Abstract:
Cardiac arrest by cardioplegia provides a reproducible and safe method to induce and maintain electromechanical cardiac quiescence. Techniques of intraoperative myocardial protection are constantly evolving. For the past three decades, modified Buckberg cardioplegia solution has been used for adult cardiac surgery at the Cleveland Clinic. This formulation serves as the crystalloid component, which is delivered 4:1 with oxygenated patient’s blood to crystalloid. Meanwhile, our use of the del Nido cardioplegia solution in adult patients, heretofore primarily used in pediatric cardiac surgical centers, has been increasing over the past several years. Single-dose, cold blood del Nido cardioplegia can be delivered antegrade if the duration of the operation will be limited and if there is no significant coronary artery disease or aortic insufficiency that would limit the distribution of cardioplegia. The addition of del Nido cardioplegia to our cardioplegia armamentarium allows us to customize our myocardial protection strategies for different surgical needs. This article aims to provide information on technical aspects of del Nido cardioplegia in adult cardiac surgery and its use at the Cleveland Clinic in the adult surgical population.
Keywords: myocardial protection and cardioplegia, del Nido solution
Most adult cardiac surgeries are performed on the arrested heart. Cardiac arrest with cardioplegia provides a reproducible and safe method to induce and maintain electromechanical cardiac quiescence. The flaccid, noncontracting heart decreases the possibility of air embolism during open procedures performed on the left side of the heart and provides a still surgical field. Aortic cross-clamping eliminates continuous coronary blood flow to the myocardium thus providing a bloodless field, enhancing visibility. Cardioplegic arrest results in a significant reduction of energy consumption by the myocardial cells, even at normothermia (1–3). Under beating-heart circumstances, the myocardium requires a constant supply of oxygen to the myocardium to meet its high-energy consumption demands. Myocardial ischemia occurs when the supply of oxygen is exceeded by the demand and can lead to myocardial cell death (infarction) or injury, which can be exacerbated on reperfusion. The mechanisms of ischemia and reperfusion injury are complex. It is important to have an understanding of the components that contribute to this damage and to develop strategies to ameliorate these factors (2–5).
Although del Nido cardioplegia has been in use for decades in pediatric surgical centers, its use in adult surgical centers is a relatively new phenomenon. For instance, the team at New York Presbyterian Hospital (Columbia campus, New York, NY) has been using this type of cardioplegia exclusively in their adult cases for the past few years. Single-dose, cold blood del Nido cardioplegia can be delivered antegrade. Given the ease of its administration and its longer redosing interval, there is increasing interest in the adult cardiac community in the use of del Nido cardioplegia (6–8). Currently, there is no prospective data on its use in adults and few reports retrospective data have been available on this topic. In animal models, there is evidence that del Nido solution may provide superior myocardial protection in aged hearts (10,11). However, use of del Nido has primarily been limited to the pediatric arena. We became interested in its use in adult patients and began using it in this arena in 2012 and recently published our experience thus far in adult isolated valve operations (12). This article aims to provide information on the technical aspects of del Nido cardioplegia in adult cardiac surgery at the Cleveland Clinic from our experience thus far.
DESCRIPTION
Cleveland Clinic Standard Setup
The standard circuit at our institution consists of a Sarns™ centrifugal pump (Terumo Cardiovascular Systems, Ann Arbor, MI) or a Revolution™ centrifugal pump (Sorin Group USA, Arvada, CO), a custom adult tubing pack with X-coating (Terumo Cardiovascular Systems Ann Arbor, MI), a CAPIOX FX25 oxygenator with hardshell reservoir (Terumo Cardiovascular Systems), and a Pall Leukoguard-6 leukocyte filter (Pall Biomedical, Portsmouth, UK). Stockert S5 roller pumps were used (Stockert, part of Sorin Group Deutschland GmbH, Munchen, Germany) for the cardioplegia, aortic vent suction, pump suction, and basket suction. A Sorin 3T heater-cooler (Sorin Group USA) is used for temperature control of the patient, cardioplegia, and heating–cooling blanket. The circuit is primed with 2000 mL of Plasma-Lyte A, 50mEq of NaHCO3, 10,000 international unit (IU) of heparin. Excess volume is pumped up to the empty Plasma-Lyte A bag. Our minimum operating priming volume is 719 mL. Fifty grams of mannitol is administered after initiation of cardiopulmonary bypass (CPB).
The Auto Log (Medtronic, Minneapolis, MN) is used for all open heart surgery cases. The retrograde autologous priming technique is used to reduce the amount of priming volume and the pump flows on bypass are maintained at a 2.0–2.4 L/mim/m2 based on body surface area of the patient. Mean arterial pressures are maintained greater than 65 mmHg. The sweep gas flow is set to maintain an arterial oxygen tension >150 mmHg and an arterial carbon dioxide tension of 35–45 mmHg. Anticoagulation is established with an initial dose of 400 IU per kilogram of body weight. Heparin is injected into the central venous line before the initiation of CPB with a target activated clotting time of over 480 seconds. At the end of CPB, heparin is reversed by protamine sulfate at a 1:1 ratio of the loading dose.
Modified Buckberg Cardioplegia for Adults at the Cleveland Clinic
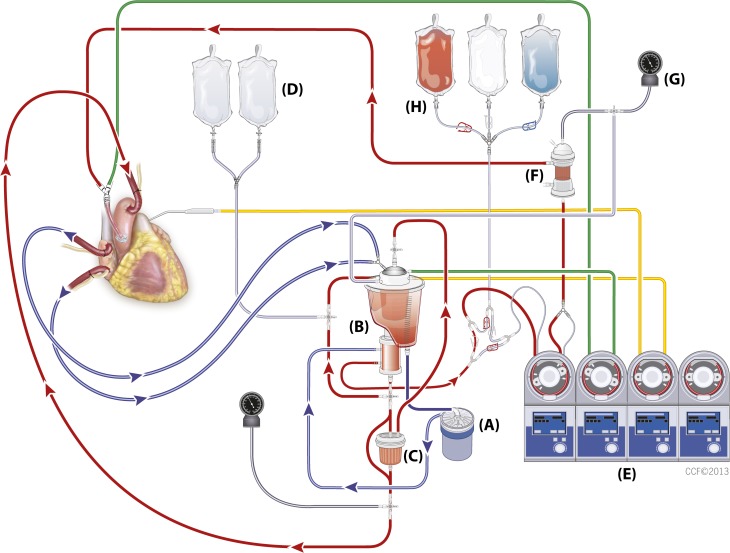
Modified Buckberg cardioplegia is a dextrose-based solution in normal saline with potassium chloride as the depolarizing agent, tromethamine as the buffer, and citrate phosphate double dextrose as a calcium chelator and delivered 4:1 oxygenated patient’s blood to crystalloid. The Buckberg cardioplegia solution is administered as three solutions: induction, maintenance, and reperfusion. During cardiac surgical cases, a dose of induction solution is given first to arrest the heart and then every 15–20 minutes during the course of the operation, the maintenance solution is delivered to maintain cardiac arrest and to replenish oxygen and nutrients to the myocytes. The induction and maintenance dose are delivered at 4°C. The reperfusion “hot shot” is delivered at 37°C just before removal of the aortic cross-clamp. The reperfusion solution contains glutamate and aspartate, which are sugar substrates to provide the heart with nutrients right before it is expected to start beating again (1,2,12). Figure 1 shows the Cleveland Clinic custom circuit for the modified Buckberg cardioplegia delivery.
Figure 1.
Cleveland Clinic dual cardioplegia circuit for the Buckberg cardioplegia delivery. (A) Centrifugal pump, (B) reservoir and oxygenator, (C) Leukocyte filter, (D) Plasma-Lyte® A solution, (E) heart–lung machine, (F) cardioplegia heat exchanger, (G) manometer for the cardioplegia system, (H) modified Buckberg solution: induction, maintenance, and reperfusion.
del Nido Cardioplegia for Adults at the Cleveland Clinic
A single dose of del Nido cardioplegia contains 26 mEq/L of potassium chloride, 13 mL of 1% lidocaine, 3.2 g/L of 20% mannitol, 2 g of 50% magnesium sulfate, 13 mEq/L of sodium bicarbonate, and 1000 mL of Plasma-Lyte A. It is delivered 1:4 with oxygenated patient’s blood to crystalloid (8–10). del Nido cardioplegia can be delivered antegrade if the duration of the operation will be limited and if there is no significant coronary artery disease or aortic insufficiency to limit the distribution of cardioplegia. It is generally used in a single-dose fashion and has been in use for nearly two decades at Children's Hospital Boston for both adult and pediatric surgeries (8). Its patent has expired and the del Nido cardioplegia can be prepared by any in-house pharmacy. Our experience with its use in adult patients at the Cleveland Clinic began in 2012. Table 1 shows a comparison between modified Buckberg cardioplegia solution (CPS) and del Nido CPS (8,13).
Table 1.
Comparison between the modified Buckberg cardioplegia solution (CPS) and del Nido CPS.1*
| Modified Buckberg CPS | del Nido CPS | ||
|---|---|---|---|
| Base solution | Induction | D5 ¼ NS 392 mL | Plasma-Lyte A 1000 mL |
| Maintenance | D5 ¼ NS 798 mL | ||
| Reperfusion | Sterile water 235mL | ||
| Total volume (approximate) | Induction | 500 mL | 1100 mL |
| Maintenance | 1000 mL | ||
| Reperfusion | 500 mL | ||
| KCL | Induction | 36 mEq/500 mL | 26 mEq |
| Maintenance | 36 mEq/L | ||
| Reperfusion | 15 mEq/500 mL | ||
| Tromethamine .3 M | Induction | 60 mL/500 mL | None |
| Maintenance | 123 mL/L | ||
| Reperfusion | 56 mL/500 mL | ||
| C-P-2-D | Induction | 30 mL/500 | None |
| Maintenance | 61 mL/L | ||
| Reperfusion | 113 mL/L | ||
| NaHCO3 | None | 13 mEq | |
| Mannitol 20% | None | 16 mL | |
| Magnesium sulfate 50% | None | 4 mL | |
| Lidocaine 2% (preservative-free) | None | 6.5 mL | |
| Additional | Dextrose 70% | 26 mL/500 mL | N/A |
| Additives in reperfusion CPS | Glutamate/aspartate | 62.5 mL/500 mL | |
| Blood:crystalloid | 4:1 | 1:4 | |
| Delivery methods | Antegrade | Antegrade (primarily) | |
| Retrograde | Coronary ostia | ||
| Coronary ostia | Retrograde | ||
| Storage | Refrigeration | Refrigeration | |
Source: Modified Buckberg cardioplegia compounded at Central Admixture Pharmacy Services (CAPS); del Nido compounded in Cleveland Clinic Foundation Pharmacy. Both CPS bags are latex-free.
N/A, not applicable.
Dual Cardioplegia Circuit and Delivery Methods
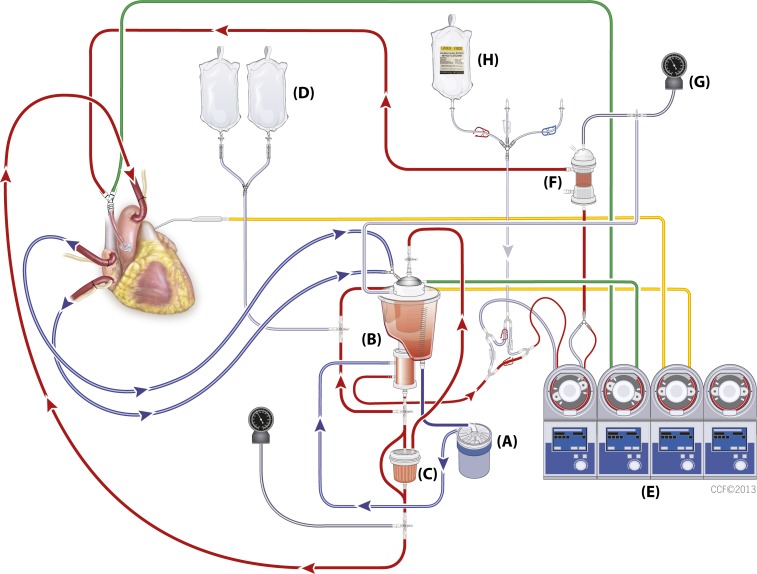
Currently, we are using a custom disposable dual circuit for del Nido cardioplegia delivery (Figure 2) adapted from our observations at New York Presbyterian Hospital Columbia.
Figure 2.
Cleveland Clinic dual cardioplegia circuit for the del Nido cardioplegia delivery. (A) Centrifugal pump, (B) reservoir and oxygenator, (C) leukocyte filter, (D) Plasma-Lyte® A solution, (E) heart–lung machine, (F) cardioplegia heat exchanger, (G) manometer for the cardioplegia system, (H) del Nido cardioplegia solution.
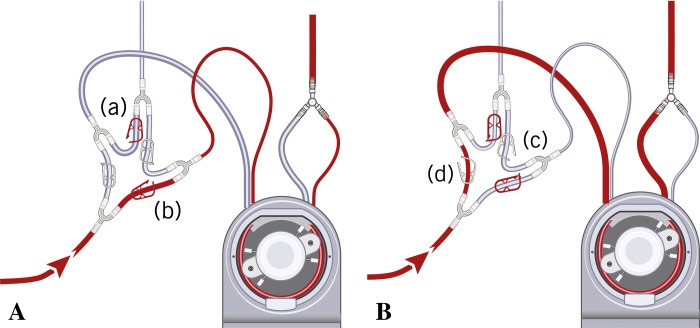
The circuit was designed with a set of Robert clamps on the blood inlet to the cardioplegia heat exchanger and a set of Robert clamps on the crystalloid cardioplegia inlet, depending on the desired cardioplegia to be used in the case. One set of Robert clamps is open and the other set is closed to deliver either 4:1 or 1:4 blood:crystalloid ratio (Figure 3A–B).
Figure 3.
(A) A set of Robert clamps ([a] and [b]) is open for del Nido cardioplegia delivery. Clamp (a) is open and del Nido cardioplegia is delivered through a ¼ line. Clamp (b) is open and blood is delivered through a 3/16 line (blood:crystalloid ratio is 1:4). (B) A set of Robert clamps ([c] and [d]) is open for the Buckberg cardioplegia delivery. Clamp (c) is open and Buckberg cardioplegia is delivered through a 3/16 line. Clamp (d) is open and blood is delivered through a ¼ line (blood:crystalloid ratio is 4:1).
The Cleveland Clinic del Nido protocol for the adult patients is to administer 20 mL/kg with a maximum dose of 1000 mL for patients larger than 50 kg. If the aortic cross-clamp time is expected to be less than 30 minutes, a half dose can be used to arrest the heart. However, even in a small-sized patient with a hypertrophic ventricle (e.g., aortic stenosis with left ventricular hypertrophy or hypertrophic cardiomyopathy), we recommend a full dose of cardioplegia (20 mL/kg) to provide satisfactory delivery of cardioplegia to provide adequate myocardial protection of the hypertrophic muscle mass. del Nido cardioplegia is given at 4°C. Once the cross-clamp is placed on the ascending aorta, a single dose of antegrade del Nido cardioplegia is delivered over 1–4 minutes at a rate of 250–450 mL/min. In the case of aortic insufficiency where aortotomy will be performed (e.g., aortic valve replacement), the aortotomy may provide exposure and access to the coronary ostia for direct cardioplegia administration. Handheld ostia cannulas are used with flows and pressures adjusted accordingly. After 90 minutes of aortic clamp time, the surgeon decides how much more del Nido cardioplegia needs to be administrated. We do not routinely use retrograde coronary sinus delivery. However, there is no contraindication to its use in this manner and retrograde administration can be a useful adjunct in the case of aortic insufficiency.
DISCUSSION
General Consideration
The initial cases in which we used del Nido cardioplegia were minimally invasive (thoracotomy or hemisternotomy) mitral valve surgeries and robotic mitral valve procedures using an Endo-balloon or a Chitwood clamp. As we gained experience, we then expanded our use of del Nido cardioplegia to include more complex cases such as double-valve procedures, maze procedures, myectomies, limited aortic repairs/replacements (with or without aortic valve repair/replacement), and robotically assisted atrial septal defect repairs or myxoma excisions. Although we continue to maintain caution as we expand our use of del Nido cardioplegia, our early experience has been encouraging (12). We have continued to avoid the use of del Nido cardioplegia in patients with coronary artery disease because adequate and uniform distribution of cardioplegia solution to the myocardium is not guaranteed when the patient has either large-vessel disease or dysfunction of the microcirculation. In such cases, no matter what form of cardioplegia delivery, uniform distribution of single-dose cardioplegia is not guaranteed. In patients with aortic insufficiency, an aortotomy and direct cardioplegia delivery through the coronary ostia are required with or without retrograde delivery.
Lidocaine Allergy
It has to be kept in mind that del Nido cardioplegia should be avoided in patients with lidocaine allergy.
Glucose Control
Modified Buckberg cardioplegia is a dextrose-based solution and causes the patients’ glucose levels to increase on administration of cardioplegia. Because the del Nido cardioplegia is not a dextrose-based solution (Plasma-Lyte A), the perfusionist should communicate with anesthesia to treat glucose levels with insulin accordingly.
Temperature
del Nido cardioplegia is delivered cold. Myocardial oxygen consumption decreases by 50% for every 10°C decrease in myocardial temperature (1,2). The del Nido cardioplegia is delivered at a temperature of 4°C and will produce myocardial cooling to less than 15°C.
The Previous Single Circuit with ¼ Y-Connector Priming Line
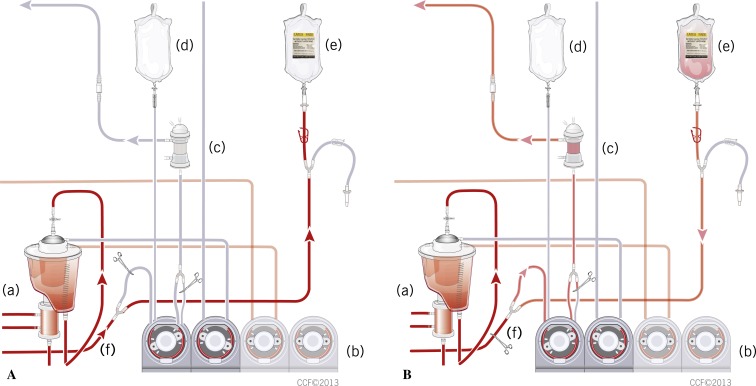
For those who does not have a dual cardioplegia circuit, a rapid prime line was aseptically cut into the cardioplegia line distal to the oxygenator using a ¼ Y-connector but proximal to the cardioplegia roller pump. The previous custom disposable single circuit we used consisted of three cardioplegia bag connection lines, a pump loop with two size tubing (¼ and 3/16), heat exchanger with a temperature monitoring port, and pressure monitoring port with a purge line with one-way valve. To modify the custom disposable single circuit from Buckberg cardioplegia to del Nido cardioplegia delivery, the original cardioplegia line was clamped out and the rapid prime line was then primed and spiked to the 1200-mL bag of del Nido cardioplegia solution. The flow of the cardioplegia pump needs be changed to ¼ tubing. The 3/16 tubing should be taken out of the raceway or be clamped at the outlet of the tubing. Once bypass was initiated and stabilized, 300 mL of oxygenated blood was transferred to the del Nido cardioplegia bag. After the addition of blood, the total volume in the del Nido cardioplegia bag was 1500 mL. The 1200 mL of crystalloid and 300 mL of patient blood allow for the proper blood-to-crystalloid ratio, 1:4 (Figure 4A–B).
Figure 4.
(A) Three hundred milliliters of oxygenated blood was transferred to the del Nido cardioplegia bag ([f] line is open). (a) Reservoir and oxygenator, (b) heart–lung machine, (c) cardioplegia heat exchanger, (d) N/S 200 mL for 3/16 cardioplegia line priming, (e) del Nido cardioplegia bag (1200 mL), (f) line open. (B) Clamping the cardioplegia line (f) in between the oxygenator and the Y-connector, which is located proximal to the inlet of the cardioplegia pump. (a) reservoir and oxygenator, (b) heart–lung machine, (c) cardioplegia heat exchanger, (d) N/S 200 mL for 3/16 cardioplegia line priming, (e) del Nido cardioplegia + blood bag (1500 mL), (f) line clamped.
del Nido Cardioplegia Delivery during the Initial Period of the Aortic Cross-Clamping
A fast cardiac arrest should preserve the adenosine-5′-triphosphate content of the myocardium in the early ischemic period and limit cell damage. Failure to produce cardiac arrest within 1–2 minutes may be the result of incomplete aortic clamping, aortic insufficiency followed by ventricular distension, or technical error such as an improperly set cardioplegia head occlusion, the cardioplegia bag is not open for delivery, or insufficient potassium in the cardioplegia solution. The perfusionist should vigilantly monitor relevant parameters and should be alert to potential delivery issues.
Aortic Insufficiency with or without Ventricular Hypertrophy
When antegrade cardioplegia is delivered, pulmonary artery pressure is monitored for ventricular distention. The subendocardium receives its flow primarily during diastole and is vulnerable to variation in blood flow. Blood flow depends on the transmural gradient, which is the difference between the aortic diastolic pressure and the intraventricular end-diastolic pressure. When the patient is hypotensive, has aortic insufficiency, ventricular fibrillation, ventricular distension, or increases ventricular end-diastolic pressure, oxygen delivery to the subendocardium may be insufficient. It is important to prevent ventricular distention, which increases wall tension and then increase oxygen consumption while at the same time decreasing oxygen delivery to the subendocardium. This is particularly dangerous in the setting of ventricular hypertrophy or fibrillation where subendocardial perfusion is already compromised (1–5). Myocardial rewarming can be an issue with a hypertrophic ventricle. Myocardial cooling is achieved through the administration of cold del Nido cardioplegia, which is usually given at a temperature of 4°C and produces myocardial cooling to less than 15°C. In our institution, a myocardial temperature probe is placed in the septal wall and should read less than 10°C. If the peak airway pressure mean is above 20 mmHg or myocardial temperature is above 15°C, the perfusionist should alert the surgeon to a potential ventricular distension or myocardial rewarming. In addition to cold cardioplegia, chilled saline or slush can be used for topical cooling of the myocardium. This method of cooling does not provide cooling to the deep myocardium but is better suited for cooling the less muscular and relatively thin right ventricle than the left ventricle.
del Nido Cardioplegia May Initially Increase the Level of Hemodilution
del Nido cardioplegia is dosed at 20 mL/kg, up to 1000 mL for patients larger than 50 kg. When it is administrated to a small patient with a relatively low hematocrit (HCT), the benefits of del Nido cardioplegia may be offset by the adverse effects of hemodilution. del Nido cardioplegia, which drains from the coronary sinus, can be suctioned into a cell saver instead of returning to systemic circulation when total bypass (bicaval cannulation with caval occlusion) is performed and the right atrium is opened. This method limits hemodilution and requires clear communication and teamwork among surgeons, physicians’ assistants, and perfusionists. In cases in which the right atrium is not opened and the cell saver cannot be used in the manner described, hemoconcentration should be considered in small patients with relatively low HCTs to maintain an acceptable patient hematocrit. However, multiple doses of modified Buckberg cardioplegia can result in similar levels of hemodilution.
Robotically Assisted Cardiac Procedures with an Endo-Balloon Clamp or Chitwood Clamp
Minimally invasive cardiac surgery has been presented as a promising technique. Various methods of mitral valve surgery through limited thoracotomy or ministernotomy incisions have been developed. A robotically assisted surgery with an Endo-aortic clamp (EAC; Edwards LifeSciences, Irvine, CA) to achieve cardioplegic arrest is now used in the clinical setting. Conventional cardiac procedures have a defined team approach with the roles of each team member well established. When the chest is open, the surgeon has both direct visual and tactile access to the heart. However, in minimally invasive surgeries, communications among team members become even more critical (14). The perfusionist becomes more responsible for both monitoring and communicating the parameters associated with proper myocardial protection.
Two minimally invasive methods have been developed to occlude the aorta when the surgical field does not allow direct clamping of the aorta. The first has been the development of the EAC that obstructs the aorta from within the aorta. The EAC is a triple-lumen catheter with an inflatable balloon at its distal end. This clamp is positioned in the ascending aorta using transesophageal echocardiography (TEE) guidance. The lumen used for balloon inflation is connected to a manometer to monitor balloon pressure. Antegrade cardioplegia is delivered through a central lumen, which also acts as an aortic root vent after cardioplegia delivery. A third lumen serves as an aortic root pressure monitor. Before performing the incision, TEE is used to confirm mitral valve dysfunction and to ensure that the aortic valve is competent. The robotically assisted mitral valve procedure requires passage of the EAC into the thoracic aorta and inflation of the EAC balloon. Therefore, aneurysms or a weakened aortic wall are contraindications. Right and left radial arterial lines are placed to monitor migration of the balloon. The second method is cardiac arrest with the Chitwood clamp. In 1997, Chitwood et al. introduced a modified aortic cross-clamp (Chitwood transaxillary clamp) that could be used in minimally invasive vascular surgery (15,16). Peripheral CPB (femoral artery–femoral vein) is used and a Chitwood clamp is introduced through the right, third, or fourth intercostal space. A 14-G, 31-cm antegrade needle (Minimally Invasive Aortic Root; Medtronic, Minneapolis, MN) is inserted through the chest wall and cardiac arrest was achieved with antegrade del Nido cardioplegia.
After systemic heparinization, the femoral artery and vein are cannulated. Additionally, a percutaneous pulmonary artery (PA) venting catheter, placed through the jugular approach, helps in ventricular decompression. CPB is initiated and adequate drainage is confirmed. The perfusionist achieves the systemic blood pressure that will be maintained during the case and the EAC is inflated under TEE. Antegrade cardioplegia delivery is started. Balloon pressure is generally 300–400 mmHg at this point. Antegrade cardioplegia flow is confirmed by using color flow Doppler echocardiography to examine the aortic root. Right and left radial arterial pressure, aortic root pressure, and balloon pressure are monitored throughout the operation.
In conclusion, del Nido cardioplegia allows customization of myocardial protection strategies for different surgical needs. We have found that del Nido cardioplegia allows us to customize myocardial protection for adult cardiac procedures. Like with any myocardial protection strategy, adequate and uniform distribution of cardioplegia solution to the myocardium is critical. This is especially important in “one-shot” cardioplegia such as del Nido cardioplegia.
ACKNOWLEDGMENTS
We thank Dr. del Nido and Mr. Greg Matte for their very kind support, insights, and guidance as we instituted our use of del Nido solution in our adult patients at the Cleveland Clinic. We also express our gratitude to the entire surgical and perfusion team at New York Presbyterian Hospital, Columbia campus, for welcoming us to visit and observe their use of del Nido solution and for sharing their cardioplegia custom tubing pack specs and experiences with us.
REFERENCES
- 1.Gravlee GP, Davis RF, Stammers AH, Ungerleider RM, eds. Cardiopulmonary Bypass: Principles and Practice, 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008:172–179, 702–4. [Google Scholar]
- 2.Hensley FA, Martin DE, Gravlee GP.. A Practical Approach to Cardiac Anesthesia, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:649–650. [Google Scholar]
- 3.Chambers DJ. Mechanisms and alternative methods of achieving cardiac arrest. Ann Thorac Surg. 2003;75:S661–S666. [DOI] [PubMed] [Google Scholar]
- 4.Cohen G, Borger MA, Weisel RD, Rao V.. Intraoperative myocardial protection: Current trends and future perspectives. Ann Thorac Surg. 1999;68:1995–2001. [DOI] [PubMed] [Google Scholar]
- 5.Vinten-Johansen J, Thourani VH.. Myocardial protection: An overview. J Extra Corpor Technol. 2000;32:38–48. [PubMed] [Google Scholar]
- 6.Patent 5,407,793. Alexandria, PA: U.S. Patent and Trademark Office; 1995. [Google Scholar]
- 7.Charette K, Gerrah R, Quaegebeur J, et al. Single dose myocardial protection utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion. 2012;27:98–103. [DOI] [PubMed] [Google Scholar]
- 8.Matte GS, Del Nido PJ.. History and use of del Nido cardioplegia solution at Boston Children's Hospital. J Extra Corpor Technol. 2012;44:98–103. [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien JD, Howlett SE, Burton HJ,O’Blenes SB, Litz DS, Friesen CL.. Pediatric cardioplegia strategy results in enhanced calcium metabolism and lower serum troponin T. Ann Thorac Surg. 2009;87:1517–1523. [DOI] [PubMed] [Google Scholar]
- 10.Govindapillai A, Hua R, Rose R, Friesen CH, O'Blenes SB.. Protecting the aged heart during cardiac surgery: Use of del Nido cardioplegia provides superior functional recovery in isolated hearts. J Thorac Cardiovasc Surg. 2013;146:940–948. [DOI] [PubMed] [Google Scholar]
- 11.O’Blenes SB, Friesen CH, Ali A, Howlett S.. Protecting the aged heart during cardiac surgery: The potential benefits of del Nido cardioplegia. J Thorac Cardiovasc Surg. 2011;141:762–769. [DOI] [PubMed] [Google Scholar]
- 12.Mick SL, Robich MP, Houghtaling PL, Gillinov AM, Soltesz EG, Johnston DR, Blackstone EH, Sabik JF.. Del nido versus Buckberg cardioplegia in adult isolated valve surgery. J Thoracic Cardiovasc Surg. 2014. In press, Published online 22 October 2014. Available at: http://www.sciencedirect.com/science/article/pii/S0022522314015943. [DOI] [PubMed] [Google Scholar]
- 13.Buckberg GD. Strategies and logic of cardioplegic delivery to prevent, avoid, and reverse ischemic and reperfusion damage. J Thorac Cardiovasc Surg. 1987;93:127–139. [PubMed] [Google Scholar]
- 14.Pisano GP, Bohmer RM.. Organizational differences in rates of learning: Evidence from the adoption of minimally invasive cardiac surgery. Manage Sci. 2001;47:752–768. [Google Scholar]
- 15.Iribarne A, Easterwood R, Chan EY, et al. The golden age of minimally invasive cardiothoracic surgery: Current and future perspectives. Future Cardiol. 2011;7:333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitwood WR Jr, Elbeery JR, Moran JF.. Minimally invasive mitral valve repair: Using a minithoracotomy and transthoracic aortic occlusion. Ann Thorac Surg. 1997;63:1477–1479. [DOI] [PubMed] [Google Scholar]