Abstract:
The Auckland Hospital cardiothoracic unit recently removed Mannitol and Voluven from its Plasma-lyte-based cardiopulmonary bypass (CPB) priming fluid. Like with any change to practice, a comprehensive audit should be performed to identify positive or negative effects. The aim of this retrospective analysis was to investigate the effect of changing the CPB prime constituents on fluid balance and clinical outcome parameters. Clinical records were reviewed for 100 consecutive patients undergoing primary, isolated coronary artery bypass grafting (CABG), 50 patients before the prime change and 50 after. All data were collated into a central database for analysis. Mean arterial pressure while on bypass was higher in the new prime group (61.5 mmHg versus 57.5 mmHg, p = .002). There was no significant difference in hematocrit, hemoglobin, serum sodium, serum potassium, or creatinine postoperatively between groups. In regard to important outcomes such as postoperative weight and fluid balance, time on ventilation, length of stay in the intensive care unit (ICU) or hospital, and mortality, there were no significant differences. Interestingly, new prime group spent a smaller proportion of their time in the ICU on mechanical ventilation (23% versus 36%, p = .022). Mannitol and Voluven, like with all drugs, carry their own potential adverse effects. This study demonstrates that removing Mannitol and Voluven from priming fluid did not have any detrimental effect on electrolytes, fluid status, and other important outcomes in this consecutive series of patients having primary isolated CABG surgery. The risk–benefit balance combined with the obvious economic benefit clearly favors removing Mannitol and Voluven from priming fluids.
Keywords: cardiopulmonary bypass, physiology
Cardiac surgery was revolutionized in the 1950s with the development of the cardiopulmonary bypass (CPB) technology. In the period since the establishment of CPB, the extracorporeal circuit has become small enough to permit the priming solution to be in the majority of cases blood-free. This priming fluid that is added to the patient’s intravascular fluid on initiation of bypass has implications. First, there is the resultant hemodilution of up to 1.5 L of fluid being added directly to the intravascular compartment. Second, there are the biochemical effects of the constituents present in the priming fluid. Although hemodilution is an unavoidable consequence of a nonblood prime, attention to reducing hemodilution during CPB through reduced circuit size and interventions such as retrograde autologous priming and ultrafiltration has been the attention of recent evidence-based recommendations (1–4). Regarding the effects of priming fluid constituents, however, there are numerous options without a clear answer. This is reflected in the large number and heterogeneity of studies investigating the use of different priming fluids (5–8). The lack of standardized guidelines on what to use in priming fluids reflects a murky evidence pool.
Intravascular administration of any fluid has the potential to modify a number of physiological processes. This becomes increasingly important when considering the fact that open heart surgery is already far outside the range of a normal physiological process. Like with any intravenous fluid, it is the constituents of the fluid that dictate the physiological effects exerted. The Green Lane Cardiothoracic Surgical Unit at Auckland City Hospital has until recently used a Plasma-Lyte 148 (Baxter Pty Ltd., New South Wales, Australia) -based priming fluid with the addition of 500 mL of hydroxyethyl starch 130/.4 (Voluven 6%; Fresenius Kabi Australia Pty Ltd., New South Wales, Australia) and 150 mL 15% Mannitol (Osmitrol; Baxter Pty Ltd.). This solution was introduced into practice out of anecdotal rather than empirical evidence. It was decided in November 2012 to remove the starch and Mannitol components from the prime fluid. The decision to remove starch was based on emerging evidence demonstrating increased renal injury and adverse events (9). The removal of Mannitol was based on advice from our intensive care unit as a result of observations of high volume and potassium requirements resulting from excessive diuresis post-CPB. There was a multidisciplinary agreement to the modification of the constituents of the fluids used in CPB prime and the aim of this study was to investigate any effect this has had on patient outcomes, in particular, renal function and fluid balance.
MATERIALS AND METHODS
Setting and Patients
In November 2012, the constituents of the priming solution used for CPB by the Green Lane Cardiothoracic Surgical Unit at Auckland City Hospital underwent a change with the removal of Voluven and Mannitol (Table 1). Clinical notes and recordings for 100 consecutive patients undergoing isolated primary coronary artery bypass grafting (CABG), 50 patients prior using the old prime (OP) and 50 patients using the new prime (NP), were retrospectively reviewed. Patients undergoing procedures other than CABG and patients having undergone previous cardiothoracic surgery were excluded. Ethics approval was not required for this retrospective review of anonymized data.
Table 1.
Prime constituents.
| Old Prime | New Prime | |
|---|---|---|
| Plasma-Lyte 148 | 700–1,000 mL | 1,350–1,650 mL |
| Voluven | 500 mL | 0 |
| Mannitol 15% | 150 mL | 0 |
| Heparin | 10,000 IU | 10,000 IU |
| Cephazolin | 1 g | 1 g |
General anesthesia with standard monitoring was induced with propofol or etomidate, a nondepolarizing muscle relaxant and fentanyl, followed by isoflurane maintenance. Only Plasma-lyte was used as intravenous fluid, limited to less than 1 L before the onset of CPB.
Cardiopulmonary bypass was conducted using a Sorin S3 heart–lung machine (Sorin Group Deutschland, Munich, Germany), a Dideco Avant 903 or EOS 905 membrane oxygenator (Sorin Group, Mirandola, Italy), and a Pall AL20 arterial line filter (Pall Corporation, Portsmouth, U.K.). Patients were cooled to 32–33°C with flows of 2.4–3.0 L min−1·m2 to achieve a DO2i > 270 mL·min−1·m2. Mean arterial pressure was maintained at > 50 mmHg and myocardial protection was achieved with 4:1 blood cardioplegia.
Data Extraction
All data were extracted from the hospital electronic records and compiled into a custom database. Variables collected included demographic data, preoperative physiological parameters, intraoperative hematological and biochemical parameters, CPB machine recordings (Stockert data management system capturing at 30-second intervals) (Sorin), postoperative recordings of fluid balance, hematological and biochemical parameters, complications, and mortality. The primary outcome measures were intensive care unit (ICU) fluid balance, hemoglobin, hematocrit, requirement for blood products, and time spent on mechanical ventilation. Secondary outcome measures included number of days to return to preoperative weight, complications, and plasma sodium, potassium, and creatinine postoperatively, and change in renal function pre-/postsurgery.
Data Analysis
All statistical analyses were conducted using SPSS statistical package 19.0.0.1 (SPSS Inc., Birmingham, AL). Independent-samples t tests were performed for all continuous variables. Categorical variable were analyzed using Pearson χ2 test. Repeated-measure mixed analyses of variance were conducted to analyze between group effects for continuous variables, which were measured in the ICU over 6-hour, 12-hour, 18-hour, and 24-hour time check points for patients who remained in the ICU for > 24 hours. Missing value correction was performed for postoperative weight change as a result of not all patients being weighed daily. Statistical significance was set at a p value < .05.
RESULTS
Demographic and Preoperative Values
All demographic and preoperative values are summarized in Table 2. The mean age of patients was 63.4 years (range, 45–80 years) and 65.2 (range, 38–88 years) in the OP and NP groups, respectively. Males made up 74% and 82% of the OP and NP groups, respectively. There were no significant differences between the OP and NP groups in any of the preoperative demographic and physiological measures. The mean Euroscore II (10) was 1.8% in the OP group and 2% in the NP group.
Table 2.
Participant demographic and preoperative values.*
| Old | New | p Value | |
|---|---|---|---|
| Age | 63.4 (8.9) | 65.2 (11.6) | .38 |
| Male | 37 | 41 | .334 |
| Height (cm) | 170 (9.4) | 172 (8.8) | .29 |
| Weight (kg) | 86.03 (22.6) | 85.05 (18.1) | .812 |
| Body mass index (kg/m2) | 29.6 (6.5) | 28.8 (5.6) | .506 |
| Renal impairment | .796 | ||
| Normal | 24 | 25 | |
| Moderate | 21 | 21 | |
| Severe | 4 | 2 | |
| On dialysis | 1 | 2 | |
| Extracardiac arteriopathy | 1 | 0 | .315 |
| Poor mobility | 0 | 0 | N/A |
| Chronic lung disease | 6 | 2 | .14 |
| Active endocarditis | 0 | 0 | N/A |
| Critical preoperative state | 0 | 0 | N/A |
| Diabetes | 17 | 18 | .589 |
| NYHA | .249 | ||
| I | 13 | 6 | |
| II | 22 | 27 | |
| III | 15 | 16 | |
| IV | 0 | 1 | |
| CCS Class 4 angina | 0 | 0 | N/A |
| LV function | .168 | ||
| Good | 37 | 29 | |
| Moderate | 11 | 17 | |
| Poor | 1 | 4 | |
| Very poor | 1 | 0 | |
| Pulmonary hypertension | 1 | ||
| None | 46 | 46 | |
| Moderate | 4 | 4 | |
| Severe | 0 | 0 | |
| Urgency | .827 | ||
| Elective | 13 | 11 | |
| Urgent | 35 | 36 | |
| Emergency | 2 | 3 | |
| Euroscore II | 1.8 (1.6) | 2 (1.5) | .557 |
| Preoperative values | |||
| Sodium (mmol/L) | 137 (2.0) | 136 (3.0) | .056 |
| Potassium (mmol/L) | 4.1 (.4) | 4 (.5) | .323 |
| Creatinine (μmol/L) | 99 (65.7) | 103 (85.9) | .758 |
| Hemoglobin (g/L) | 129 (17.5) | 129 (16.3) | .953 |
| Hematocrit | .4 (.05) | .39 (.05) | .811 |
| Glucose (mmol/L) | 6.3 (.13) | 6.3 (.14) | .864 |
Renal impairment (normal = creatinine clearance > 85 mL/min, moderate = creatinine clearance 50–85 mL/min, severe = creatinine clearance < 50 mL/min).
NYHA, New York Heart Association; CCS, Canadian Cardiovascular Society; LV function (good = left ventricular ejection fraction > 50%, moderate = left ventricular ejection fraction 31–50%, poor = left ventricular ejection fraction 21–30%, very poor = left ventricular ejection fraction ≤ 20%). Pulmonary hypertension (moderate = pulmonary artery systolic pressure 31–55 mmHg, severe = pulmonary artery systolic pressure > 55 mmHg); N/A, not applicable.
Operative Data
A summary of operative recordings is summarized in Table 3. Time on bypass and aortic cross-clamp time were not significantly different between the OP and NP groups. Although the requirement for red blood cells (RBCs) between the OP and NP groups was not significantly different (four of 50 versus seven of 50, respectively, p = .579), the mean peak hemoglobin during surgery was higher in the NP group compared with the OP group (99.7 g/L versus 93.8 g/L, p = .043). The mean nadir hemoglobin during surgery, however, was not significantly different between the groups nor was the peak or nadir hematocrit values. The mean nadir serum sodium during surgery was lower in the OP group compared with the NP group (134.2 mmol/L versus 135.1 mmol/L, p = .027) yet there was no significant difference in the peak serum sodium or peak or nadir serum potassium between the groups. The mean arterial pressure while on CPB was higher in the NP group compared with the OP group (61.5 mmHg versus 57.5 mmHg, p = .002). This increased perfusion pressure did not have a corresponding significant difference in CPB flow rate.
Table 3.
Operative values.
| Old | New | p Value | |
|---|---|---|---|
| Bypass time (minutes) | 78.3 (24.4) | 81.8 (25.6) | .495 |
| Crossclamp time (minutes) | 50.1 (18.6) | 52.6 (20.5) | .511 |
| Average mean arterial pressure on CPB (mmHg) | 57.5 (7.5) | 61.5 (5.2) | .002* |
| Average flow rate (L/min−2·m−2) | 4.8 (.8) | 4.8 (.6) | .786 |
| Peak hematocrit | .29 (.04) | .3 (.04) | .249 |
| Nadir hematocrit | .26 (.04) | .27 (.04) | .322 |
| Peak hemoglobin (g/L) | 93.8 (12.5) | 99.7 (16.2) | .043* |
| Nadir hemoglobin (g/L) | 85.6 (13.4) | 88.5 (13.3) | .281 |
| Peak sodium (mmol/L) | 134.2 (2.1) | 135.1 (3.0) | .089 |
| Nadir sodium (mmol/L) | 132.4 (2.4) | 133.7 (3.4) | .027* |
| Peak potassium (mmol/L) | 5.4 (.69) | 5.4 (.78) | .914 |
| Nadir potassium (mmol/L) | 4.6 (.59) | 4.6 (.65) | .847 |
| Peak glucose (mmol/L) | 10.4 (2.8) | 10.7 (3.1) | .608 |
| Nadir glucose (mmol/L) | 8.3 (2.4) | 8.4 (2.7) | .862 |
| Patients requiring RBCs | 4 | 7 | .579 |
Statistically significant (p < .05).
CPB, cardiopulmonary bypass; RBCs, red blood cells.
Postoperative Data
A summary of ICU parameters of hemoglobin, hematocrit, serum sodium, serum potassium, and serum creatinine is summarized in Table 4. There were no significant differences between groups in regard to any of these measurements indicating that the difference observed between groups during surgery do not persist during ICU stay.
Table 4.
Postoperative values..
| Old | New | p Value | |
|---|---|---|---|
| New renal failure requiring dialysis | 1 | 0 | .315 |
| Reoperation | 2 | 2 | 1 |
| Thromboembolic event | 1 | 0 | .315 |
| Atrial fibrillation | 9 | 15 | .16 |
| Mediastinal re-exploration | 2 | 2 | 1 |
| Neurological complication | 1 | 0 | .315 |
| Graft site wound healing complication | 1 | 2 | .558 |
| Sternal wound healing complication | 1 | 1 | 1 |
| Death | 0 | 0 | N/A |
| ICU hemoglobin peak (g/L) | 121.5 (13.0) | 123.5 (12.5) | .441 |
| ICU hemoglobin nadir (g/L) | 94.4 (17.5) | 97.0 (15.9) | .431 |
| ICU hematocrit peak | .36 (.05) | .37 (.04) | .238 |
| ICU hematocrit nadir | .31 (.05) | .31 (.04) | .695 |
| ICU peak sodium (mmol/L) | 138.7 (2.5) | 138.4 (2.7) | .703 |
| ICU nadir sodium (mmol/L) | 135.7 (2.5) | 135.0 (2.9) | .175 |
| ICU peak potassium (mmol/L) | 5.0 (.4) | 5.0 (.5) | .672 |
| ICU nadir potassium (mmol/L) | 4.0 (.3) | 3.9 (.3) | .347 |
| ICU creatinine peak (μmol/L) | 99.6 (67.5) | 97.0 (72.1) | .848 |
| Change in creatinine (pre-/postoperative) | −1 | 6.4 | .059 |
| 6-hour ICU blood products (old n = 50, new n = 50) | |||
| Patients requiring RBCs | 1 | 4 | .169 |
| Patients requiring platelets | 4 | 1 | .169 |
| Patients requiring FFP | 1 | 1 | 1 |
| 12-hour ICU blood products (old n = 50, new n = 50) | |||
| Patients requiring RBCs | 1 | 5 | .092 |
| Patients requiring platelets | 4 | 2 | .4 |
| Patients requiring FFP | 1 | 1 | 1 |
| 18-hour ICU blood products (old n = 45, new n = 49) | |||
| Patients requiring RBCs | 2 | 6 | .176 |
| Patients requiring platelets | 3 | 3 | .914 |
| Patients requiring FFP | 0 | 2 | .171 |
| 24-hour ICU blood products (old n = 26, new n = 31) | |||
| Patients requiring RBCs | 4 | 4 | .9 |
| Patients requiring platelets | 3 | 2 | .577 |
| Patients requiring FFP | 0 | 2 | .171 |
| Days to return to preoperative weight | 5.3 (2.4) | 5.4 (2.3) | .899 |
| LOS ICU | 43.4 (55.9) | 49.8 (111.5) | .719 |
| Ventilation time | 16.9 (41.2) | 16.0 (62.1) | .928 |
| Percentage of time in ICU on ventilator | 31.5 (19.0) | 22.6 (19.0) | .022 |
| LOS in the hospital | 10.7 (5.9) | 12.7 (8.0) | .165 |
ICU, intensive care unit; RBCs, red blood cells; FFP, fresh-frozen plasma; LOS, length of stay; N/A, not applicable.
Requirements for blood products (RBC, platelets, and/or fresh-frozen plasma [FFP]) were also not significantly different between the OP and NP groups. RBCs were administered to four patients in the OP group and six patients in the NP group, platelets were administered to four patients in the OP group and three patients in the NP group, and FFP was administered to one patient in the OP group and two patients in the NP group.
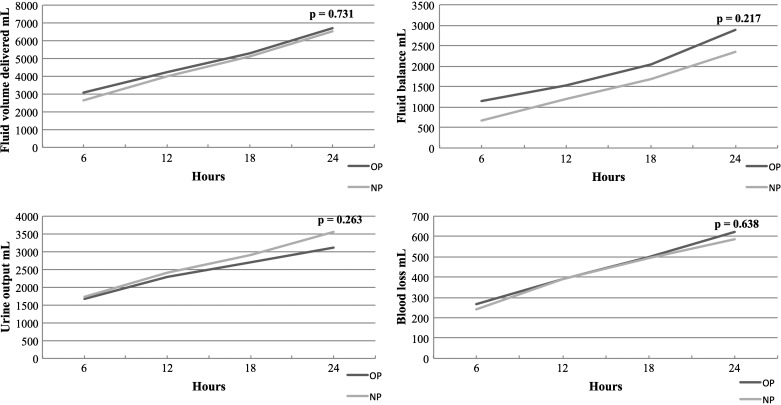
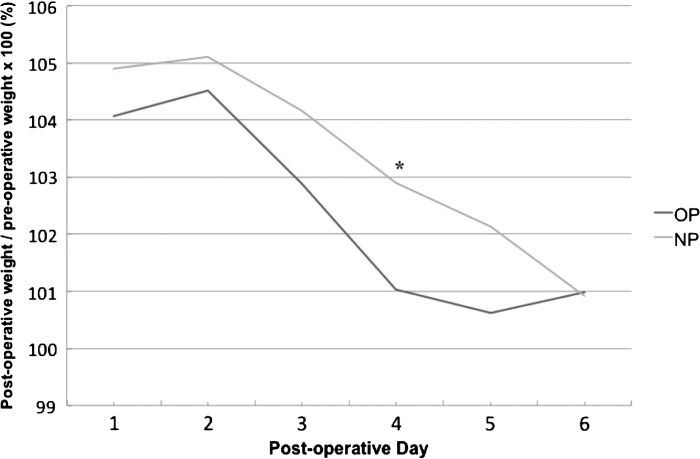
Fluid balance recordings were documented at intervals of 6, 12, 18, and 24 hours and these are summarized in Figure 1. There was no significant difference in repeated-measure analysis between the OP and NP groups in volume of fluid delivered, urine output, blood loss, or fluid balance, although fluid balance remained between 330 and 530 mL higher in the OP group across the four time points. There was also no significant difference between these parameters at each time point individually. On average, it took patients 5.3 and 5.4 days to reach their preoperative weight in the OP and NP groups, respectively, and although repeated-measures analysis of postoperative weight relative to preoperative weight was not significantly different, there was a significant difference on Day 4 postoperatively (NP = 103% versus OP = 101%, p = .044) (Figure 2).
Figure 1.
Cumulative fluid balance recordings over 24 hours in the intensive care unit.
Figure 2.
Postoperative weight relative to preoperative weight.
Other outcome measures related to complications, time in the ICU, time on mechanical ventilation, and length of stay in hospital are summarized in Table 4. Although individually there is no significant difference between OP and NP groups in any of these measures individually, the average percentage of time in the ICU spent on mechanical ventilation was lower in the NP group compared with the OP group (22.6% versus 31.5%, p = .022). There were no deaths in either group and the most frequent complication was postoperative atrial fibrillation (nine and 15 in the OP and NP groups, respectively).
DISCUSSION
This study provides a retrospective analysis on the effect of removing Voluven and Mannitol from a Plasma-lyte 148 CPB priming solution. Studies like this highlight the value of rigorous data capture of important outcome variables for quality control analysis to enable easy identification of variation in performance over time and during changes to practice. From a clinical audit standpoint, this study validates the decision to alter the prime constituents in this manner and should be encouraging for other centers considering making such a change. Perhaps the most significant finding in these data is the demonstration that since the prime change, patients are spending less of their time in the ICU on ventilation, which clearly reduces the likelihood of ventilator-associated adverse events (11).
An unavoidable consequence of using fluid replacement products to prime the CPB machine is hemodilution. For example, in this study, patients’ hemoglobin was diluted by 44 g/L (34%) and 40 g/L (31%) in the OP and NP groups, respectively. It has long been known that mortality and morbidity increase with the extent of hemodilution. Lower nadir hematocrit values have been well correlated with requirement for increased transfusion, intra-aortic balloon pump, stroke, myocardial infarction, cardiac arrest, renal failure, bleeding, sepsis, pulmonary edema, and mortality (4,12–14). With this in mind, reducing the extent of hemodilution is a worthy goal. Condensing the bypass circuit (4) and retrograde autologous priming (3) has proven to be effective in reducing the extent of hemodilution in a very simplistic manner. Less simplistic is the role of changing priming constituents. The proposed (yet controversial) (15) benefit of colloid fluids pivots on the reduced ability for the larger molecules to permeate blood vessels and therefore support the colloid osmotic gradient between the intra- and extravascular compartments and reducing fluid shift into interstitial tissues. It is therefore plausible that removing Voluven from the priming solution could reduce the extent of hemodilution with a greater portion of administered fluid shifting into the interstitial tissue resulting from the reduction in vascular colloid osmotic pressure. Although the mean values in this study of our surrogate markers for hemodilution (hematocrit and hemoglobin) were consistently higher in the NP group both in the operating room and ICU, most of these differences were not statistically significant. The peak hemoglobin during CPB was higher in the NP group (99.7 g/L versus 93.8 g/L, p = .043). This difference did not remain significant in the ICU. Although this is an interesting observation, the true relevance lies in whether this difference translates to any clinical significance in requirement for blood products, fluid balance/distribution parameters, complication rates, or time to recovery.
The provision of blood transfusion is a potentially life-saving treatment. It is however a measure that needs to be well considered not only as a result of the limited supply, but also because it carries substantial risk. Patients requiring blood products face increased risks of adverse effects including infection, renal injury/failure, increased length of hospital stay, and mortality (16–22). With the close link between the extent of hemodilution associated with CPB and requirement for blood transfusions discussed already (13), this should be a careful consideration when altering prime constituents. In this study, although we have not seen a reduction in the requirement of blood transfusions that might accompany a reduction in hemodilution, it is reassuring that there has not been an increase. This lack of significant difference between groups in requirement for all blood products is consistent across subproducts of transfusions, RBC, platelets, FFP, and cryoprecipitate.
Another important consideration is fluid balance and fluid distribution. To this point in the discussion we have focused primarily in the intravascular fluid compartment. However, when changing to a Voluven- and Mannitol-free priming solution, there are a number of factors to consider. Simple physiology tells us that removing the colloid Voluven will increase the shift of fluid from the blood vessels into the extravascular compartment and this is reflected in the literature where increased concentration of hydroxyethyl starch in the prime reduces interstitial fluid expansion (23). Mannitol was also removed from our priming solution. It has been demonstrated that urine output is greater when patients receive a CPB prime containing Mannitol even after it has been entirely cleared from the body, suggesting improved postoperative renal function by including Mannitol in the prime (24). The final consideration is the effect of priming constituents on coagulation and therefore blood loss. Hydroxyethyl starch containing primes has been shown to increase blood loss, reoperations for bleeding, and requirement for blood products and despite suggestions, there is no evidence to prove these effects are mitigated by the use of lower molecular-weight starches (6). In summary of these three factors, it would be reasonable to hypothesize that we might see an increase in requirement for fluids resulting from reduced intravascular colloid osmotic pressure, a reduction in urine output as a result of the removal of Mannitol, and a reduction in blood loss resulting from the removal of Voluven. This would result in patients in the NP group having an increased fluid balance and postoperative weight, therefore carrying a higher fluid load. The reality in this study is quite different as can be seen in Figures 1 and 2. The fluid volume delivered in the ICU, urine output, blood loss, and fluid balance over 24 hours does not reflect any significant difference between groups at individual 6-, 12-, 18-, and 24-hour time points or as a repeated-measures analysis. Interestingly, removal of Mannitol has not reduced urine output. A proposed mechanism for this is that the starch in the OP group reduced filtration in the kidney and so although there may have been a reduction in urine output as a result of the removal of Mannitol, there has also been an increase resulting from the removal of the Voluven; therefore, urine output remains relatively unchanged. Further to this, the mean fluid balance (although not statistically significant) remained between 330 and 530 mL (Figure 1) lower in the NP group over the first 24 hours despite being heavier on postoperative Day 4 relative to preoperative weight (Figure 2). This point demonstrates a conflicting relationship between the measures of fluid balance and postoperative weight where ideally there should be a close link between the two. Although it might be expected that the weight gain in the NP group would be greater than the OP group as a result of more fluid shifting to the interstitial tissues, the fact that this is not reflected in the fluid balance recordings does bring the role of fluid balance in guiding fluid status into question. Finally, as already discussed, there was no significant difference in requirement for blood products nor was there a difference in blood loss between the groups suggesting that the removal of the Voluven has not had any clinically significant effect on coagulation.
The true question is whether this change in prime constituents has had any effect on the patients and the cost of treating them. It is encouraging that there was no significant difference between groups for any complications, length of stay in the ICU, length of time on ventilation, and length of stay in the hospital (Table 4). One finding of interest is since the prime change, the percentage of time while in the ICU spent on ventilation has decreased by nearly 10%. Although it is acknowledged there are a number of confounders that may have contributed to this finding, it is of interest considering time spent on ventilation is clearly related to increase risks such as iatrogenic respiratory alkalosis, increased intracranial pressure, gastric distention, impaired hepatic and renal function, pneumothoraces, and pneumonia (11).
This study is limited by the retrospective method introducing the potential for biases and confounders that are not easily identified or controlled for. An extension of this limitation lies in the fact that we were forced to exclude some variables as a result of concerns about the reliability of their recordings. Most notably this applies to the variables pertaining to fluid balance while in the operating room such as the precise recordings of crystalloid and colloid fluids administered and the actual volumes of any blood products administered and blood loss.
Priming fluids for CPB remain a controversial topic and this is a common issue in the field of fluid resuscitation medicine. The wide range of options for fluids and variables involved in the use of CPB makes it unlikely that a definitive answer can be elicited as to what the optimal priming fluid is and it is likely to be different between patients of differing physiology and pathology. However, this study certainly has demonstrated that Mannitol and Voluven as used in our CPB prime offer no appreciable benefit in regard to any functional, clinical, or biochemical outcome measure. Removing these elements from the priming solution obviously saves money also, estimated to be $21,000 NZD ($17,500 USD) per year in our unit. In summary, it has been encouraging that we have been able to remove Mannitol and Voluven from our Plasma-lyte 148-based priming solution with no adverse effects.
REFERENCES
- 1.Ferraris VA, Brown JR, Despotis GJ, et al. . 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. Ann Thorac Surg. 2011;91:944–982. [DOI] [PubMed] [Google Scholar]
- 2.Menkis AH, Martin J, Cheng DCH, et al. . Drug, devices, technologies, and techniques for blood management in minimally invasive and conventional cardiothoracic surgery: A consensus statement from the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) 2011. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery. 2012;7:229–241. [DOI] [PubMed] [Google Scholar]
- 3.Rosengart TK, DeBois W, O’Hara M, et al. . Retrograde autologous priming for cardiopulmonary bypass: A safe and effective means of decreasing hemodilution and transfusion requirements. J Thorac Cardiovasc Surg. 1998;115:426–439. [DOI] [PubMed] [Google Scholar]
- 4.Shann KG, Likosky DS, Murkin JM, et al. . An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–290. [DOI] [PubMed] [Google Scholar]
- 5.Himpe D.. Colloids versus crystalloids as priming solutions for cardiopulmonary bypass: A meta-analysis of prospective, randomised clinical trials. Acta Anaesthesiol Belg. 2003;54:207–215. [PubMed] [Google Scholar]
- 6.Navickis RJ, Haynes GR, Wilkes MM.. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: A meta-analysis of randomized trials. J Thorac Cardiovasc Surg. 2012;144:223–230. [DOI] [PubMed] [Google Scholar]
- 7.Russell JA, Navickis RJ, Wilkes MM.. Albumin versus crystalloid for pump priming in cardiac surgery: Meta-analysis of controlled trials. J Cardiothorac Vasc Anesth. 2004;18:429–437. [DOI] [PubMed] [Google Scholar]
- 8.Wilkes MM, Navickis RJ, Sibbald WJ.. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: A meta-analysis of postoperative bleeding. Ann Thorac Surg. 2001;72:527–533; discussion 534. [DOI] [PubMed] [Google Scholar]
- 9.Myburgh JA, Finfer S, Bellomo R, et al. . Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 10.Nashef SAM, Roques F, Sharples LD, et al. . EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–745. [DOI] [PubMed] [Google Scholar]
- 11.Pierson DJ.. Complications associated with mechanical ventilation. Crit Care Clin. 1990;6:711–724. [PubMed] [Google Scholar]
- 12.Carson J, Spence R, Poses R, Bonavita G.. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;331:727–729. [DOI] [PubMed] [Google Scholar]
- 13.Dial S, Delabays E, Albert M, et al. . Hemodilution and surgical hemostasis contribute significantly to transfusion requirements in patients undergoing coronary artery bypass. J Thorac Cardiovasc Surg. 2005;130:654–661. [DOI] [PubMed] [Google Scholar]
- 14.Roth-Isigkeit A. Borstel TV, Seyfarth M, Schmucker P.. Perioperative serum levels of tumour-necrosis-factor alpha (TNF-), IL-1, IL-6, IL-10 and soluble IL-2 receptor in patients undergoing cardiac surgery, with cardiopulmonary bypass without and with correction for haemodilution. Clin Exp Immunol. 1999;118:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perel P, Roberts I, Ker K.. Colloids versus crystalloids for fluid resuscitation in critically ill patients [Update of Cochrane Database Syst Rev. 2012;6:CD000567; PMID: 22696320]. Cochrane Database Syst Rev. 2013;2:CD000567. [DOI] [PubMed] [Google Scholar]
- 16.Chelemer SB, Prato BS, Cox PM Jr, O’Connor GT, Morton JR.. Association of bacterial infection and red blood cell transfusion after coronary artery bypass surgery. Ann Thorac Surg. 2002;73:138–142. [DOI] [PubMed] [Google Scholar]
- 17.Engoren MC, Habib RH, Zacharias A, et al. . Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–1186. [DOI] [PubMed] [Google Scholar]
- 18.Fransen E, Maessen J, Dentener M, Senden N, Buurman W.. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–1239. [DOI] [PubMed] [Google Scholar]
- 19.Habib RH, Zacharias A, Schwann TA, et al. . Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: Implications on operative outcome. Crit Care Med. 2005;33:1749–1756. [DOI] [PubMed] [Google Scholar]
- 20.Leal-Noval SR, Rincon-Ferrari MD, Garcia-Curiel A, et al. . Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461–1468. [DOI] [PubMed] [Google Scholar]
- 21.Michalopoulos A, Tzelepis G, Dafni U, Geroulanos S.. Determinants of hospital mortality after coronary artery bypass grafting. Chest. 1999;115:1598–1603. [DOI] [PubMed] [Google Scholar]
- 22.Vamvakas EC, Carven JH.. RBC transfusion and postoperative length of stay in the hospital or the intensive care unit among patients undergoing coronary artery bypass graft surgery: The effects of confounding factors. Transfus (Paris). 2000;40:832–839. [DOI] [PubMed] [Google Scholar]
- 23.Ali MA, Saleh M.. Selection of optimal quantity of hydroxyethyl starch in the cardiopulmonary bypass prime. Perfusion. 2004;19:41–45. [DOI] [PubMed] [Google Scholar]
- 24.Fisher AR, Jones P, Barlow P, et al. . The influence of mannitol on renal function during and after open-heart surgery. Perfusion. 1998;13:181–186. [DOI] [PubMed] [Google Scholar]