.2) | <.001 |
| ≥1 month to 1 year | 24 (18.9) | 15 (20.3) | |
| ≥1–7 years | 4 (3.2) | 18 (24.3) | |
| ≥8–18 years | 11 (8.7) | 12 (16.2) | |
| Male, no. (%) | 66 (51.9) | 35 (47.3) | .523 |
| ECMO indication, no. (%) | <.001 | ||
| Respiratory failure | 75 (59.1) | 27 (36.5) | |
| Cardiac failure | 27 (21.3) | 19 (25.7) | |
| Sepsis | 9 (7.1) | 2 (2.7) | |
| ECPR | 16 (12.6) | 26 (35.1) | |
| ECMO mode, no. (%) | |||
| VA-ECMO/CPS | 114 (89.8) | 69 (93.2) | .405 |
| VV-ECMO | 13 (10.2) | 5 (6.8) | |
| Epinephrine use before ECMO, no. (%) | 72 (56.7) | 53 (71.6) | .035 |
| Fluid administered in first 24 hours on ECMO (mL/kg) | 242 (191–311) | 269 (178–405) | .115 |
| ECMO duration (days), median (IQR) | 5 (3–9) | 7 (3–13) | .42 |
| Intracranial hemorrhage, no. (%) | 14 (11.0) | 20 (27.0) | .004 |
| Arterial blood gas values | |||
| pH pre-ECMO, median (IQR) | 7.34 (7.28–7.44) | 7.26 (7.07–7.37) | <.001 |
| pH post-ECMO, median (IQR) | 7.49 (7.40–7.57) | 7.39 (7.21–7.48) | <.001 |
| Delta-pH (mmHg), median (IQR) | .14 (.04–.24) | .13 (.05–.24) | .889 |
| paCO2 pre-ECMO (mmHg), median (IQR) | 40 (34–54) | 47 (37–68) | .019 |
| paCO2 post-ECMO (mmHg), median (IQR) | 28 (23–34) | 32 (23–40) | .034 |
| Delta-paCO2 (mmHg), median (IQR) | 14 (5–23) | 17 (5–35) | .889 |
| paO2 pre-ECMO (mmHg), median (IQR) | 63 (44–133) | 74 (39–245) | .424 |
| paO2 post-ECMO (mmHg), median (IQR) | 293 (110–400) | 279 (86–445) | .943 |
Data are presented as median and interquartile range (IQR) unless otherwise indicated. Differences between survivors and nonsurvivors were analyzed with the Wilcoxon rank sum test and χ2 test.
ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; VA, venoarterial; CPS, mode of cardiopulmonary support for ECPR; VV, venovenous; IQR, interquartile range; Delta-pH = (last pH pre-ECMO - first pH post-ECMO initiation); Delta-paCO2 = (last paCO2 pre-ECMO - first paCO2 post-ECMO initiation); paCO2, arterial carbon dioxide content measured by blood gas; paO2, arterial oxygen content measured by blood gas.
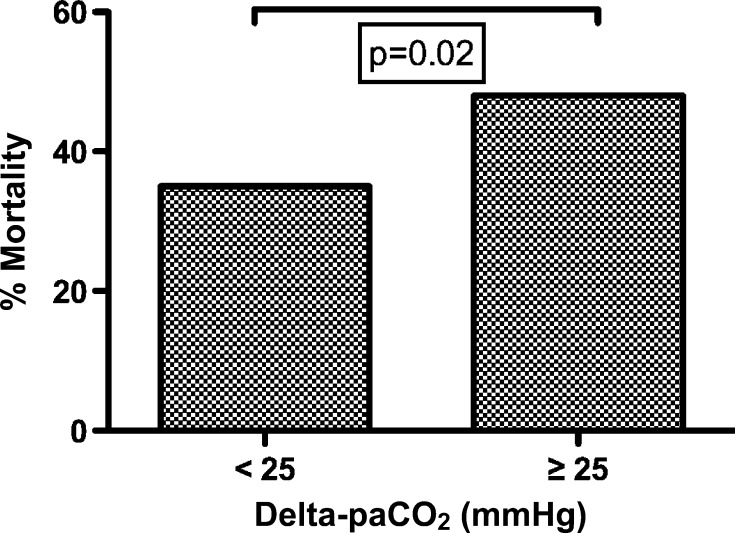
Complete ABG data within 3 hours of ECMO initiation were available in 169 of 201 (84%) patients. We found a decrease in paCO2 from the pre-ECMO ABG to the post-ECMO initiation ABG in 147 of 169 (87%) patients, and an increase in paCO2 occurred in 22 of 169 (13%) patients. The median delta-paCO2 was 14 mmHg (interquartile range, 5–26 mmHg). Percentages of mortality by delta-paCO2 comparing those patients with <25 mmHg to ≥25 mmHg alteration were higher in those patients with a greater decrease in paCO2 (p = .02, see Figure 1). Simple logistic regression showed a significant association between the magnitude of delta-paCO2 at the time of ECMO initiation and mortality (unadjusted OR, 2.18; 95% CI, 1.21–3.92; p = .01). After adjusting for potential confounding factors (age, indication for ECMO, including respiratory failure, cardiac failure, sepsis and ECPR, year of ECMO, epinephrine use before ECMO, volume of fluid administered in the first 24 hours of ECMO, and ECMO duration), the magnitude of paCO2 decrease (≥25 mmHg) was significantly associated with mortality (adjusted OR, 2.21; 95% CI, 1.06–4.63; p = .036). Bivariate and multivariable logistic regression results for all significant variables are presented in Table 2.
Figure 1.
Mortality and the magnitude of arterial carbon dioxide (paCO2) decrement caused by the initiation of extracorporeal membrane oxygenation (ECMO) support. The percent mortality for delta-paCO2 greater than and less than 25 mmHg is shown, demonstrating a significant increase in mortality with the greatest paCO2 change, p = .02. Delta-paCO2 = (last paCO2 pre-ECMO — first paCO2 post-ECMO initiation).
Table 2.
Bivariate and multivariable regression analysis for mortality.
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Delta-paCO2 (mmHg) | 2.18 (1.21–3.92) | 2.21 (1.06–4.63) |
| Age | ||
| 0 to <1 month | Reference | Reference |
| ≥1 month to 1 year | 1.89 (.88–4.09) | 1.26 (.49–3.21) |
| ≥1–7 years | 13.7 (4.27–43.6) | 12.9 (3.56–46.9) |
| ≥8–18 years | 3.31 (1.32–8.30) | 3.25 (1.07–9.88) |
| Indication for ECMO | ||
| Respiratory failure | Reference | Reference |
| Cardiac failure | 1.95 (.94–4.07) | 1.76 (.67–4.61) |
| Sepsis | .62 (.13–3.04) | .48 (.08–2.87) |
| ECPR | 4.51 (2.11–9.68) | 3.46 (1.22–9.79) |
| Year of ECMO | .83 (.73–.94) | .83 (.72–.97) |
| Epinephrine use before ECMO | 1.93 (1.04–3.57) | 1.71 (.79–3.68) |
| Fluid administered in first 24 hours on ECMO (mL/kg) | 1.00 (.99–1.00) | 1.00 (.99–1.00) |
| ECMO duration (days) | 1.03 (.99–1.07) | 1.05 (.99–1.11) |
| Intracranial hemorrhage | 2.98 (1.40–6.37) | 2.23 (.89–5.59) |
OR, odds ratio; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; Delta-paCO2 = (last paCO2 pre-ECMO - first paCO2 post-ECMO initiation); paCO2, arterial carbon dioxide content; Reference, reference group for age and indication for ECMO analysis.
DISCUSSION
Extracorporeal membrane oxygenation support is increasingly but inconsistently used across ECLS centers for children and adults. Institutional differences in ECMO-practice and patient selection have resulted in variable survival rates for ECMO programs (14–16). In this heterogeneous study cohort, our institutional survival to discharge rate of 63%was inclusive of all age groups and ECMO indications. Survival was significantly associated with age, epinephrine use before ECMO, ICH, pH, and paCO2. When controlling for known confounding factors, the magnitude of change in paCO2 at ECMO initiation remained associated with mortality. Furthermore, mortality was highest in those patients with the greatest magnitude of delta-paCO2 (≥25 mmHg) during ECMO initiation in our study cohort.
Pre-ECMO variables such as younger age, better oxygenation status, and shorter courses of mechanical ventilation before ECMO initiation have been associated with improved survival (17–19). Studies of institutional experience with ECMO in patients <1 month of age with respiratory failure have demonstrated 75% survival to hospital discharge, whereas survival from cardiac failure was 39% (16). Older infants and children have a reported 56% survival from ECMO for respiratory failure and 48% survival for cardiac failure (20–22). In our cohort, the majority of survivors were younger children with respiratory failure (59%), whereas the nonsurvivors tended to be older children divided among respiratory failure (37%), cardiac failure (26%), and cardiac arrest with support (35%).
ECMO support is associated with a high risk for brain injury, including ICH, brain infarction, and brain death. Neurologic complications were prominent in those who received neuroimaging (n = 49). Intracranial hemorrhage detected by neuroimaging was the most prominent neurologic complication of ECMO in our cohort, affecting 11% of survivors and 27% of nonsurvivors in this study. The impact of ICH on survival was prominent and agreed with other ECMO outcome studies (8). Acute neurologic injury occurs in 22% of patients undergoing ECPR compared with other indications for ECMO (23). In our study, inclusion of patients undergoing ECPR in our analysis could partially explain the higher rate of ICH. Furthermore, patients who did not receive neuroimaging (n = 152) may have had ICH or infarctions that were unrecognized and could have occurred either before or during the ECMO procedure. Whether these injuries were present before ECMO initiation is unknown because there was no standardized protocol for timing of neurologic imaging or formal neurologic assessment.
Measured pH on ABG before and after ECMO initiation was significantly higher in survivors. However, pH and delta-pH were not associated with survival when controlling for confounding factors. Low pre-ECMO pH has been reported as an independent predictor of survival (7,24). As speculated in these studies, low pH can be attributable to worsening metabolic acidosis from poor oxygen delivery and organ perfusion in the period before ECMO initiation. Future studies are needed to better evaluate the importance of the relationship between pH and ECMO outcomes.
Hypercarbia and pre-ECMO elevations of paCO2 have been associated with poor outcomes from ECMO. Studies evaluating paCO2 values with ECMO have demonstrated pre-ECMO elevated paCO2 (>50 mmHg) to be associated by univariate analysis with ICH in neonates rescued with ECMO (8,10). In other studies of pediatric ECMO, duration of mechanical ventilation and serum pH less than 7.29 before ECMO initiation have been associated with increased mortality (11). There is no clear mechanism for elevated paCO2 to “actively” contribute to increased mortality except through the physiologic effect of paCO2 on the brain. Although neurologic injury in our study cohort was not significantly associated with alterations in paCO2 by multivariable analysis, the potential for large-magnitude changes in paCO2 to negatively impact cerebral blood volume and flow could provide a potential mechanism for nonsurvivable neurologic injury with ECMO initiation. Our ability to demonstrate this relationship was limited by the inconsistent acquisition of neuroimaging by the clinical care teams in our cohort. Animal studies of sustained hypercarbia demonstrated an increase in cerebral blood flow that returned to baseline over a 24-hour period as the pH of cerebrospinal fluid (CSF) normalized (25). Immediate restoration of normocarbia in patients at the initiation of ECMO has not been shown to cause cerebral ischemia or intracranial hemorrhage. However, if these associations between ABG measurements and outcome reflect the severity of illness prompting the application of ECMO, then there is no evident ECMO strategy to improve outcome. Alternatively, if these associations occur because of sudden and harmful changes in the pH of CSF, then strategies to introduce ECMO with a goal of controlling paCO2 alterations warrant investigation.
In both the survivors and nonsurvivors, the directional change in delta-paCO2 was predominantly a decrease between the pre- to post-ECMO initiation ABG. This suggests higher pre-ECMO paCO2 at our institution and most likely represents adjustment of ventilation to normocarbia or hypercarbia in caring for patients with respiratory failure. Other centers still practicing hyperventilation for neonates with persistent pulmonary hypertension or diaphragmatic hernias before ECMO may find a different result than the one presented here. In animal models of prolonged hypocarbia, cerebral blood flow is decreased for several hours before normalizing. Sudden restoration of normocarbia in these animals causes a sustained and apparently pathologic doubling of cerebral blood flow (26). Based on these animal studies, it is possible that paCO2-mediated changes in either direction could result in neurologic injury.
Although pre-ECMO ABG findings may be the result of disease severity or issues of failing hemodynamic or respiratory management, our institution had no consistent strategy to match ECMO circuit prime characteristics to these patients’ paCO2 before ECMO initiation. Even within a single institution, during the study period, we did not routinely adjust the prime to minimize changes in pH or paCO2. There are numerous potential clinical strategies to minimize paCO2 alterations with ECMO initiation that could mitigate the observed changes in paCO2. Since the end of this study period, we have been balancing the ECMO priming solution to match a patient’s ABG in both pH and paCO2. In addition, in-line continuous blood gas analyzers are available that could facilitate correction of serum pH at a controlled rate during the initiation and maintenance of ECMO. Additional manipulations of CO2 clearance control on ECMO can occur with adjustment of either the oxygenator sweep rate or the concentration of CO2 in the sweep gas mixture as long as safeguards against hypoxic gas mixtures are in place. Combining CO2 and pH regulatory mechanisms on ECMO could provide oxygenation and cardiac output augmentation in this way without altering the patient serum pH. Future studies are needed to confirm the time course for lowering the paCO2 to demonstrate that it is feasible. One review of ECMO practices has suggested a correction of paCO2 over 24 hours (27). This recommendation is consistent with data from rabbits, showing that pial artery diameter returns to normal 20–24 hours after an abrupt and sustained reduction in paCO2 (28). Although speculative, these approaches could be of value in reducing neurologic injuries and improving survival.
Our study has the limitations inherent to the retrospective design and single-center experience. Although our findings reflect our institutional practice, they may not be generalizable to other ECLS centers. Blood gas data in the ELSO registry are difficult to differentiate between source (i.e., patient vs. ECMO circuit), whether the samples are venous or arterial, and the exact time of sample acquisition relative to the initiation of ECMO. Currently, the ELSO registry does not assess delta-paCO2 with ECMO initiation, although this could be added to the database and would allow multi-institutional evaluation of our finding. Although the course and outcome of those patients who had similar diagnoses but did not receive ECMO are relevant, this is beyond the scope of our study. However, unlike other factors (pre-ECMO and patient-specific) associated with outcome from ECMO support, we have identified a potentially modifiable factor that could be monitored and controlled during ECMO initiation. A stronger case for the relationship of paCO2 alterations with survival would have been made if an association between large delta-paCO2 and acute neurologic injury had been demonstrated. However, our retrospective chart review is unable to test this association given the inconsistent application of neuroimaging in this cohort of patients on ECMO. Future studies could confirm our findings in other cohorts and at other ECLS centers.
CONCLUSION
ECMO is an important support option for pediatric patients with refractory respiratory or hemodynamic failure. Our study has demonstrated an institutional survival rate of 63% from ECMO therapy and found improved survival was associated with smaller changes of paCO2 at the time of ECMOinitiation. The association between mortality and paCO2 decrement was greatest in the highest quartile change of paCO2 by multivariable analysis controlling for other factors associated with death in the setting of ECMO. Although our findings need to be prospectively validated in other cohorts, they have implications for the development of ECMO priming and sweep strategies that would focus on minimizing an individual’s changes in paCO2 with ECMO initiation.
ACKNOWLEDGMENTS
The project described was supported by an American Heart Association Clinical Research Program Grant (for R.B.E.) and Grants No. UL1 RR 025005 and 1KL2RR025006-01 (for M.M.B.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at www.ncrr.nih.gov/. This project was partially supported by the NCRR and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant No. 101872 (biostatistical support).
REFERENCES
- 1.Dalton HJ, Butt WW.. Extracorporeal life support: An update of Rogers’ Textbook of Pediatric Intensive Care. Pediatr Crit Care Med. 2012;13:461–471. [DOI] [PubMed] [Google Scholar]
- 2.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A.. Extracorporeal Life Support Registry Report 2008: Neonatal and pediatric cardiac cases. ASAIO J. 2009;55:111–116. [DOI] [PubMed] [Google Scholar]
- 3.Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: The Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J. 2012;58:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta P, McDonald R, Chipman CW, et al. 20-year experience of prolonged extracorporeal membrane oxygenation in critically ill children with cardiac or pulmonary failure. Ann Thorac Surg. 2012;93:1584–1590. [DOI] [PubMed] [Google Scholar]
- 5.Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B.. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008;17(Suppl 4):S41–S47. [DOI] [PubMed] [Google Scholar]
- 6.Grist G, Thomas D.. Blood anion gaps and venoarterial carbon dioxide gradients as risk factors in long-term extracorporeal support. J Extra Corpor Technol. 1997;29:6–10. [PubMed] [Google Scholar]
- 7.Mehta NM, Turner D, Walsh B, et al. Factors associated with survival in pediatric extracorporeal membrane oxygenation—A single-center experience. J Pediatr Surg. 2010;45:1995–2003. [DOI] [PubMed] [Google Scholar]
- 8.Hardart GE, Fackler JC.. Predictors of intracranial hemorrhage during neonatal extracorporeal membrane oxygenation. J Pediatr. 1999;134:156–159. [DOI] [PubMed] [Google Scholar]
- 9.Bembea MM, Savage W, Strouse JJ, et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12: 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardart GE, Hardart MK, Arnold JH.. Intracranial hemorrhage in premature neonates treated with extracorporeal membrane oxygenation correlates with conceptional age. J Pediatr. 2004;145: 184–189. [DOI] [PubMed] [Google Scholar]
- 11.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL.. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. [DOI] [PubMed] [Google Scholar]
- 12.de Mol AC, Gerrits LC, van Heijst AF, Straatman H, van der Staak FH, Liem KD.. Intravascular volume administration: A contributing risk factor for intracranial hemorrhage during extracorporeal membrane oxygenation? Pediatrics. 2008;121:e1599–e1603. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer DW, Lemeshow S.. Applied Logistic Regression, 2nd edition New York, NY: Wiley; 2000:1–94. [Google Scholar]
- 14.Weber TR, Kountzman B.. Extracorporeal membrane oxygenation for nonneonatal pulmonary and multiple-organ failure. J Pediatr Surg. 1998;33:1605–1609. [DOI] [PubMed] [Google Scholar]
- 15.Vats A, Pettignano R, Culler S, Wright J.. Extracorporeal life support in pediatric acute respiratory failure: We can afford it AND need it. Crit Care Med. 2000;28:1690–1691. [DOI] [PubMed] [Google Scholar]
- 16.Moler FW, Custer JR, Bartlett RH, et al. Extracorporeal life support for severe pediatric respiratory failure: An updated experience 1991–1993. J Pediatr. 1994;124:875–880. [DOI] [PubMed] [Google Scholar]
- 17.Wagner K, Risnes I, Abdelnoor M, Karlsen HM, Svennevig JL.. Is it possible to predict outcome in cardiac ECMO? Analysis of preoperative risk factors. Perfusion. 2007;22:225–229. [DOI] [PubMed] [Google Scholar]
- 18.Wagner K, Risnes I, Abdelnoor M, Karlsen HM, Svennevig JL.. Is it possible to predict outcome in pulmonary ECMO? Analysis of preoperative risk factors. Perfusion. 2008;23:95–99. [DOI] [PubMed] [Google Scholar]
- 19.Walters HL 3rd, Hakimi M, Rice MD, Lyons JM, Whittlesey GC, Klein MD.. Pediatric cardiac surgical ECMO: Multivariate analysis of risk factors for hospital death. Ann Thorac Surg. 1995;60:329–336; discussion 36–37. [DOI] [PubMed] [Google Scholar]
- 20.Lequier L.. Extracorporeal life support in pediatric and neonatal critical care: A review. J Intensive Care Med. 2004;19:243–258. [DOI] [PubMed] [Google Scholar]
- 21.Maclaren G, Butt W.. Extracorporeal membrane oxygenation and sepsis. Crit Care Resusc. 2007;9:76–80. [PubMed] [Google Scholar]
- 22.Maclaren G, Butt W, Best D, Donath S, Taylor A.. Extracorporeal membrane oxygenation for refractory septic shock in children: One institution’s experience. Pediatr Crit Care Med. 2007;8: 447–451. [DOI] [PubMed] [Google Scholar]
- 23.Barrett CS, Bratton SL, Salvin JW, Laussen PC, Rycus PT, Thiagarajan RR.. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10:445–451. [DOI] [PubMed] [Google Scholar]
- 24.Swaniker F, Kolla S, Moler F, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35:197–202. [DOI] [PubMed] [Google Scholar]
- 25.Warner DS, Turner DM, Kassell NF.. Time-dependent effects of prolonged hypercapnia on cerebrovascular parameters in dogs: Acid-base chemistry. Stroke. 1987;18:142–149. [DOI] [PubMed] [Google Scholar]
- 26.Gleason CA, Hamm C, Jones MD Jr.. Cerebral blood flow, oxygenation, and carbohydrate metabolism in immature fetal sheep in utero. Am J Physiol. 1989;256:R1264–R1268. [DOI] [PubMed] [Google Scholar]
- 27.Short BL.. The effect of extracorporeal life support on the brain: A focus on ECMO. Semin Perinatol. 2005;29:45–50. [DOI] [PubMed] [Google Scholar]
- 28.Muizelaar JP, van der Poel HG, Li ZC, Kontos HA, Levasseur JE.. Pial arteriolar vessel diameter and CO2 reactivity during prolonged hyperventilation in the rabbit. J Neurosurg. 1988;69:923–927. [DOI] [PubMed] [Google Scholar]