Abstract:
Acquired antithrombin (AT) deficiency has been associated with patients on extracorporeal membrane oxygenation (ECMO) as a result of hemodilution, blood coagulation activation, and the use of heparin. Replacement of AT has been typically utilized through the use of fresh-frozen plasma or AT concentrate. Antithrombin alfa (ATryn®) is a recombinant form of AT (rAT) with an identical amino acid sequence as that of plasma-derived antithrombin. The primary objective of this study is to examine the relationship of rAT dose to measured plasma antithrombin activity in a small series of patients who received rAT while on ECMO. A retrospective chart review was performed of all patients at Medical City Children’s Hospital who received ATryn while supported on ECMO between December 2011 and April 2012. Five patients were identified and the patients’ weight, bolus dose of ATryn, drip rate of ATryn, and AT blood levels were collected for analysis. The median age of these patients was 1 month (range, 1 day to 3.75 years). Because no dosing guidelines exist for pediatric ECMO, a starting dose of ATryn was chosen based on the manufacturer’s labeled indication (prevention of thromboembolic events in patients with AT hereditary deficiency). The median dose of rAT was 368 IU/kg/day (range, 104–520 IU/kg/day) to obtain AT activity level of 80–120%. The average time to reach the targeted AT activity level (80–120%) was 12.7 hours (range, 11–17 hours). Our findings suggest that the published ATryn dose may be inadequate to reach desired AT activity concentrations for pediatric patients on ECMO. Difference in patient population, use of extracorporeal circuits, and the use of heparin are likely explanations for this finding. We would also recommend frequent checking of AT levels while delivering this drug because making timely adjustments is necessary for achieving and maintaining the target AT activity level.
Keywords: recombinant antithrombin, ATryn, heparin resistance, extracorporeal membrane oxygenation
Clinicians are always seeking the optimal state of balance between anticoagulation and hemostasis in patients being supported by extracorporeal circulation (ECMO). The ideal state would limit clot formation within the circuit, which may increase the lifespan of the ECMO circuit as well as reducing the risk of emboli being delivered to the patient while minimizing the risk of hemorrhage (1,2).
Unfractionated heparin is the most widely used method of anticoagulation for patients on ECMO support. Heparin has a relatively short half-life and exerts its anticoagulation effect by binding to antithrombin (AT), a potent endogenous anticoagulant (1,3). AT is a naturally occurring glycoprotein produced by the liver. It is the primary inhibitor of coagulation with the body. AT inhibits coagulation by irreversibly binding to factors IIa and Xa and to a lesser extent by inhibiting factors IXa, XIa, and XIIa. When heparin binds to AT, it causes a conformational change in the AT molecule exposing the reactive site and accelerates the formation of the thrombin– antithrombin (TAT) complex by more than a thousand fold (1,4,5).
When a patient is placed on ECMO, consumption of endogenous AT occurs from blood exposure to both the artificial surface of the ECMO circuit as well as heparin. If this consumption continues untreated, it will lead to AT deficiency and consequently heparin resistance (2,6). Studies suggest that using AT supplementation to maintain normal AT activity levels may attenuate the consumption of coagulation factors, platelets, and potentially the associated inflammatory response that occurs secondary to inadequate anticoagulation (7–9). Additionally, neonates have reduced AT activity levels that do not reach those of healthy adults until 3–6 months of age (10,11). Coupled with data demonstrating deteriorating AT activity levels over time while supported on ECMO, these factors have led some to suggest that pediatric patients on ECMO may benefit from AT supplementation (12,13).
AT supplementation is currently available through three sources. Fresh-frozen plasma (FFP) contains AT at an approximate concentration of 1 unit/mL of FFP. Concentrated pooled human AT is available (Thrombate III®; Grifols Therapeutics Inc., Los Angeles, CA) as a lyophilized powder that is prepared from multiple donor plasma units and when reconstituted has an approximate concentration of 50 IU/mL (14). A final source of AT replacement is recombinant AT (rAT) (ATryn®; rEVO Biologics, Framingham, MA). rAT is produced through transgenic technology, which results in a purified AT substitute that is collected from genetically engineered animals rather than from pooled human blood. It is available as a lyophilized powder and when reconstituted has a concentration of 175 IU/mL (15).
The objective of this study was to examine a small series of patients who received rAT to treat AT deficiency while on ECMO. Specifically, we intended to observe the relationship of rAT dose to measured plasma AT activity.
PATIENTS AND METHODS
Once Institutional Review Board approval was obtained, a retrospective review was performed on all patients on ECMO from December 2011 to April 2012. We identified five patients who received rAT while on ECMO. All of the patients received loading doses and maintenance infusion of rAT as described by the packet insert for prevention of thromboembolic events in nonpregnant surgical patients with hereditary deficiency (HD) of AT (Table 1). AT activity levels were monitored and the maintenance infusion was adjusted as suggested by the manufacturer (Table 2). AT activity levels, rAT infusion rates, and patient demographics were collected for analysis.
Table 1.
rAT dosing table for surgical patients.
| Dosing Table for Surgical Patients | |||
|---|---|---|---|
| Loading Dose (IU) | Maintenance Dose (IU/h) | ||
Manufacturer’s dosing recommendations for adult hereditary AT deficient patients for the prevention of thromboembolism. It is recommended that the loading dose is given as a 15 minute infusion after which the maintenance dose is given as a continuous infusion.
IU, international units; h, hour; AT, antithrombin. AT, antithrombin.
Table 2.
AT monitoring table.
| Initial Monitoring Time | AT Level | Dose Adjustment | Recheck AT Level |
|---|---|---|---|
| 2 hour after initial treatment | <80% | Increase by 30% | 2 Hours after each dose adjustment |
| 80–120% | None | 2 hours after initiation of AT replacement of 2 hours after dose adjustment | |
| >120% | Decrease by 30% | 2 hours after each dose adjustment |
It is recommended to check AT levels two hours after initiation of the maintenance infusion as well as after any changes to the infusion rate. Once steady state is reach it is recommended that clinicians check AT levels at least once a day.
AT, antithrombin.
Circuit Description
Two ECMO circuits were used during the study period. A neonate circuit, which consisted of a ¼-inch heparin–albumin-coated tubing (Bioline; Maquet Cardiopulmonary AG, Hirrlingen, Germany), and a pediatric circuit, which consisted of 3/8-inch heparin–albumin-coated tubing. Both circuits also consist of a centrifugal pump (Rotoflow; Maquet Cardiopulmonary AG) and a heparin–albumincoated polymethylpentene oxygenator (Quadrox D;Maquet Cardiopulmonary AG).
The ECMO circuits were primed with a balanced electrolyte solution (Plasma-Lyte A; Baxter Healthcare Corporation, Deerfield, IL). The neonate circuit has an approximate prime volume of 350 mL and the pediatric circuit has a prime volume of approximately 425 mL. Before placing a patient on ECMO, this solution was displaced with 300–350 mL of packed red blood cells, approximately 100 mL of FFP, 50 mL of 25% albumin, 300 units of heparin, and 10 mEq of sodium bicarbonate.
Anticoagulation and Transfusion Protocols
Heparin was used for anticoagulation in all of the patients. Patients received a 100-unit/kg bolus of heparin before cannulation. The continuous heparin infusion was started at a rate of 20 units/kg/h once the activated clotting time (ACT) on ECMO was <300 seconds. The desired ACT range varied depending on the level or risk of bleeding as determined by the treating intensivist. If the patient had an average risk (nonsurgical patients) of bleeding, the ACT range was set at 180–220 seconds. If the patient was experiencing active bleeding or had high risk of bleeding (surgical patients, immediately postoperative), the ACT range was set at 160–180 seconds. If the patient had life-threatening active bleeding, the target ACT range was 150–170 seconds.
Blood product transfusions were based on laboratory values and coagulation study results. Platelets were transfused to maintain a platelet count >100,000 or considered as a result of a low maximum amplitude (MA) on a thromboelastograph (TEG). Cryoprecipitate was transfused to maintain a fibrinogen >100 mg/dL or considered as a result of a low angle on a TEG. Packed red blood cells were transfused to reach desired hemoglobin levels set by the treating intensivist (typically 10–12 mg/dL). FFP was given to maintain a prothrombin time based on bleeding risk (<16 seconds if average risk, <15 seconds if high risk, or <14 seconds if life-threatening) or considered when a TEG showed an extended R (reaction rate) or an extended TEG ACT on a heparinase rapid TEG.
AT replacement was considered when a patient’s AT activity level was below the normal threshold of 80% or when patients appeared to be heparin-resistant, requiring a high infusion of heparin (>40 U/kg/h) with little to no extension of the ACT. AT replacement was carried out by FFP, human AT concentrate, or rAT.
CASE REPORTS
Case 1: Pulmonary Hypertension after Gastroschisis Repair
A 4-day-old girl was transferred to our center as a result of worsening respiratory failure after repair of gastroschisis on the second day of life. Initially after surgical repair the child was reported to be doing well. However, later that evening she started to have desaturations and widening differential between the pre- and postductal saturations. The patient was provided maximal medical support including inhaled nitric oxide, high-frequency oscillatory ventilation, and inotropic support; however, despite multiple interventions, the child continued to exhibit low oxygen saturation. The pediatric surgeons evaluated the patient for possible abdominal compartment syndrome and felt that etiology of the child’s continued desaturations was most likely pulmonary in origin; therefore, the patient was transferred to our hospital (fourth day of life). On arrival at our facility, the patient exhibited a progressive hypoxemia with saturations as low as 49%; therefore, venoarterial ECMO was instituted. The initial cardiac echocardiogram showed mild to moderate right ventricular dilation and hypertrophy with good right ventricle function. There was no tricuspid valve regurgitation present so it was not possible to accurately assess the right ventricle or pulmonary artery pressure. On ECMO Day 2, an AT level of 50.2% was noted and the decision to start a rAT infusion was made. On ECMO Day 8, it was felt that the patient’s pulmonary hypertension had resolved to the point where she could be removed from ECMO support. The patient remained on the rAT infusion until ECMO was successfully weaned 144 hours after the initial loading dose was given. During the time period in which this patient was treated, our laboratory was unable to run sequential AT levels, which resulted in delayed adjustments to the rAT infusion. The patient’s AT level never reached the target range of 80–120%. The highest AT activity was 70.8%. The patient experienced no issues with excessive bleeding or clotting and no circuit or component changes were necessary.
Case 2: Cardiac Arrest
A 2-month-old boy born at an outside facility was transferred to our hospital on the third day of life as a result of suspicion of hypoplastic left heart syndrome. On the eighth day of life he underwent a Norwood with Sano modification. The patient had a protracted hospital course exacerbated by feeding intolerance with gastroesophageal reflux requiring Nissen fundoplication and gastrostomy tube placement and left diaphragm dysfunction requiring diaphragm plication. On hospital Day 65, the patient experienced an aspiration event, which led to acute desaturation and bradycardia requiring chest compressions. The patient was emergently placed on ECMO. AT replacement through rAT was started at ECMO Hour 6. The AT level before starting the rAT was 45.4% and 11 hours after the infusion was started, the patient’s AT level reached 84.6%. The rAT infusion continued until the patient was successfully weaned from ECMO 4 days later. This patient experienced no issues with excessive bleeding or clotting and needed no circuit or component change-out.
Case 3: Cardiac Dysfunction Status Postsurgical Repair
A 3-year-old boy was admitted to our center for respiratory issues relating to metapneumovirus as well as status asthmaticus. This patient was well known to us as he was being followed for aortic stenosis and aortic insufficiency following resection of a subaortic membrane at 2 years of life. While being treated he was noted to have worsening cardiovascular insufficiency, mitral and tricuspid regurgitation, and tunnel-like left ventricle. On hospital Day 19, the patient developed hypotension, pulseless ventricular arrhythmias requiring cardiopulmonary resuscitation (CPR), and electrocardioversion, both of which were nonsuccessful and the patient was placed on ECMO. The patient was supported on ECMO for 3 days after which the patient was successfully weaned. The post-ECMO period was complicated by continued myocardial depression and a bloodstream infection. It was felt that the patient would require a surgical intervention to address his continued cardiac dysfunction. So on hospital Day 48, he returned to the operating room to have a Ross-Konno procedure. After an extensive repair, the patient was unable to be weaned from bypass and was transferred to ECMO support. Five hours after ECMO was initiated, the patient’s AT activity was 52.2% and it was decided to start AT replacement therapy using rAT. At ECMO Hour 15, the AT activity reached 81.5% falling into the desired target range. The patient remained on the rAT infusion for the duration of the ECMO run, which lasted 6 days. The patient’s myocardial function did not improve and progressively worsened. On ECMO Day 6 the patient was separated from ECMO and died. Although the patient experienced mild to moderate bleeding during periods of his ECMO support, adjustment of the patient’s coagulation and anticoagulation parameters provided adequate hemostasis. The circuit showed no signs of clotting and no component needed to be changed out.
Case 4: Cardiac Dysfunction Status Postsurgical Repair
A 1-month-old boy was brought to our center with the diagnosis of Scimitar syndrome with partial anomalous pulmonary venous return with right pulmonary veins returning to the inferior vena cava and left veins returning to the left atrium. After surgical repair the patient was unable to be weaned from bypass and was placed on ECMO support. The AT activity 5 hours postoperatively was 46.7% and rAT was chosen to correct the AT deficiency in this patient. At ECMO Hour 25, the AT activity level reached 82.5%. The rAT infusion was continued until the patient was weaned off ECMO 7 days later. Ten hours after being removed from ECMO support, the patient experienced a steady decrease in systolic blood pressure that was unresponsive to medications and CPR was initiated; the decision was made not to place the patient back on ECMO and after 2 hours of attempted resuscitation, the patient died. There was significant bleeding during the ECMO run necessitating multiple blood product infusions and ultimately surgical exploration. There were no clotting incidents and no component of the ECMO circuit required change-out.
Case 5: Respiratory Distress Syndrome with Pulmonary Hypertension
This patient was born at our facility and was noted to be cyanotic at birth with poor respiratory effort necessitating intubation. The patient remained hypoxemic despite inhaled surfactant and high-frequency ventilation. A cardiac echocardiogram showed normal left ventricular function with a dilated right ventricle, flattened septum, and moderate tricuspid regurgitation. A patent foramen ovale was noted with right-to-left shunt flow and a large patent ductus arteriosus with bidirectional flows; however, the majority of the shunt was right to left. Despite pharmacologic and ventilator support, the patient’s oxygenation continued to deteriorate and the decision was made to place the patient on ECMO. The patient demonstrated low AT activity levels on ECMO ranging from 34% to 39.8% despite multiple infusions of FFP. On ECMO Day 2, the decision was made to start rAT replacement. The patient reached a maximum AT activity level of 49.4% 10 hours after the infusion of rAT was started. The patient received the rAT for 29 hours; however, as a result of a backorder of product, the pharmacy at our center ran out of rAT and the patient was switched to AT concentrate boluses for the remainder of his ECMO run. The patient was successfully weaned off on ECMO Day 7. Throughout his ECMO support, he experienced no issues with clotting or bleeding and no component of the circuit required change-out.
RESULTS
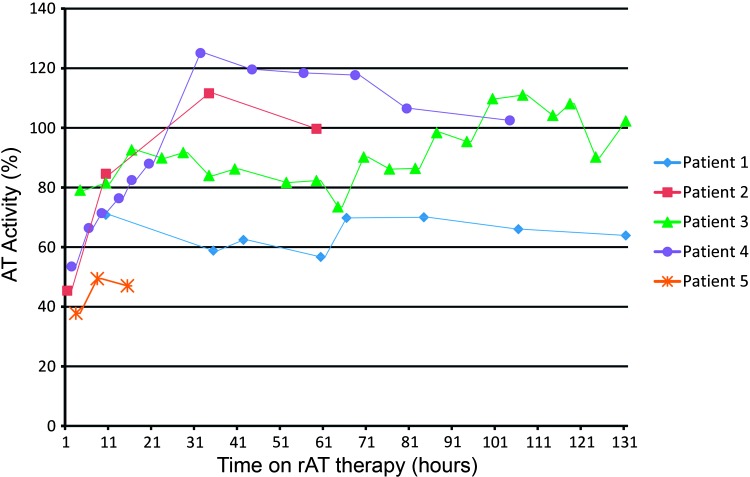
Between December 2011 and April 2012, 14 patients were supported by ECMO; one patient was supported twice on ECMO. Of these 14 patients, five of them received rAT as AT replacement therapy while being supported on ECMO. During this time period, there were no major changes to our anticoagulation or transfusion protocols for patients supported on ECMO. Demographic, rAT infusion rates, and AT activity of these patients are presented in Table 3. The median age of these patients was 1 month (range, 1 day to 3.75 years) with a median weight of 4 kg (range, 3–15 kg). Two patients (Patients 1 and 5) failed to reach the desired AT activity level despite being on a continuous infusion for 147 hours and 29 hours, respectively, and therefore were not included in the data for median infusion rate and time until target level was achieved. The median rAT infusion rate was 480 IU/kg/day (range, 104–520 IU/kg/day). The median time to the desired AT level was 11 hours (range, 10–17 hours) (Figure 1). All of the patients who received rAT during this time period were placed on venoarterial ECMO.
Table 3.
Comparison of patients receiving rAT while on ECMO.
| Patient No. | Weight (kg) | Age | Baseline AT Activity Level (%) | rAT Bolus Dose (units) | rAT Infusion Rate (U/kg/day) | Maximum AT Activity Level Achieved (%) | Time Until Target Level Achieved (hours) |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 days | 50.2 | 65 | 320 | 70.8 | † |
| 2 | 4 | 2.5 months | 45.4 | 95 | 480 | 111.6 | 10 |
| 3 | 15 | 3 years | 52.2 | 312 | 104 | 111 | 11 |
| 4 | 3 | 1 month | 46.7 | 70 | 520 | 125.1 | 17 |
| 4 | 5 | 1 day | 39.8 | 131 | 177 | 49.4 | ‡ |
Patients 1 and 5 did not achieve the desired AT activity level and therefore were not included in calculating the median values reported in this paper.
Patient 1 did not achieve the targeted AT activity level despite being on rAT for 147 hours.
Patient 2 did not achieve the targeted AT activity level despite being on rAT for 29 hours.
IU, international units; h, hour; AT, antithrombin.
Figure 1.
AT activity (%), antithrombin activity level measured as a percent of normal; rAT, recombinant antithrombin. The target AT activity range was 80–120% for our patients supported on ECMO. Patient 1 and Patient 5 failed to reach our desired range throughout their rAT therapy. The median time to reach the targeted AT activity range was 11 hours (range, 10–17 hours). ECMO, extracorporeal membrane oxygenation.
DISCUSSION
Of the many important lessons learned, one was the importance of monitoring and the need for frequent adjustments to achieve and maintain target AT activity levels. Our experience suggests that monitoring of AT levels frequently during the beginning of the infusion may be helpful because the use of AT to supplement anticoagulation during ECMO is a more dynamic clinical environment than AT administration for the prevention of thromboembolism. We would suggest following the manufacturer’s recommendation of checking AT activity levels 2 hours after the initiation of therapy and again every 2 hours after subsequent changes to the rAT infusion. Once steady state has been achieved, the manufacturer recommends that AT levels be drawn at least every 24 hours; it is our policy to check steady-state levels at least every 12 hours as a result of rapid elimination of rAT during ECMO. The ability to continuously monitor and adjust the rAT infusion is imperative; therefore, we advise any center using rAT that they check with their laboratory to assure that they are able to run AT levels in a timely fashion. Despite our initial delays in adjustment to the infusion, one notable trend across all of our patients was a lengthy period (median, 11 hours; range, 10–17 hours) between the time of initiation and reaching the target AT level. We believe that this delay in achieving the desired AT level may be the result of the current rAT dosing regimen underestimating the effects of ECMO and its added volume of distribution. It is important to note that the dosing strategy from the manufacturer is for prophylaxis of thromboembolism in surgical adult patients with HD; however, this was the only regimen that is available at this time for a center to use as a starting point for AT replacement therapy using rAT. It is important to note that all of our examination of this case series is just observational and no data were analyzed for statistical significance.
Other authors have published data outlining their use of rAT as a replacement therapy for AT-deficient patients. Tiede and colleagues examined rAT therapy in adult hereditary antithrombin-deficient patients who were undergoing high-risk situations including elective surgery, childbirth, or cesarean delivery. They reported an average infusion of 135.8 IU/kg/day to restore and maintain AT activity in surgical patients, whereas obstetric patients required an average of 279 IU/kg/day to achieve and maintain AT activity at 100% (16). Konkle and associates studied patients with hereditary AT deficiency treated with rAT during surgery. Although they reported infusion rates in 4- to 8-hour increments, when extrapolated to a full 24 hours, they showed an average infusion rate of 170 IU/kg/day to achieve target AT activity levels of 80–120%(17). The average infusion rates reported in these two studies are much lower than the median 480 IU/kg/day to achieve a target goal of 80–120% AT activity found in the current series.
We would offer that our results differ from that reported for multiple reasons. The exposure of the patient’s blood to the artificial surface of the ECMO circuit will activate the hemostatic system leading to both a production and consumption of TAT complexes, which would also result in a diminished half-life for circulating AT (18,19). In the presence of heparin, the half-life of AT is markedly reduced. Therefore, the actual half-life of rAT may be significantly shorter than the 11.6–17.7 hours reported in the prescribing information (15). Therefore, it is very likely that our increased rAT requirement maybe attributable in part to the increased metabolism and elimination of rAT as a result of its interaction with heparin. Finally, patients who are on ECMO have an increased circulating blood volume resulting from additional volume of the extracorporeal circuit, and this increased blood volume is not taken into account when using the current weight-based dosing strategies. Therefore, significant underdosing may occur if the total circulating blood volume (patient + circuit) is not factored into the dosing calculations.
Support for these theories comes from the prescribing information published by the manufacturer, which states that pregnant patients require a higher dose of rAT as a result of their increased clearance and volume of distribution (Table 4). Using this regimen, pregnant patients receive almost twice as much rAT as surgical patients to compensate for the factors listed here. We believe that because both of these situations also occur with patients being supported by ECMO (higher clearance and greater volume of distribution), it is very likely that they will also require a higher dose of rAT to achieve desired AT levels. It is likely as well that this effect will be greater for smaller patients on ECMO because the extracorporeal circuit represents a greater proportion of their total circulating blood volume (patient + circuit). Our findings support this in that the largest patient (15 kg) required 104 IU/kg/day compared with one of the smallest (3 kg) who required 520 IU/kg/day.
Table 4.
rAT dosing table for pregnant patients.
| Dosing Table for Pregnant Patients | |||
|---|---|---|---|
| Loading Dose (IU) | Loading Dose (IU/h) | ||
The different dose factors for pregnant patients and surgical patients which will result in an almost doubling of rAT given to pregnant patients when compared to the same size surgical patients.
IU, international units; h, hour; AT, antithrombin.
For these reasons we believe that the current dosing strategies published may be inadequate for AT replacement for pediatric patients being supported by ECMO. We would advise any center using rAT to determine if it is possible to run AT levels in a timely fashion because monitoring AT activity levels is imperative to appropriately dose this drug. We strongly feel that there needs to be future examination of the pharmacokinetics of rAT in patients supported on ECMO so that a more accurate dosing regimen can be developed.
REFERENCES
- 1.Oliver WC.. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009;13:154–175. [DOI] [PubMed] [Google Scholar]
- 2.Urlesberger B, Zobel G, Zenz W, et al. Activation of the clotting system during extracorporeal membrane oxygenation in term newborn infants. J Pediatr. 1996;129:264–268. [DOI] [PubMed] [Google Scholar]
- 3.Bembea MM, Annich G, Rycus P, et al. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: An international survey. Pediatr Crit Care Med. 2013;14:e77–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson ST, Bjork I, Sheffer R, et al. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin–proteinase reactions. J Biochem. 1992;267:12528–12538. [PubMed] [Google Scholar]
- 5.Rosenberg RD.. Biochemistry of heparin antithrombin interactions, and the physiologic role of this natural anticoagulant mechanism. Am J Med. 1989;87:2s–9s. [DOI] [PubMed] [Google Scholar]
- 6.Grossmann R, Babin-Ebell J, Misoph M, et al. Changes in coagulation and fibrinolytic parameters caused by extracorporeal circulation. Heart Vessels. 1996;11:310–317. [DOI] [PubMed] [Google Scholar]
- 7.Koster A, Chew D, Kuebler W, et al. High antithrombin III levels attenuate hemostatic activation and leukocyte activation during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;126:906–907. [DOI] [PubMed] [Google Scholar]
- 8.Despotis GJ, Joist JH, Hogue CW, et al. The impact of heparin concentration and activated clotting time monitoring on blood conservation. A prospective, randomized evaluation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg. 1995;100:46–54. [DOI] [PubMed] [Google Scholar]
- 9.Avidan MS, Levy JH, van Aken H, et al. Recombinant human antithrombin III restores heparin responsiveness and decreases activation of coagulation in heparin-resistant patients during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;130:107–113. [DOI] [PubMed] [Google Scholar]
- 10.Guzzetta NA, Miller BE, Todd K, et al. Clinical measures of heparin’s effect and thrombin inhibitor levels in pediatric patients with congenital heart disease. Anesth Analg. 2006;103:1131–1138. [DOI] [PubMed] [Google Scholar]
- 11.Newall F, Ignjatovic V, Summerhayes R, et al. In vivo age dependency of unfractionated heparin in infants and children. Thromb Res. 2009;123:710–714. [DOI] [PubMed] [Google Scholar]
- 12.Urlesberger B, Zobel G, Zenz W, et al. Activation of the clotting system during extracorporeal membrane oxygenation in term newborn infants. J Pediatr. 1996;129:264–268. [DOI] [PubMed] [Google Scholar]
- 13.Niebler RA, Christensen M, Brenes R, et al. Antithrombin replacement during extracorporeal membrane oxygenation. Artif Organs. 2011;35:1024–1028. [DOI] [PubMed] [Google Scholar]
- 14.Thrombate III prescribing information. GTC Biotherapeutics, Inc., October 2012. Available at: www.thrombate.com. Accessed August 1, 2013.
- 15.ATryn prescribing information. GTC Biotherapeutics, Inc., November 2010. Available at: www.atryn.com. Accessed August 1, 2013.
- 16.Tiede A, Campbell T, Shaffer DW, et al. Antithrombin alfa in hereditary antithrombin deficient patients: A phase 3 study of prophylactic intravenous administration in high risk situations. Thromb Haemost. 2008;99:616–622. [DOI] [PubMed] [Google Scholar]
- 17.Konkle BA, Bauer KA, Weinstein R, et al. Use of recombinant human antithrombin in patients with congenital antithrombin deficiency undergoing surgical procedures. Hemostasis. 2003;43:390–394. [DOI] [PubMed] [Google Scholar]
- 18.Gruenwald CE, Manlhiot C, Crawford-Lean L, et al. Management and monitoring of anticoagulation for children undergoing cardiopulmonary bypass in cardiac surgery. J ECT. 2010;42:29–19. [PMC free article] [PubMed] [Google Scholar]
- 19.Stammers AH, Willett L, Fristoe L, et al. Coagulation monitoring during extracorporeal membrane oyxgenation: The role of thrombelastography. J ECT. 1995;27:137–145. [PubMed] [Google Scholar]