Abstract
Depending on the population examined, from 6 to 83% of people with diabetes mellitus exhibit symptoms of altered gut motility, manifesting as dysphagia, reflux, early satiety, nausea, abdominal pain, diarrhea, or constipation. Hyperglycemia-induced cell loss within the enteric nervous system has been demonstrated in both diabetic rodents and patients with diabetes. Glycemic control is recommended to prevent diabetic gastroenteropathy but is often difficult to achieve with current treatment modalities. We asked if hepatic insulin gene therapy (HIGT) could inhibit the development of diabetic gastroenteropathy in mice. Bowel length, bowel transit, colonic muscle relaxation, and the numbers of both stimulatory and inhibitory neurons in the colonic myenteric plexus were compared in groups of diabetic mice (DM), control nondiabetic mice (Con), and diabetic mice treated with HIGT (HIGT). Delivery of a metabolically responsive insulin transgene to the liver of STZ-diabetic mice with an adeno-associated virus, sero-type 8 (AAV8) produced near-normal blood sugars for over 1 month and prevented anatomic, functional, and neurohistologic changes observed in diabetic mice. We conclude that in addition to normalizing oxidative metabolism in diabetic rodents, HIGT is sufficient to prevent the development of diabetic gastroenteropathy.
Introduction
Approximately 29 million people in the United States have diabetes mellitus,1 and many exhibit some form of diabetes-associated enteropathy presenting as altered gut motility.2,3 The frequency of clinical complaints varies with the population studied, ranging from 6–27% in unselected patients with diabetes in the community setting to 76–83% in patients referred to tertiary care facilities.2,3 Symptoms include dysphagia, reflux, early satiety, nausea, abdominal pain, diarrhea, or constipation, either alone or in concert.2,3 The breadth of clinical symptoms displayed in diabetic gastroenteropathy reflects observations that all portions of the gastrointestinal tract can be affected by diabetes.4 Moreover, within a given anatomical segment of the gut, the etiology of dysfunction is likely multifactorial, ranging from dysfunction of either the autonomic nervous system (sympathetic and parasympathetic) or enteric nervous system (ENS), to altered trophic factor signaling resulting in enteric myopathy and neural apoptosis.2,4
Among the multiple factors producing bowel dysfunction in diabetes, hyperglycemia-induced cell loss within the ENS appears to play a significant role.5,6 The ENS, an arm of the peripheral nervous system with at least as many neurons as the spinal cord, is primarily responsible for autonomously mediating gut motility responses to the physical and biochemical composition of bowel contents.7 The ENS, with its assortment of neuronal and glial cells coordinating the action of both stimulatory and inhibitory signals, is localized within two plexuses, one between the longitudinal and circular smooth muscle layers, the myenteric plexus, and one within the submucosa, the submucosal plexus.7 Recent observations indicate that hyperglycemia-induced neuronal loss, particularly of inhibitory neurons within these plexuses, plays a significant role in altered gut function.3,5,6
Insulin therapy is necessary to control glycemia in all patients with type 1 diabetes mellitus and in ~30% of patients with type 2 diabetes.1 Treatment with exogenous insulin improves blood sugars but is often insufficient to prevent long-term complications.8,9 While intensification of insulin regimens reduces hyperglycemia, it also increases the incidence of insulin-induced hypoglycemia.9
Gene therapy may provide a means to improve and simplify treatment of diabetes mellitus. Hepatic insulin gene therapy (HIGT) has been shown to produce near-normal random blood glucose in multiple rodent models of diabetes mellitus.10–13 Significantly, HIGT-treated animals tolerate chow deprivation without lethal hypoglycemia and exhibit normal fluctuations of diurnal oxidative metabolism.14 Moreover, the glycemic control induced by HIGT was sufficient to prevent diabetes-associated macrovascular dysfunction.15
We undertook this study to determine if HIGT could inhibit the development of diabetic gastroenteropathy in mice. We compared bowel length, bowel transit, muscle relaxation, and the numbers of both stimulatory and inhibitory neurons in the myenteric plexus of diabetic mice (DM), control nondiabetic mice (Con), and diabetic mice treated with HIGT (HIGT). Delivery of a metabolically responsive insulin transgene to the liver of STZ-diabetic mice with an adeno-associated virus, sero-type 8 (AAV8) produced near-normal blood sugars for over 1 month, and this level of glycemic control was sufficient to prevent anatomic, functional, and neurohistologic changes observed in diabetic mice. We conclude that in addition to normalizing oxidative metabolism in diabetic rodents, HIGT is sufficient to prevent the development of diabetic gastroenteropathy.
Results
Blood glucose and body weight
Average body weights and blood glucose of control (Con), diabetic (DM), and HIGT-treated (HIGT) mice were similar at baseline prior to induction of diabetes (Table 1). On day 0, following STZ or buffer injection, body weights of DM and HIGT declined and blood glucose values increased to consistently greater than 200 mg/dl, a diagnostic threshold for diabetes mellitus chosen a prior (Figure 1). Analysis post hoc of random blood sugar values obtained for Con (n = 1,016; average ± SD = 151 ± 22 mg/dl), and calculation of (average + (2× SD)) provided an upper limit for normal of 195 mg/dl, confirming the utility of the selected threshold value. At day 0, average blood sugars were higher among DM than HIGT (Table 1). All mice underwent laparotomy and received similar quantities of virus, AAV2/8-SC-(GlRE3) BP-1 2xfur to HIGT, and AAV2/8-SC-CMV GFP to both Con and DM, administered by hepatic portal system injection on day 0. The average time between STZ and virus administration was similar among HIGT (7.4 ± 3.7 SD days) and DM (6.63 ± 1.8 SD days) mice (P = 0.47 for difference). All mice were sacrificed 50 days after virus administration.
Table 1. Baseline body weight and glycemia of subject animals.
| Body weight | Con | DM | HIGT |
|---|---|---|---|
| Initial | 29.61 ± 0.42 | 30.35 ± 0.20 | 30.30 ± 0.62 |
| Day 0 | 30.95 ± 0.45 | 28.70 ± 0.21** | 29.35 ± 0.45* |
| Final | 36.74 ± 0.79 | 30.29 ± 0.52** | 37.35 ± 0.44*** |
| Blood glucose | |||
| Initial | 160 ± 4.29 | 164 ± 5.6 | 165 ± 4.6 |
| Day 0 | 156 ± 5.36 | 538 ± 10.2** | 403 ± 27.7**,*** |
| Final | 149 ± 3.5 | 586 ± 5.5** | 148 ± 15.6 |
Data are presented as mean ± SD, n = 30–35 for each condition.
Con, control nondiabetic mice; DM, diabetic mice; HIGT, hepatic insulin gene therapy.
*P < 0.05 versus DM; **P < 0.01 versus Con; ***P < 0.01 versus DM by Tukey’s multiple comparison test after one-way analysis of variance with P < 0.05.
Figure 1.
HIGT effects on blood sugar, growth, and fat depots. (a) Blood glucose and (b) body weight of control (open circles), diabetic (open triangles), and HIGT-treated (closed triangles) mice. Data are presented as mean ± SEM of 6–35 values for each day depicted. (c) Mesentery and (d) epididymal fat weights of Con, DM, and HIGT mice. Data are presented as mean ± SEM, n = 18–46, *P < 0.05 versus Con; #P < 0.05 vs. DM. Con, control nondiabetic mice; DM, diabetic mice; HIGT, hepatic insulin gene therapy.
Treatment of Con and DM mice with AAV 2/8 SC CMV GFP failed to modify blood sugars. Blood glucose among Con mice remained below 200 mg/dl, and blood glucose among DM mice remained persistently above 500 mg/dl (Figure 1). In contrast, treatment of HIGT with AAV 2/8 SC G3 2xfur reduced blood glucose beginning 4 to 6 days following viral injection. HIGT mice consistently achieved normal blood glucose levels (<200 mg/dl) beginning ~11 days after receiving virus (Figure 1a). After declining to less than 200 mg/dl, blood sugars in HIGT-treated mice maintained a mean of 133 ± 3.87 mg/dl, less than the mean blood glucose of 147 ± 1.01 mg/dl for control mice (P < 0.01). However, variance among HIGT blood sugars was greater than Con (F-test, P < 0.0001).
Following abdominal surgery and viral injections, all mice lost weight. With the support of exogenous insulin injections, DM mice gained weight beginning 3 days after virus administration but weighed consistently less than Con mice. In contrast, despite receiving no insulin injections once blood glucose values began to decline, HIGT mice gained weight beginning 4 days after virus injection, achieved normal weight by 12 days postsurgery, and subsequently grew similarly to Con mice. Final body weight of Con and HIGT mice was similar (Table 1; Figure 1b; P > 0.05).
Consistent with lower body weights and prolonged hyperglycemia, mesentery weight was diminished among DM (Figure 1c). In contrast, mesentery weight in HIGT mice was similar to Con (Figure 1c). The average weight of epididymal fat pads was reduced in DM (Figure 1d). Epididymal fat pad weight was greater in HIGT than in DM (P < 0.01). However, despite similar body weights and blood glucose values, epididymal fat pad weight in HIGT was less than Con (P < 0.01).
Serum hormones and adipokines
Serum insulin levels were diminished and glucagon levels were increased in DM compared to Con mice (Table 2). Despite having also received STZ, insulin concentrations among HIGT mice measured with a mouse insulin assay were similar to Con and greater than DM mice. As expected, human insulin was undetectable in Con and DM mice but was also not detected in HIGT mice (HIGT 0.42 ± 0.24 mU/ml (n = 10), DM 0.58 ± 0.39 mU/l (n = 19), and Con 0.31 ± 0.17 mU/l (n = 11); analysis of variance, P = 0.728). Transgenic human insulin expression was confirmed in HIGT animals and excluded in Con mice by RT-PCR (Figure 2a,b). Consistent with extant insulin levels, serum glucagon concentrations in HIGT mice were similar to Con, and lower than that in DM mice (P < 0.001). While serum leptin concentrations among DM mice were diminished compared to Con mice, HIGT treatment increased serum leptin concentrations (P < 0.01 vs. DM). However, serum leptin values in HIGT remained below those found among Con mice (P < 0.05). No differences in serum concentrations of resistin, IL-6, PAI-1, or adiponectin were observed. Amounts of TNFα and MCP-1 remained below the level of detection for all groups.
Table 2. Hormones and adipokines determined in sera of control, diabetic, and HIGT-treated mice.
| Control | DM | HIGT | |
|---|---|---|---|
| Insulin (pmol/l) | 1,584.90 ± 302 | 5,97.50 ± 234* | 1,850.15 ± 226 |
| Glucagon (pmol/l) | 53.93 ± 9.2 | 119.50 ± 19* | 36.94 ± 6.7** |
| Leptin (pmol/l) | 3,588.52 ± 282 | 740.77 ± 347* | 2,492.73 ± 324*,** |
| Resistin (pg/ml) | 3,482.78 ± 198 | 2,594.15 ± 274 | 3,478.99 ± 433 |
| Adiponectin (µg/ml) | 8.18 ± 0.6 | 7.17 ± 0.7 | 8.36 ± 0.9 |
| PAI-1 (pg/ml) | 2,633 ± 293 | 2,985 ± 590 | 4,654 ± 1099 |
| IL-6 (pg/ml) | 23.22 ± 5.1 | 16.41 ± 5.0 | 28.12 ± 5.3 |
Value of insulin in HIGT-treated mice reflects cross-reactivity in murine insulin assay. Data are presented as mean ± SD, n = 8–20 for each condition.
Con, control nondiabetic mice; DM, diabetic mice; HIGT, hepatic insulin gene therapy.
*P < 0.05 versus control; **P < 0.01 versus DM, by t-test after one-way analysis of variance with P < 0.05.
Figure 2.

Transgenic human proinsulin expression in livers of HIGT mice. (a) Human proinsulin expression in liver of HIGT mice is greater than in Con mice (n = 4, each group). (b) An agarose gel of the human proinsulin amplicon in livers of HIGT mice, and the absence of this amplicon in RT-PCR product from Con mice (n = 3). Con, control nondiabetic mice; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HIGT, hepatic insulin gene therapy.
Gut anatomy, gastric emptying, and insulin signaling
Percent gastric emptying was similar across groups (Figure 3a). Total bowel length was increased among DM mice compared to both Con and HIGT mice (Figure 3b). In contrast, bowel length was similar between HIGT and Con mice. Thirty-minute dye transit distance was similar across groups (Figure 3c). However, due to the increased intestinal length, percent intestinal transit among DM mice was lower than either Con or HIGT mice (Figure 3d).
Figure 3.
HIGT effects on diabetes-induced alterations in gut function and anatomy. (a) Gastric emptying, (b) total bowel length, (c) intestinal dye transit distance, and (d) percent intestinal dye transit for control, diabetic, and HIGT-treated mice. Data are presented as mean ± SEM of n = 5–19 for each value. *P < 0.05; **P < 0.01. Con, control nondiabetic mice; DM, diabetic mice; HIGT, hepatic insulin gene therapy.
We assessed insulin signaling by western blot and phosphoprotein assay of insulin receptor (IR), AKT, and GSK3 in subject mice. Tissue content of insulin receptor (IR)β was similar across groups (Figure 4a,b). However, phosphorylated IRβ was diminished in both DM and HIGT mice (Figure 4c). Despite the reduction in IRβ phosphorylation, the amount of phosphorylated (p-) AKT in ilea of DM mice assessed by western blot tended to be elevated compared to Con, but similar in HIGT and Con (Figure 4d,e). Analysis by phosphoprotein assay confirmed similar amounts of p-AKT in Con and HIGT ilea, and increased p-AKT in DM mice compared to both Con and HIGT (Figure 4f). We observed a similar pattern of GSK3 phosphorylation among the three groups (Figure 4g).
Figure 4.
Insulin signaling in ilea of control, DM, and HIGT-treated mice. (a,b) Total β-subunit of the insulin receptor (IRβ) was compared across groups by controlling for β-actin content. (c) Phosphorylated IRβ was assayed in a Luminex-based multiplex bead analyzer. (d,e) The amount of phosphorylated AKT was compared to total AKT based on the presented western blot or (f) assayed using assay in a Luminex-based multiplex bead analyzer. (g) The quantities of phosphorylated GSK3 α/β were measured using a Luminex-based multiplex bead analyzer. n = 3–4; *P < 0.05 versus Con; #P < 0.05 versus DM. Con, control nondiabetic mice; DM, diabetic mice; HIGT, hepatic insulin gene therapy; IR, insulin receptor.
Colonic function, neuronal assessment, and insulin signaling
We assessed colonic function by measuring isometric contraction and relaxation of proximal colonic muscle strips in response to EFS and by measuring stool frequency and water content. Colonic smooth muscle contraction was similar among Con, DM, and HIGT mice (Figure 5a). In contrast, colonic smooth muscle relaxation was impaired in DM mice and normalized by HIGT treatment (Figure 5b). Stool frequency was similar across groups (Figure 5c). However, stool water content was diminished among DM mice and corrected by HIGT treatment.
Figure 5.
Colonic function assessed by colonic muscle isometry and determination of stool frequency and water content. (a) Representative isometry curve and graph of summed data from colonic muscle segments pretreated with NG-nitro-l-arginine methyl ester to inhibit nitric oxide–induced relaxation revealed no difference in contraction following electrical field stimulation (EFS). n = 4–9 (b) Representative isometry curve and graph of summed data from colonic muscle segments following precontraction 5-hydroxytryptamine (5-HT). EFS induces less relaxation in colonic muscle from DM than from Con. n = 8–10. (c) Stool frequency was similar across groups. Stool water content in DM animals was diminished compared to controls. *P < 0.05 versus Con, n = 6–25. Con, control nondiabetic mice; DM, diabetic mice; HIGT, hepatic insulin gene therapy.
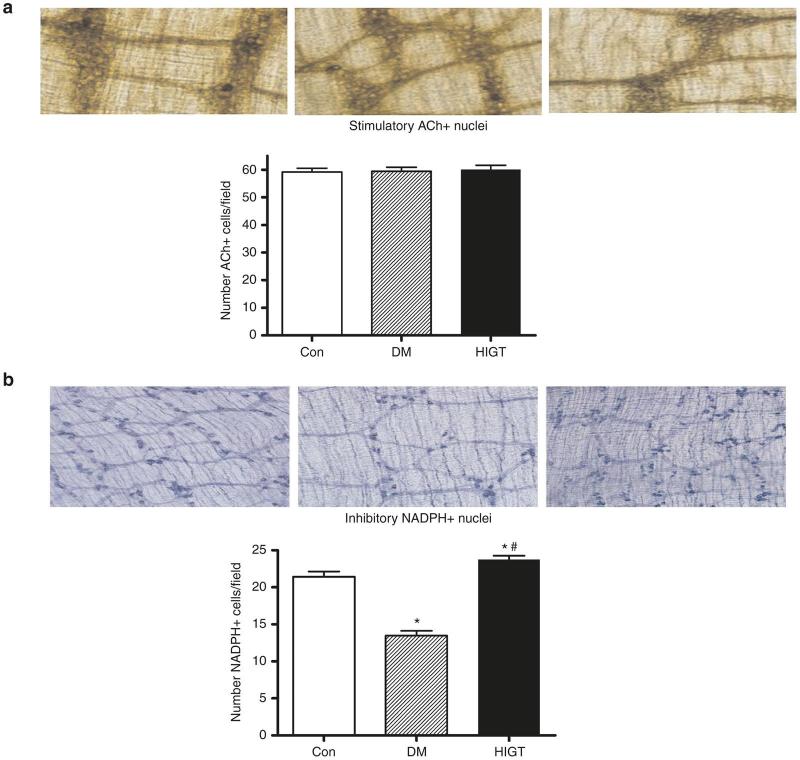
Sustained EFS-induced colonic smooth muscle contraction combined with impaired relaxation suggested a selective impairment of inhibitory neurons with relative sparing of stimulatory neurons. We observed no difference in the number of nuclei staining for ACh, i.e., stimulatory neurons within the colon (Figure 6a). In contrast, the number of NADPH diaphorase staining nuclei, i.e., inhibitory neurons, was diminished among DM mice (Figure 6b). This deficit in inhibitory neurons was prevented by HIGT treatment.
Figure 6.
Abundance of stimulatory (ACh) and inhibitory (NADPH oxidase) staining nuclei were assessed in colonic segments from control, DM, and HIGT-treated mice. (a) There was no difference in the number of nuclei of stimulatory (ACh) neurons across conditions. (b) The number of inhibitory (NADPH oxidase) neurons was diminished among DM animals, and this diminution was abrogated among HIGT treated animals. *P < 0.05 versus Con; #P < 0.05 versus DM, n = 6–10. Con, control nondiabetic mice; DM, diabetic mice; HIGT, hepatic insulin gene therapy.
Colonic abundance of IRβ was similar across groups (Figure 7a,b). Similar to the ileum, phosphorylation of IRβ was reduced in both DM and HIGT (Figure 7c). Western blotting indicated a profound reduction in p-AKT in the colons of DM and HIGT compared to Con (Figure 7d,e). While phosphoprotein assay revealed a tendency toward a reduction in p-AKT among HIGT, it failed to confirm any reduction in DM (Figure 7f). The pattern of phosphorylated GSK3 across groups was similar to p-AKT (Figure 7g).
Figure 7.
Insulin signaling in proximal colon of control, DM, and HIGT-treated mice. (a,b) Total β-subunit of the insulin receptor (IRβ) was compared across groups by controlling for β-actin content. (c) Phosphorylated IRβ was assayed in a Luminex-based multiplex bead analyzer. (d,e) The amount of phosphorylated AKT was compared to total AKT based on the presented western blot or (f) measured using assay in a Luminex-based multiplex bead analyzer. (g) The quantities of phosphorylated GSK3 α/β were measured using a Luminex-based multiplex bead analyzer. Con, control; DM, diabetic mice; HIGT, hepatic insulin gene therapy.
Discussion
We have demonstrated that treatment of diabetic mice with a metabolically responsive insulin transgene prevents the development of abnormal gastrointestinal anatomy, colonic loss of inhibitory neurons, and altered colonic muscle function, while normalizing blood sugars, body weights, and growth. These data demonstrate the efficacy of HIGT to inhibit the development of diabetic enteropathy.
Enteropathy associated with diabetes mellitus presents in up to 75% of patients and encompasses symptoms of nausea, bloating, abdominal pain, diarrhea, or constipation.5 This diversity of symptoms suggests that multiple processes are impacted by diabetes. However, accumulating evidence indicates that impairment of the ENS may be a common mediator of gastrointestinal dysfunction in diabetes.2,4,5,16 The ENS autonomously controls and coordinates the motility, blood flow, and secretion of the gastrointestinal system and displays rapid dysfunction when exposed to hyperglycemia associated with diabetes.2,4,5,16 The ENS network of sensory, motor, and interneurons, connected via the myenteric and submucosal plexuses, inervates the entire GI system, responding to luminal content and smooth muscle tension.2,5 Animal studies suggest variable susceptibility to diabetes-associated neuronal impairment and damage depending on the duration of hyperglycemia, the type of neuron, and anatomic location.2,5 However, in general, inhibitory, nitrergic neurons appear to be more susceptible to damage imposed by diabetes than stimulatory, cholinergic neurons.2,5
In this study, insulin transgene delivery by adeno-associated virus (AAV) restored average random blood sugars to normal in STZ-diabetic mice. Similar to delivery with adenovirus, delivery by AAV induces insulin expression in the liver (Figure 2; Thulé et al. manuscript submitted)10,12 but fails to fully normalize blood glucose variance.15 Serum insulin concentrations appeared to normalize in HIGT-treated mice. However, the modified human insulin produced by our transgene exhibits a concentration-dependent enhancement of cross-reactivity with the mouse insulin assay utilized in these studies (Thulé, data not shown). In addition, the transgene product in HIGT mice is poorly assessed by human insulin–specific ELISA, as indicated by the similar concentrations observed in HIGT, DM, and Con animals. Consequently, the actual concentration of circulating human insulin in the HIGT-treated mice remains unclear. Irrespective of the measured insulin concentration, HIGT treatment successfully normalized average blood sugars and body weight, as well as circulating glucagon and adiponectin levels. In contrast to HIGT mice treated with adenovirus, HIGT mice treated with AAV failed to exhibit increased serum resistin levels, suggesting that the adenoviral vector, and not the insulin transgene, were responsible for previously documented elevations.12 Interestingly, circulating leptin levels in HIGT treated mice increased compared to DM, but remained less than Con, a pattern previously observed in HIGT treated diabetic rats.15 Circulating leptin is produced by adipose tissue.17 It is thus noteworthy that epididymal fat pads were smaller in HIGT-treated mice than in controls, despite similar body weights, confirming another finding in HIGT-treated rats.14 HIGT-treated rats produce more heat during both feeding and fasting than control animals, possibly due to greater expression of uncoupling protein-3 in muscle.14 This phenomenon remains to be confirmed in HIGT-treated mice.
The etiology of diabetic gastrointestinal complications involves multiple factors.2,5 However, damage to the ENS is likely involved in both human subjects with diabetes mellitus2,3,5,18 and in diabetic animal models.6,19,20 Anitha et al. demonstrated delayed gastric emptying, aberrant colonic muscle relaxation, and diminished inhibitory colonic neurons secondary to enteric neuronal apoptosis in STZ-diabetic mice.6,19,20 Our findings of intact gastric emptying among DM may reflect strain differences between B6/CBA, and C57 in-bred mice, both of which exhibit prolonged gastric emptying following exposure to hyperglycemia, and the out-bred CD-1mice used in these studies.6,19–21 Consistent with multiple prior studies, our DM mice exhibited impaired colonic muscle relaxation and diminished numbers of inhibitory colonic neurons in the myenteric plexus.2,5
While our study suggests that controlling glycemia is sufficient to inihibit diabetes associated gastrointestinal dysfunction, we did not evaluate other potentially influencial factors. Alterations and interactions between intestinal permeability, microbiome, and the immune system may impact gut function, either directly or indirectly through effects on the ENS. An altered intesitinal microbiome clearly impacts disease incidence among rodent models of autoimmune diabetes, and alterations in gut bacteria appear to preceed development of type 1 diabetes in children.22–24 Moreover, intestinal permeability to gut flora, and the intestinal immune response, are abnormal in subjects with type 1 diabetes, raising the possibility that subsequent intestinal inflammation may underly gastrointestinal dysfunction.23 Similarly, it remains possible that STZ treatment may have produced gastrointestinal dysfunction that was exacerbated by hyperglycemia. We were unable to identify literature reporting a direct effect of STZ on either enteric neurons or gastrointestinal function. However, a study similar to ours in non–STZ-mediated model of diabetes would be required to exclude an STZ effect.
The DM mice in this study also exhibited bowel elongation and a consequent percent delay in bowel transit. Because we only measured total bowel lengths, we cannot determine if the large or small bowel was elongated, or whether both segments were affected. Mayhew and Carson demonstrated that 12 weeks of hyperglycemia is sufficient to induce small bowel hyperplasia, increasing circumference, crypt, and villus height, as well as length in STZ-diabetic Sprague Dawley rats.25 The increase in small bowel length (from 114 to 121 cm) failed to reach statistical significance, but their diabetic rats were severely catabolic, losing 1.8 gm body weight per day.25 In contrast, Domènech et al.26 observed significant elongation in both the small intestine and colon of diabetic mice. However, the specific drivers of bowel elongation remain poorly defined.
Interestingly, oral insulin reduces the diabetes-induced mucosal hypertrophy observed in STZ-treated rats,27 raising the possibility that hepatic insulin may be secreted into the bile of HIGT-treated mice and restrict bowel growth. However, this remains speculation.
Similar to Anitha et al.,6 we observed a decline in colonic inhibitory neurons associated with impaired relaxation of colonic muscle in DM mice, and both of these findings were prevented by HIGT treatment, consistent with normalization of blood sugars. These results stand in contrast to those of Domènech et al.,26 who failed to detect differential effects of hyperglycemia on nNOS or ACh staining neurons in vivo but documented a reduction in total myenteric neurons. In their study, Anitha et al.6 demonstrated that hyperglycemia-induced apoptosis was associated with diminished phosphorylation of PI3K and AKT, an effect that was inhibited both in cell culture and in vivo by glial derived neurotrophic factor. Interestingly, insulin signaling was not universally restored in gut tissue of HIGT-treated mice. Indeed, in ilea and colons, phosphorylation of IRβ was reduced compared to Con, and in colons p-AKT was diminished, consistent with diminished signaling through PI3K. None the less, phosphorylation of AKT and GSK3 were normalized in ilea of HIGT animals, and function was preserved in the ilea and colons of HIGT mice. However, it should be noted that the current study assessed insulin signaling in full-thickness ileum and colon samples, rather than the isolated enteric neurons examined by Anitha et al.6 Furthermore, increased amounts of p-AKT in whole-thickness ileum DM samples suggest increased activity of non–IR-mediated AKT activation, raising the potential for a combination of insulin and non–IR-mediated events in HIGT samples.28 Consequently, it remains possible that HIGT differentially affects insulin signaling within myenteric neurons, or induces local secretion of alternative neurotropic factors, such as glial derived neurotrophic factor, that counter the effects of diminished insulin signaling and excessive non–insulin-mediated signaling. Alternatively, preservation of enteric neurons and bowel function may reflect the glycemic control afforded by HIGT. Whether the aberrant intestinal insulin signaling observed in HIGT animals remains sufficient to preserve function over time periods longer than the current study will have to be investigated.
The capacity of metabolically responsive HIGT to effectively control glycemia has been established in multiple rodent models and a large animal model of diabetes mellitus.10–13,29 However, evidence that HIGT can effectively prevent morbidities associated with diabetes mellitus is sparse. Utilizing impaired endothelium-mediated aortic ring contractility as a surrogate marker for hyperglycemia associated vasculopathy, Thulé et al.15 demonstrated that HIGT can prevent diabetes-induced large vessel disease. In addition, Chen et al.29 were able to prevent the development of diabetic nephropathy and ocular cataract formation in pigs following hepatic insulin transgene production. The current study expands the efficacy of HIGT to prevent diabetic complications to the intestinal tract.
In summary, we successfully established a model of diabetic enteric neuropathy using STZ in CD-1 mice with autonomic, functional, and molecular changes. We also demonstrated that HIGT can prevent autonomic, functional, and molecular aspects of diabetic enteric neuropathy aside from normalization of blood glucose, weight growth, and hormone level with single injection of AAV2/8 SC G3 2xfur. Compared to other therapies, HIGT still has some advantage for treating STZ-induced diabetic mice. However, questions remain for hepatic insulin gene therapy. First of all, the precise mechanism of how HIGT can prevent enteric neuropathy remains unclear. Second, whether HIGT can reverse diabetic enteric neuropathy is still unknown. Last but not least, whether HIGT can prevent diabetic enteric neuropathy in large animals or human requires further study.
Materials and Methods
Animals
Male CD-1 virus-antigen free (VAF plus) mice (Charles River Laboratories, Wilmington, MA) were group-housed in an AAALAC-accredited animal care facility under a 12:12 light–dark cycle. Water and chow was provided ad libitum. Mice in the DM and HIGT groups received i.v. streptozotocin (STZ, 200 mg/kg; Sigma, St Louis, MO) dissolved in 0.05 mol/l citrate buffer (pH 4.5) via penile vein injection. Control mice received injections of citrate buffer alone. Blood sugar and body weight was monitored on tail-tip venous plexus blood every 1 to 3 days using a hand-held blood glucose monitor (Freestyle; Abbott, Abbott Park, IL). Mice were diagnosed with diabetes mellitus upon two consecutive random daily blood glucose determinations greater than 200 mg/dl. In response to two consistent findings of body weight decline, STZ-treated mice received a single s.c. injection of glargine insulin (usually 0.2 U) calculated to prevent further weight loss, but not reduce blood sugars. All procedures were approved by the VA/Emory University Institutional Animal Care and Use Committee.
Virus production and administration
Pseudotyped AAV8 virus was produced by first cloning coupled promoter and expression sequences, CMV GFP or (GlRE3)BP-1 2xfur, into plasmids between adeno-associated virus, serotype 2, inverted terminal repeats, of which the terminal resolution sequence of the 5′ inverted terminal repeats had been excised, permitting viral packaging of self-complementary, double-stranded viral genomes, to create pSC-CMV GFP and pSC-(GlRE3)BP-1 2xfur.30 Triple cotransfection of 120 μg total DNA containing equimolar ratios of pSC-CMV GFP or pSC-(GlRE3)BP-1 2xfur, pAAV2rep-AAV8cap (graciously provided by Dr James Wilson, University of Pennsylvania, Center for Gene Therapy), and pDF (a gift from Roland Herzog and Weidong Xiao, The Children’s Hospital of Philadelphia, Philadelphia, PA), which expresses adenoviral helper-proteins, using calcium phosphate (Profection Transfection Kit, Promega) into subconfluent HEK 293 cells (ATTC, Manassas, VA) cultured on 150 mm dishes in Dulbecco's Modified Eagle Medium supplemented with 10% fetal calf serum provided all essential packaging and helper functions necessary for the generation of rAAV8 pseudotyped virus particles. Medium was changed the following day, and cells harvested 3 days after transfection. Recombinant AAV8 particles were purified by two rounds of CsCl density gradient centrifugation, as previously described.31 Concentration of viral genomes was determined lysing viral particles in sodium dodecyl sulfate and quantifying the number of genomes by real-time PCR. The (GlRE3)BP1 2xfur insulin transgene used to construct pSC (GlRE3)BP1 2xfur consisted of a glucose and insulin responsive, liver-specific promoter and a human proinsulin gene modified to permit posttranslational furin processing (2xFur, gift of Genentech, South San Francisco, CA). Construction of the (GlRE3)BP-1 promoter has been previously described.10,32 The pSC-CMV GFP was constructed by removing the CMV promoter and enhanced GFP fragments from pIRES-EGFP (Clontech Laboratories, Mountain View, CA).
The evening prior to surgery, STZ-treated mice received a s.c. injection of glargine insulin (0.5 U) to prevent volume depletion. All mice received hepatic portal system venous injections of AAV following induction of O2/isoflurane inhalation anesthesia. Following midline ventral laparotomy, using a surgical microscope, virus was delivered into a mesenteric vein via a hand drawn polyethylene catheter (PE-50 tubing; Instech Laboratories, Plymouth Meeting, PA) attached to 1 cc syringe in a uniform volume (800 μl) of normal saline. HIGT mice received AAV2/8-SC-(GlRE3) BP-1 2xfur (2.2 × 1010 vg) by mesenteric vein injection after developing hyperglycemia. Control and DM mice received mesenteric vein injections of AAV2/8-SC-CMV GFP at a similar dose, unless otherwise indicated. All mice were euthanized 50 days after virus administration.
Liver real-time RT-PCR
Total RNA was extracted from tissues flash frozen in liquid nitrogen and stored at −70 °C using TRI reagent (Ambion, TX, USA) or Trizol (Gibco BRL, Gaithersburg, MD) and treated with DNA-Free (Ambion) per manufacturer’s instructions. After quantification of RNA by spectrophotometry, cDNA was reverse transcribed from 1.0 µg of total RNA in a 20-µl reaction using an oligo-dT17 primer, recombinant RNAsin, and Moloney-Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) (all from Promega, Madison, WI). M-MLV RT was inactivated at 95 °C. Quantitative real-time PCR was performed on a MyiQ Single Color Real-Time PCR Detection System using iQ SYBR Green Supermix (Bio-Rad, CA). A 20 µl reaction mix containing 3 μl of cDNA (at 1:5 dilution) was exposed to 30 thermal cycles. All samples were run in triplicates. The relative amount of individual cDNAs was determined by comparison to a standard curve and normalized to a house-keeping gene (GAPDH). PCR primers used were: human proinsulin (fwd) GCAGCCTTTGTGAACCAACAC and (rev) CCCCGCACACTAGGTAGAGA and glyceraldehydephosphate dehydrogenase (GAPDH) (fwd) GACCACAGTCCATGCCATCAC and (rev) GACCTTGCCCACAGCCTTG. Insulin expression normalized to GAPDH expression was compared using the ΔΔCt method.33
Gastric emptying and bowel length measurement
At sacrifice, 5 or 30 minutes after oral gavage of 0.1 ml methylene blue labeled 10% dextrose solution, the entire gut from distal esophagous to anus was excised en bloc. Stomach contents were confined by ligating the pylorus and the lower esophageal sphincter. The distance from the pylorus to the most distal migration of dye was measured in centimeter, and transit expressed as a percentage of total intestinal length (pylorus to anus). The stomach was frozen at −70 °C until analysis of gastric emptying. Gastric emptying was determined as described,6 by homogenizing minced stomachs in 15 ml of 0.1 N sodium hydroxide (NaOH) for 30 seconds, incubating 1 hour at room temperature with an additional 5 ml of 0.1 N NaOH. Five microliters of this suspension was centrifuged for 20 minutes at 1,250g at 4 °C, and absorbance at 570 nm of the supernatant determined. Absorbance at 5-minutes served as a reference. The percent gastric emptying was calculated by the following formula: ((A570 reference − A570 sample)/A570 reference) × 100. Serum obtained from retro-orbital blood sampled from mice before and 30 minutes after gavage confirmed absence of intestinal methylene blue absorption (data not shown).
Stool frequency and water content
Mice placed individually in a clean cage were observed throughout a 60-minute collection period. Fecal pellets were collected upon expulsion, counted, and placed into sealed, preweighed 1.5 ml tube. Tubes were weighed to obtain the wet weight of the stool, which was then dried overnight at 65 °C and reweighed to obtain the dry weight. Stool water content was calculated from the difference between the wet and dry stool weights.
Colonic segment isometric muscle recordings
Longitudinal strips of proximal colons of Control, DM, and HIGT mice suspended on hooks linked to an isometric force transducer were placed between platinum electrodes in 15 ml chambers containing 37 °C Krebs buffer (NaCl, 118 mmol/l; KCl, 4.7 mmol/l; KH2PO4, 1.2 mmol/l; MgCl2, 1.2 mmol/l; NaHCO3, 23 mmol/l; EDTA, 0.3 mmol/l; glucose, 10 mmol/l; and CaCl2, 2.5 mmol/l; pH 7.4) continuously gassed with 95% O2 and 5% CO2. After a 1-hour equilibration at 4.9 mN, tension was continuously recorded using the PowerLab recording software (version 5.2.1; AD Instruments), as relaxation was induced by transmural electrical field stimulation (EFS) (24 V, 4 Hz, repeating 0.03 millisecond pulses for a duration of 20 seconds) in the presence of atropine (1 μmol/l) and guanethidine sulfate (1 μmol/l) to block cholinergic- and adrenergic-mediated responses, respectively. Strips were precontracted with 5-hydroxytryptamine (10 μmol/l) for 30 seconds prior to EFS. The first induced relaxation was used for quantitation of each sample. Relaxation was abolished by coincubation with tetrodotoxin (10–7 mol/l), indicating mediation by a neuronal pathway. Constraction was measured by applying EFS 20 minutes after incubation with NG-nitro-l-arginine methyl ester (100 μmol/l). Contraction or relaxation was expressed as a % change from baseline muscle tone. For quantitative determination of contraction or relaxation, intestinal strips from at least three mice were used, and the average of contractions or relaxations was obtained from EFS.
Histochemical tissue staining
At sacrifice, three 4-cm serial segments of colon, beginning at the cecum, were freed of mesentery, cut longitudinally to expose the mucosa and pinned mucosa side down onto hardended silicone gel in a glass dish. Subsequent to fixing intestinal segments 1 hour at room temperature in 4% paraformaldehyde, the mucosa and circular muscle layer were separated from the preparation, while the longitudinal muscle layer and the myenteric plexus were subjected to histologic staining. The most distal ileum was stained for peripherin, the next most distal segment for NADPH diaphorase, and the most proximal segment for acetylcholinesterase (ACh) staining. For NADPH diaphorase staining, fixed tissue was washed in PBS, incubated in diaphorase solution (β-NADPH diaphorase, 1 mg/ml; nitroblue tetrazolium, 0.1 mg/ml; and 0.3% Triton-X 100 in PBS) for 1 hour at 37 °C, rewashed in PBS, and mounted on a glass slide. For ACh staining, fixed tissue was washed in PBS, incubated in fresh copper buffer solution (100 ml dH2O, 7.2 mg ethopropazine, 115.6 mg acetylthiocholine iodide, 75.0 mg glycine, 50.0 mg copper sulfate pentahydrate, 885.0 mg sodium acetate trihydrate; pH to 5.6 with glacial acetic acid) for 2 hours, washed in dH2O, incubated for 1 minute in 1.25% sodium sulfide nonahydrate solution, re-washed in dH2O, then mounted on a glass slide. For both NADPH diaphorase– and ACh-stained tissues, nuclei of myenteric neuronal fibers were manually counted within 20 randomly selected squares of a 0.1 mm2 microscope grid. Samples from 6–10 mice for each experimental condition were assessed.
Fat weight and hepatic glycogen content
Right and left epididymal fat pads and bowel mesenter were harvested at sacrifice and weighed immediately on a Mettler MS1100 analytical balance. Right and left epididymal fat pads from each animal were weighed independently, and statistics performed on the entire data set. The entire bowel mesentery from the ileum to the cecum, excluding the spleen and pancreas, was stripped manually from the excised bowel and weighed immediately. Liver samples were obtained at sacrifice from normal (Con), diabetic (DM), and HIGT-treated mice, by flash freezing in liquid N2, and stored at −70 °C until analysis. Tissue glycogen assays were performed with modification of a glycogen assay kit (Sigma) as previously described.34 Briefly, 25–50 mg of liver were boiled in 500 µl 30% KOH saturated with Na2SO4 for 60 minutes. After cooling, glycogen was precipitated by the addition of 1.5 ml 95% ethanol, was cleaved by the addition of O-amyloglucosidase, and the produced glucose quantified by colorimetric assay per the manufacturer’s instructions (Sigma). Background values of non–glycogen-derived glucose were simultaneously determined by excluding O-amyloglucosidase in parallel samples and subtracted from glycogen-derived values prior to calculating glycogen content.
Serum analysis
At sacrifice, serum was obtained by centrifugation of whole blood collected in microcentrifuge tubes containing clot activator, and frozen at −70 °C until analysis, using Milliplex multiplex assays (MADPK-71K-ADPN, MADPK-71K, MENDO-75K; EMD Millipore, Billerica, MA) run on a Luminex-100/200 device using MasterPlex QT software (MiraiBio/Hitachi Solutions America, San Bruno, CA). In addition, serum was assayed for transgenic human insulin using the Mercodia Ultrasensitive Human Insulin ELISA (Catalog nr 10-1132-01; Mercodia, Winston Salem, NC).
Western blotting and phosphoprotein assay
Mouse ileum and proximal colon homogenates of control, empty virus-treated diabetic or HIGT-treated diabetic mice were isolated using T-PER tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL) with Phosphatase Inhibitor Cocktail Set I (Calbiochem, Darmstadt, Germany). Total protein was quantitated using the Pierce BCA Protein Assay Kit (Thermo Scientific). Aliquots containing equal amounts of protein combined with prestained molecular weight markers were resolved on 12-well 1.0 mm NuPAGE Novex 4–12% Bis–Tris Gels using 1× NuPAGE MES SDS running buffer (Invitrogen, Carlsbad, CA). Proteins were transferred onto nitrocellulose membranes using the XCell blot module (Invitrogen) with 1× NuPAGE transfer buffer and probed with antibodies directed against IRβ, AKT, or phospho-AKT (Ser473) (1:1,000; Cell Signaling, Danvers, MA), or β-actin HRP (1:4,000; Genescript, Piscataway, NJ) in a solution of 5% nonfat milk, 50 mmol/l Tris–HCl pH 7.4, 150 mmol/l NaCl, and 0.1% v/v Tween-20 (TBS/T) at 4 °C during continuous, gentle rotary agitation overnight. After five 10-minute washes in TBS/T solution, membranes were incubated with 10 ml of SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) for 5 minutes. Relative levels of immunoreactive proteins were quantified using the ChemiDoc XRS imaging system and Quantity One software (Bio-Rad Laboratories).
Quantities of phospho-IRβ (Tyr 1186), phospho-AKT (Ser 473), and phospho-GSK3α/β (Ser 21/Ser 9) were assessed using Bio-Plex phosphoprotein assays on full-thickness ileum and proximal colon homogenates per manufacturer’s instructions (Bio-Rad Laboratories). Homogenates were processed from tissue samples flash frozen in liquid N2 at sacrifice and stored until use at −70 °C, utilizing a Bio-Plex cell lysis kit, treated with Bio-Plex phosphoprotein assays and detectection reagents, and read on a Luminex 100/200 analyzer using MasterPlex QT software (MiraiBio/Hitachi Solutions America, San Bruno, CA).
Statistics
Unless otherwise specified, data are presented as mean ± SEM with the utilized n. Comparisons were made following one-way analysis of variance using the statistics program resident in GraphPad Prism 4 (GraphPad Software) and Tukey’s multiple comparison test if analysis of variance delivered a P < 0.05.
Acknowledgments
This work was supported by a Research Grant, Juvenile Diabetes Research Foundation International 1-2000-401, American Diabetes Association Innovative Award, GTEC Center for Engineering of Living Tissues/NSF EEC-9731643, and a VA Merit Award to P.M.T., by NIH/NRSA DKO 7298 and a VA VISN7 CDA to D.E.O., and NIH RO1 DK080684 and a VA Merit Award to S.S.
The authors declare no conflict of interest. No author owns any intellectual property associated with this research, sits on an advisory board, or has received travel or accommodations from entities involved with this research. No author, or family member, has financial investments with commercial entities involved with this research.
References
- Services, U.D.o.H.a.H., Centers for Disease Control and Prevention. 2014National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States 2014 Centers for Disease Control and Prevention; Atlanta, Georgia. [Google Scholar]
- Ordög T, Hayashi Y, Gibbons SJ. Cellular pathogenesis of diabetic gastroenteropathy. Minerva Gastroenterol Dietol. 2009;55:315–343. [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–8, e26. doi: 10.1111/j.1365-2982.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin JH, Chang EB. Therapy insight: gastrointestinal complications of diabetes–pathophysiology and management. Nat Clin Pract Gastroenterol Hepatol. 2008;5:162–171. doi: 10.1038/ncpgasthep1054. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951–960. doi: 10.1111/j.1365-2982.2007.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA. 2014;311:2315–2325. doi: 10.1001/jama.2014.5951. [DOI] [PubMed] [Google Scholar]
- Thulé PM, Liu JM. Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Ther. 2000;7:1744–1752. doi: 10.1038/sj.gt.3301297. [DOI] [PubMed] [Google Scholar]
- Dong H, Altomonte J, Morral N, Meseck M, Thung SN, Woo SL. Basal insulin gene expression significantly improves conventional insulin therapy in type 1 diabetic rats. Diabetes. 2002;51:130–138. doi: 10.2337/diabetes.51.1.130. [DOI] [PubMed] [Google Scholar]
- Zhang JA, Jia D, Olson DE, Campbell AG, Thulé PM. Hepatic insulin gene therapy diminishes liver glycogen despite insulin responsive transcriptional effects in diabetic CD-1 mice. J Gene Med. 2009;11:588–597. doi: 10.1002/jgm.1341. [DOI] [PubMed] [Google Scholar]
- Olson DE, Paveglio SA, Huey PU, Porter MH, Thulé PM. Glucose-responsive hepatic insulin gene therapy of spontaneously diabetic BB/Wor rats. Hum Gene Ther. 2003;14:1401–1413. doi: 10.1089/104303403769211628. [DOI] [PubMed] [Google Scholar]
- Olson DE, Campbell AG, Porter MH, Freeman KG, Kelso E, Flatt WP. Hepatic insulin gene therapy normalizes diurnal fluctuation of oxidative metabolism in diabetic BB/Wor rats. Mol Ther. 2008;16:1235–1242. doi: 10.1038/mt.2008.97. [DOI] [PubMed] [Google Scholar]
- Thulé PM, Campbell AG, Kleinhenz DJ, Olson DE, Boutwell JJ, Sutliff RL. Hepatic insulin gene therapy prevents deterioration of vascular function and improves adipocytokine profile in STZ-diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E114–E122. doi: 10.1152/ajpendo.00134.2005. [DOI] [PubMed] [Google Scholar]
- Phillips LK, Rayner CK, Jones KL, Horowitz M. An update on autonomic neuropathy affecting the gastrointestinal tract. Curr Diab Rep. 2006;6:417–423. doi: 10.1007/s11892-006-0073-0. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent Prog Horm Res. 2004;59:225–244. doi: 10.1210/rp.59.1.225. [DOI] [PubMed] [Google Scholar]
- He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- Fregonesi CE, Miranda-Neto MH, Molinari SL, Zanoni JN. Quantitative study of the myenteric plexus of the stomach of rats with streptozotocin-induced diabetes. Arq Neuropsiquiatr. 2001;59:50–53. doi: 10.1590/s0004-282x2001000100011. [DOI] [PubMed] [Google Scholar]
- Furlan M, Molinari S, de Miranda Neto M. Morphoquantitative effects of acute diabetes on the myenteric neurons of the proximal colon of adult rats. Arq Neuropsiquiatr. 2002;60:576–581. doi: 10.1590/s0004-282x2002000400012. [DOI] [PubMed] [Google Scholar]
- Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–384. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS ONE. 2011;6:e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Carson FL. Mechanisms of adaptation in rat small intestine: regional differences in quantitative morphology during normal growth and experimental hypertrophy. J Anat. 1989;164:189–200. [PMC free article] [PubMed] [Google Scholar]
- Domènech A, Pasquinelli G, De Giorgio R, Gori A, Bosch F, Pumarola M. Morphofunctional changes underlying intestinal dysmotility in diabetic RIP-I/hIFNß transgenic mice. Int J Exp Pathol. 2011;92:400–412. doi: 10.1111/j.1365-2613.2011.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhotnik I, Shamir R, Bashenko Y, Mogilner JG, Chemodanov E, Shaoul R. Effect of oral insulin on diabetes-induced intestinal mucosal growth in rats. Dig Dis Sci. 2011;56:2566–2574. doi: 10.1007/s10620-011-1654-6. [DOI] [PubMed] [Google Scholar]
- Mahajan K, Mahajan NP. PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. J Cell Physiol. 2012;227:3178–3184. doi: 10.1002/jcp.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NK, Wong JS, Kee IH, Lai SH, Thng CH, Ng WH. Nonvirally modified autologous primary hepatocytes correct diabetes and prevent target organ injury in a large preclinical model. PLoS ONE. 2008;3:e1734. doi: 10.1371/journal.pone.0001734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- Haberman R, Kroner-Lux G, Samulski R.1999. Production of adeno-associated viral vectors. In: Dracopoli, N, Current Protocols in Human Genetics John Wiley & Sons; New York. pp. 12.9.1–12.9.16. [Google Scholar]
- Thulé P, Liu JM, Phillips L. Glucose regulated production of human insulin in rat heptocytes. Gene Ther. 2000;7:205–214. doi: 10.1038/sj.gt.3301076. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- van Dijk TH, van der Sluijs FH, Wiegman CH, Baller JF, Gustafson LA, Burger HJ. Acute inhibition of hepatic glucose-6-phosphatase does not affect gluconeogenesis but directs gluconeogenic flux toward glycogen in fasted rats. A pharmacological study with the chlorogenic acid derivative S4048. J Biol Chem. 2001;276:25727–25735. doi: 10.1074/jbc.M101223200. [DOI] [PubMed] [Google Scholar]