Abstract:
Cardioplegia is an integral and essential method of myocardial protection for patients of all ages requiring cardiac surgery in which the heart must be stopped. Numerous cardioplegia solutions and delivery methods have been developed. The del Nido cardioplegia solution has been in use for 18 years at Boston Children’s Hospital. This is a unique four parts crystalloid to one part whole blood formulation that is generally used in a single-dose fashion. Although the formulation was originally developed for use in pediatric and infant patients, its use for adult cardiac surgery has been expanding. National and international inquiries to our institution regarding this cardioplegia have been increasing over the last 2 years. We present the developmental history, supporting theory, and current protocol for use of what is now referred to as del Nido cardioplegia.
Keywords: del Nido solution, cardioplegia, myocardial protection, Boston Children’s Hospital, pediatrics, infants
Cardioplegia is an integral and essential method of myocardial protection for patients of all ages requiring cardiac surgery in which the heart must be stopped. Researchers at the University of Pittsburgh (Pittsburgh, PA) developed a novel formulation for myocardial protection in the early 1990s. This teamled by Pedro del NidoHung Cao-DanhK. Eric Sommersand Akihiko Ohkadoeventually patented this solution (1). Modifications have been made to the original solution and it is now referred to as del Nido cardioplegia in the literature and in clinical practice. It is available from several compounding companies in the United States or it can be prepared by an in-house pharmacy because the University of Pittsburgh patent has now expired. National and international inquiries to our institution regarding this cardioplegia have been increasing over the last 2 years. We present the developmental history, supporting theory, and current protocol for use of del Nido cardioplegia at Boston Children’s Hospital.
HISTORY
Cardioplegia for infant and pediatric patients was originally the same as that used for adults and was simply adjusted for volume, flow, and pressure (2). St. Thomas’ Hospital cardioplegia solution (Plegisol; Abbott Laboratories, Chicago, IL) was widely used in the 1980s and 1990s in this fashion (3). This is still the case at many centers throughout North America and Europe. Researchers at the University of Pittsburgh recognized the need for a cardioplegia solution that more specifically addressed the needs and differences of the immature heart.
The differences and requirements of immature myocardium have been described in contradictory terms. The immature heart has been described both as more tolerant to ischemia (4–6) and less so (7,8). A 1989 study by Kempsford and Hearse (9) may have explained this contradiction by imparting that the efficacy of cardioplegia in the immature myocardium may be more related to the cardioplegia solution than the physiology of the neonatal heart. However, the contradictions continue with St. Thomas’ solution being shown as both effective in the neonatal heart (10,11) and ineffective (7). Furthermore, the neonatal heart has been shown experimentally to prefer single-dose cardioplegia (10,12,13), whereas others have concluded no difference when compared with multidose cardioplegia (14).
Contradictions may be expected in the cited studies because all were animal models with varying and/or undefined delivery protocols. The University of Pittsburgh researchers focused on the increasingly sophisticated and accurate measurements of intracellular calcium and its regulation, myocardial high-energy phosphates such as adenosine triphosphate (ATP), lactate production, and intracellular buffering when developing their cardioplegia. Although many of the concepts were developed in rodent models, the efficacy of protection and applicability to hearts from different age animals was also tested in large animal models.
SUPPORTING THEORY
Normal contractile function in its most basic form relies on high-energy phosphates, maintenance of intracellular pH, and ionic and cell membrane homeostasis, all contributing to aerobic metabolism. A disruption of any of these may lead to irreversible damage after myocardial ischemia (15). Furthermore, the promotion of anaerobic glycolysis, scavenging of oxygen-free radicals, and prevention of intracellular calcium accumulation are thought to be germane in preserving function during the arrest period (15,16). Cardioplegia solutions generally rely on metabolic arrest coupled with hypothermia to address these concerns (17). The most commonly used method to achieve contractile arrest is by providing a high concentration of potassium ions into the extracellular space. Although the advantage of this strategy is its simplicity and the rapid onset of arrest, washout of the potassium-containing solution and the fact that potassium causes cardiomyocyte depolarization are the main drawbacks of this approach. From early on it was recognized that hyperpolarizing cells during ischemia slowed down the rate of energy consumption and intracellular accumulation of the detrimental calcium ion. For this purpose, polarizing agents such as procaine and lidocaine, along with calcium-competing ions such as magnesium, were added to the formulation of these solutions. The addition of red blood cells in varying amounts evolved from the concept of oxygen and energy delivery during ischemia, although the exact mechanism by which red blood cells aid in myocardial protection remains controversial. Numerous cardioplegia solutions and protocol combinations based on these principles have been developed (18). It is beyond the scope of this article to describe the many variations. However, discussion is provided that describes the manner in which del Nido cardioplegia solution addresses these concerns. It is important to note that although the ingredients were developed with the immature myocardium in mind, its use in adult patients with acquired cardiovascular disease has been reported (New York Presbyterian Hospital and Cleveland Clinic Foundation, personal communication). The del Nido cardioplegia solution is used on all patients at our institution from neonates to older adults with congenital heart disease.
Base Solution of Plasma-Lyte A
The del Nido cardioplegia contains a base solution of Plasma-Lyte A, which has an electrolyte composition similar to the extracellular fluid (Baxter Healthcare Corporation, Deerfield, IL). The concentrations of electrolytes before the addition of cardioplegic additives are 140 mEq/L sodium, 5 mEq/L potassium, 3 mEq/L magnesium, 98 mEq/L chloride, 27 mEq/L acetate, and 23 mEq/L gluconate. The manufacturer lists a pH value of 7.4. The cardioplegia additives to this base solution are listed Table 1. This formulation serves as the crystalloid component, which is mixed with blood in a ratio of four parts crystalloid to one part fully oxygenated patient whole blood (usually obtained from the bypass circuit). It is important to note that there is no calcium in the base solution. The final calcium concentration of this cardioplegia can be described as trace because 20% of the delivered volume contains patient blood. This is an important consideration because trace calcium in cardioplegic solutions has been shown to be preferable as compared with acalcemic or normal levels (15,19–21).
Table 1. Crystalloid component of del Nido cardioplegia solution.
| 1 L Plasma-Lyte A base solution to which the following are added: |
| Mannitol 20%, 16.3 mL |
| Magnesium sulfate 50%, 4 mL |
| Sodium bicarbonate 8.4%, 13 mL |
| Potassium chloride (2 mEq/mL), 13 mL |
| Lidocaine 1%, 13 mL |
Mannitol
Myocardial injury during cardioplegic arrest and subsequent reperfusion may be in part the result of oxygen-free radicals including superoxide anion, hydrogen peroxide, and hydroxyl. These radicals are normally countered enzymatically within the cell but this is inhibited during myocardial arrest (16,22). Additionally, myocardial edema has also been implicated in postischemic myocardial impairment. Hyperosmotic mannitol has been shown to both scavenge free radicals and reduce myocardial cell swelling (23). To note, at Boston Children’s Hospital, we also deliver .5 g/kg mannitol to the bypass circuit shortly before the removal of the cross-clamp owing to these properties and also its osmotic diuretic effects.
Magnesium Sulfate
Myocardial function is intimately related to intracellular calcium concentration. The normal calcium flux in the myocardium increases intracellular calcium for contraction and decreases it for relaxation. If calcium is allowed to accumulate in the myocardium, relaxation may be interrupted and diastolic stiffness with poor recovery may result (24). Magnesium has been shown to be a natural calcium channel blocker (25). This effect is likely how magnesium has been shown to improve ventricular recovery in hypothermic cardioplegia solutions when coupled with a low calcium level (19,26).
Sodium Bicarbonate
Aerobic metabolism is not usually possible for the entire myocardial arrest period. Therefore, anaerobic glycolysis must be supported. Anaerobic glycolysis and its production of ATP has been shown to be inhibited by excess hydrogen ion accumulation (17,27,28). The del Nido cardioplegia mix incorporates sodium bicarbonate as a buffering solution to scavenge excess hydrogen ions and to assist in maintaining intracellular pH. It is also important to note that red blood cells contain a high concentration of carbonic anhydrase, an enzyme that facilitates the scavenging of hydrogen ions with bicarbonate to generate carbon dioxide and water. This property of red blood cells may in fact be its most important role in cardioplegia.
Potassium Chloride
Hyperkalemia is the most common arresting method for cardiac surgery because it provides rapid arrest (29,30) and reliable recovery but it has been shown to have limitations. It provides a depolarized arrest. Depolarized arrest has been associated with poor myocardial recovery as a result of intracellular sodium and calcium accumulation during the arrest period (30). Lidocaine likely inhibits these negative effects while enhancing the period of time in which electromechanical activity is absent. The potassium level in del Nido cardioplegia is 24 mEq/L (see subsequent equation).
* Potassium added to the plasmalyte base solution 13 ml or 26 mEq. Total solution volume 1059ml.
** 5mEq is the potassium concentration in the Plasmalyte base solution used to formulate the del Nido solution.
*** 4.5 mEq/L is an estimate of the patients serum potassium level
Lidocaine
Lidocaine is classified as a sodium channel blocker and is a frequently used antiarrhythmic. Sodium channel blockade increases the refractory period of the cardiac myocyte (24). When cardioplegia is given in an ideal environment without washout, this action is prolonged because the lidocaine remains in adequate concentrations to continually affect the myocardium. Additionally, sodium channel blockade helps counteract the negative effects of a hyperkalemic depolarized arrest by polarizing the cell membrane to some degree and preventing sodium and calcium accumulation within the cell. Depolarized arrest can allow for sodium and calcium accumulation through exchange mechanisms and blocking the sodium channels helps prevent this (30). A 2009 study by O’Brien et al. (31) showed that del Nido cardioplegia reduced calcium accumulation during myocardial ischemia in a setting of a depolarized arrest. It may be helpful to note that del Nido cardioplegia can therefore be classified as a modified depolarizing agent, primarily as a result of the properties of lidocaine and magnesium.
Patient Blood Additive
The del Nido cardioplegia is delivered with 20% by volume fully oxygenated patient blood, which supports aerobic metabolism for a finite period of time and provides buffering properties to promote anaerobic glycolysis as well. Blood in cardioplegia has also been shown to improve coronary perfusion during cardioplegia delivery (32). Furthermore, studies have shown blood cardioplegia to preserve myocardial metabolism and function (33) and result in less metabolic ischemic stress and reperfusion injury when compared with asanguineous cardioplegia in a varied population of patients undergoing congenital heart surgery (34).
The cardioplegia hematocrit can be calculated by multiplying the blood component hematocrit (drawn from the bypass circuit) by the 20% portion. The delivery hematocrit of del Nido cardioplegia will be 6% if the 20% blood portion has a hematocrit of 30% (.3 bypass circuit hematocrit +.2 portion of cardioplegia mix = hematocrit of 6%).
Hypothermia
Decreasing the myocardial metabolic rate with hypothermia is a common practice for cardioplegia delivery (24,29,35). Hypothermia decreases oxygen and high-energy phosphate consumption while providing its own additional cardioplegic effect at low temperatures (5). In our circuit, the del Nido cardioplegia passes through a cooling coil in ice. The delivery temperature with this simple method is usually 8–12°C.
BOSTON CHILDREN’S HOSPITAL CARDIOPLEGIA PROTOCOL
The Circuit
We use a recirculating system for cardioplegia delivery at Boston Children’s Hospital. This system does not connect to the standard bypass circuit nor does it draw blood from it directly. Our recirculating system has a dead space volume of just 1 mL (plus the root needle volume) and this is an important design consideration. The dead space volume in a custom circuit used for smaller patients can actually hinder the performance of a cardioplegia because the dead space volume may equate to a significant percentage of the delivery dose while harboring poorly mixed and room temperature cardioplegia solution.
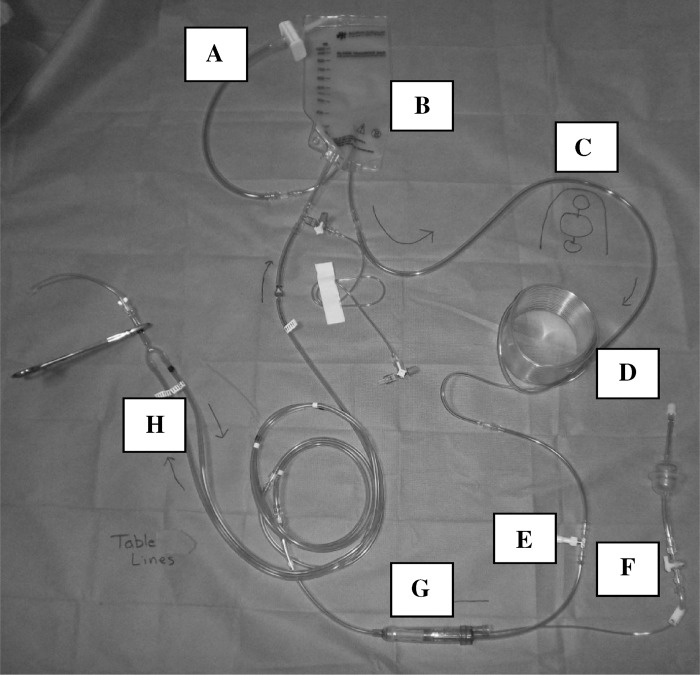
The custom disposable tubing set we use (Figure 1) consists of a cardioplegia bag connection linewith integral .2-μm crystalloid filter, cardioplegia reservoir, pump loop, cooling coil, temperature monitoring site, pressure monitoring site, and a bubble trap with an integral 270-μm filter, all with tubing that connects to sterile lines at the surgical field. We use several different custom tubing packs for our bypass circuits. Each pack contains the same sterile cardioplegia table lines, which are connected to the custom cardioplegia tubing set during preparation of the heart–lung machine. Final delivery is most often through a 14-Fr or 18-Fr aortic root needle (Medtronic Inc., Minneapolis, MN). Coronary perfusers are used as needed.We do not routinely use retrograde coronary sinus delivery.
Figure 1.
(A) Cardioplegia bag connection line with an integral .2-μm crystalloid filter. (B) Cardioplegia reservoir. (C) Pump loop. (D) Cooling coil. (E) Temperature monitoring site. (F) Pressure monitoring site. (G) Bubble trap with an integral 270-μm filter. (H) Sterile field table lines shown connected to circuit.
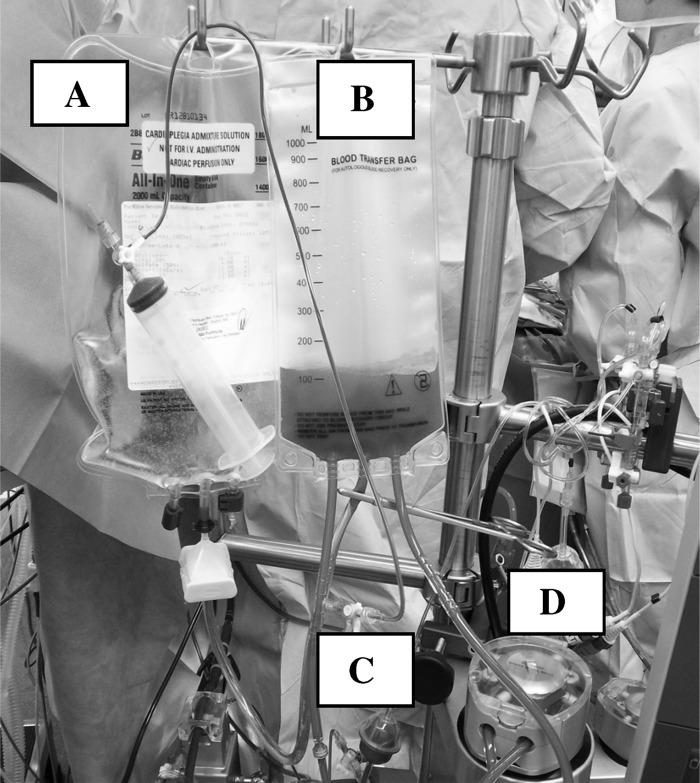
The prime of the entire cardioplegia circuit (table lines connected to cardioplegia tubing set) is 125 mL. We maintain a minimum 25-mL volume in the cardioplegia reservoir to prevent air entrainment. Therefore, we use a prime volume of 150 mL for calculation purposes. Figure 2 shows our custom cardioplegia set in use.
Figure 2.
(A) Cardioplegia bag with crystalloid component as provided by pharmacy. (B) Cardioplegia reservoir bag where the 4:1 (crystalloid: blood) components are mixed and recirculated. (C) Stopcock, line, and syringe used to inject bypass circuit blood into the cardioplegia circuit. (D) Cardioplegia roller head.
Dose
The del Nido cardioplegia solution is generally given as a single 20-mL/kg dose. The maximum arresting dose is usually limited to 1 L for patients larger than 50 kg. Additional cardioplegia volume may be given for hypertrophied hearts, those with aortic insufficiency, or those with known coronary disease based on the effectiveness of the initial dose and surgeon preference. A smaller arresting dose of 10 mL/kg may be used for procedures requiring a cross-clamp time less than 30 minutes. Subsequent doses are not normally given except for the rare occurrence of electrical activity or for exceptionally long cross-clamp times (greater than 3 hours) at the surgeon’s discretion.
It is important to mix the crystalloid cardioplegia component correctly with the patient’s blood to achieve the desired ratio of parts: four parts crystalloid to one part patient blood. Clinically, we multiply the patient’s weight in kilograms by 20 mL/kg to get the total cardioplegia dose volume. We then add the circuit prime volume of 150 mL and divide the total by five parts to arrive at the volume of the blood component. Then, by taking the delivery dose volume, adding 25 mL to account for the minimum reservoir level, and subtracting the blood component, we arrive at the crystalloid volume that should be in the cardioplegia reservoir before the addition of blood. Fully oxygenated patient blood is drawn off the bypass circuit once the patient has been on the heart–lung machine for at least 1–2 minutes. This precise volume is drawn from the bypass circuit through a manifold syringe and then injected into the separate recirculating cardioplegia delivery system. The result is that only the 20-mL/kg cardioplegia dose, plus the reservoir minimum of 25 mL, is in the cardioplegia reservoir bag recirculating awaiting delivery. Tables 2 and 3 include calculation examples. To note, additional doses only require the perfusionist to calculate the proper amount of blood and crystalloid that should be allocated to the delivery reservoir because the circuit prime has already been accounted for.
Table 2. Cardioplegia calculation for a 3-kg patient.
| • 3 kg × 20 mL/kg = 60 mL cardioplegia dose volume |
| • 60-mL dose volume + 150-mL prime volume = 210-mL total system volume |
| • 210 total system volume/5 = 42-mL blood component volume |
| • (60-mL dose volume + 25-mL minimum reservoir volume) − (42-mL blood component volume) = 43-mL crystalloid cardioplegia volume in the reservoir before the addition of blood that will result in the proper 4:1 mixture for this 3-kg patient |
| • The perfusionist will have the proper dose amount and mixture for this patient by recirculating 43 mLin the cardioplegia reservoir before bypass and then adding 42 mL of patient whole blood once on bypass. Once the 60mL cardioplegia dose is given, the user would be left with the minimum operating level of 25mL in the cardioplegia reservoir. |
Table 3. Cardioplegia calculation for a 50-kg patient.
| • 50 kg × 20 mL/kg = 1000-mL cardioplegia dose volume |
| • 1000-mL dose volume + 150-mL prime volume = 1150-mL total system volume |
| • 1150-mL total system volume/5 = 230-mL blood component volume |
| • (1000-mL dose volume + 25-mL minimum reservoir volume) − (230-mL blood component volume) = 795-mL crystalloid cardioplegia volume in the reservoir before the addition of blood that will result in the proper 4:1 mixture for this 50-kg patient |
| • The perfusionist will have the proper dose amount and mixture for this patient by recirculating 795 mL in the cardioplegia reservoir before bypass and then adding 230 mL of patient whole blood once on bypass. Once the 1000-mL cardioplegia dose is given, the user would be left with the minimum operating level of 25 mL in the cardioplegia reservoir. |
Delivery
Delivery is initiated with the surgeon moving the clamp on the table lines from the outlet to the return limb. Flow is controlled at the heart–lung machine by the perfusionist. We generally give the 20-mL/kg cardioplegia dose over 1–2 minutes with a system pressure of 100–200 mmHg. We do not monitor aortic root pressure although the surgeon monitors aortic root distention closely during delivery to prevent capillary damage from high shear forces with too rapid a delivery. This method results in a cardioplegia delivery flow rate of 10–20 mL/kg/min in infants and toddlers. In other words, it is clinically rather simple to estimate the initial delivery rate by taking half of the arresting dose volume and using that as the flow rate. For example, in a 12-kg patient, the dose volume would be 240 mL (20 mL/kg cardioplegia × 12 kg). An initial infusion rate of half that (120 mL/min) would be a proper estimate to achieve an infusion rate of 1–2 minutes with a system pressure of 100–200 mmHg, at least with our custom cardioplegia circuit. The initial flow rate for root administration is limited to 300 mL/min in larger patients. The initial flow rate for ostial administration can be classified as “barely on.” Cardioplegia flow for all methods is adjusted from the initial flow based on the surgeon’s observation of the heart and electrical activity. Cardioplegia system pressures may be higher with increased flows, smaller root needles, and ostial delivery. These higher system pressures are not linearly related to root pressure, but it is still imperative that the surgeon visually monitor delivery.
SUMMARY
The del Nido cardioplegia solution was originally developed for infant and pediatric patients and has been in use for 18 years at Boston Children’s Hospital. This cardioplegia is generally given as a single 20-mL/kg dose antegrade at 8–12°C through a recirculating delivery system. The unique formulation reduces energy consumption, blocks calcium entry into the intracellular environment, scavenges hydrogen ions, preserves high-energy phosphates, and promotes anaerobic glycolysis during myocardial arrest. O’Blenes et al. (21) has shown experimentally in a senescent rat heart model that del Nido cardioplegia results in lower intracellular calcium levels and less frequent spontaneous contractions. That same center has also shown lower troponin T release in pediatric patients when compared with their adult cardioplegia strategy (31).Most recently, Charette et al. (36) published a single-surgeon retrospective study of matched congenital heart patients with a cross-clamp time greater than 90minutes. That study showed no significant difference in postoperative complications with del Nido cardioplegia as compared with that institution’s prior multidose cardioplegia strategy. We have seen, in our subjective opinion, excellent myocardial outcomes in our congenital heart disease population of newborns through those in their 60s. The use of del Nido cardioplegia has been expanding into adult cardiac centers over the last decade with anecdotally reported success. However, there are no prospective randomized controlled trials of this cardioplegia strategy matched against othermethods with which to compare our results.
ACKNOWLEDGMENTS
The authors are grateful to the Indian Journal of ExtraCorporeal Technology for permitting publication of this work in the Journal of ExtraCorporeal Technology as an invited editorial.
REFERENCES
- 1.Patent 5,407,793. 1995. U.S. Patent and Trademark Office; Alexandria, VA. [Google Scholar]
- 2.Allen BS.. Pediatric myocardial protection: Where do we stand? J Thorac Cardiovasc Surg. 2004;128:11–13. [DOI] [PubMed] [Google Scholar]
- 3.Bilfinger TV, Moeller JT, Kurusz M, Grimson RC, Anagnostopoulos CE.. Pediatric myocardial protection in the United States: A survey of current clinical practice. Thorac Cardiovasc Surg. 1992;40:214–218. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu T, Zund G, Schermerhorn ML, Shinoka T, Minura T, Mayer JE Jr.. Age differences in effects of hypothermic ischemia on endothelial and ventricular function. Ann Thorac Surg. 1995;60:S501–S504. [DOI] [PubMed] [Google Scholar]
- 5.Bove EL, Stammers AH.. Recovery of left ventricular function after hypothermic ischemia: Age-related differences in the isolated working rabbit heart. J Thorac Cardiovasc Surg. 1986;91:115–122. [PubMed] [Google Scholar]
- 6.Baker JE, Boerboom LE, Olinger GN.. Age-related changes in the ability of hypothermia and cardioplegia to protect ischemic rabbit myocardium. J Thorac Cardiovasc Surg. 1988;96:717–724. [PubMed] [Google Scholar]
- 7.Wittnich C, Peniston C, Ianuzzo D, Abel JG, Salerno TA.. Relative vulnerability of neonatal and adult hearts to ischemic injury. Circulation. 1987;76:V156–V160. [PubMed] [Google Scholar]
- 8.Parrish M, Payne A, Fixler DE.. Global myocardial ischemia in the newborn, juvenile, and adult isolated isovolemic rabbit heart. Circ Res. 1987;61:609–615. [DOI] [PubMed] [Google Scholar]
- 9.Kempsford RD, Hearse DJ.. Protection of the immature myocardium during global ischemia. A comparison of four clinical cardioplegic solutions in the rabbit heart. J Thorac Cardiovasc Surg. 1989;97:856–863. [PubMed] [Google Scholar]
- 10.Magovern JA, Pae WE Jr, Waldhausen JA.. Protection of the immature myocardium. J Thorac Cardiovasc Surg. 1988;96:408–413. [PubMed] [Google Scholar]
- 11.Konishis T, Apstein CS.. Comparison of three cardioplegic solutions during hypothermic ischemic arrest in neonatal blood-perfused rabbit hearts. J Thorac Cardiovasc Surg. 1989;98:1132–1137. [PubMed] [Google Scholar]
- 12.Kohman LJ, Veit LJ.. Single-dose versus multidose cardioplegia in neonatal hearts. J Thorac Cardiovasc Surg. 1994;107:1512–1518. [PubMed] [Google Scholar]
- 13.Sawa Y, Matsuda H, Shimazaki Y, et al. Comparison of single dose versus multiple dose crystalloid cardioplegia in neonate. J Thorac Cardiovasc Surg. 1989;97:229–234. [PubMed] [Google Scholar]
- 14.Bove EL, Stammers AH, Gallagher KP.. Protection of the neonatal myocardium during hypothermic ischemia. J Thorac Cardiovasc Surg. 1987;94:115–123. [PubMed] [Google Scholar]
- 15.Jennings RB, Reimer KA.. Lethal myocardial ischemic injury. Am J Pathol. 1981;102:241–255. [PMC free article] [PubMed] [Google Scholar]
- 16.Werns SW, Shea MJ, Lucchesi BR.. Free radicals and myocardial injury: Pharmacologic implications. Circulation. 1986;74:1–5. [DOI] [PubMed] [Google Scholar]
- 17.Ohkado A, Cao-Danh H, Sommers KE, del Nido PJ.. Evaluation of highly buffered low-calcium solution for long-term preservation of the heart: Comparison with the University of Wisconsin solution. J Thorac Cardiovasc Surg. 1994;108:762–771. [PubMed] [Google Scholar]
- 18.Chambers DJ, Hearse DJ.. Developments in cardioprotection: Polarized arrest as an alternative to depolarized arrest. Ann Thorac Surg. 1999;68:1960–1966. [DOI] [PubMed] [Google Scholar]
- 19.Rebeyka IM, Axford-Gatley RA, Bush BG, et al. Calcium paradox in an in vivo model of multidose cardioplegia and moderate hypothermia. J Thorac Cardiovasc Surg. 1990;99:475–483. [PubMed] [Google Scholar]
- 20.Swanson DK, Pasaoglu I, Berkoff HA, Southard JA, Hegge JO.. Improved heart preservation with UW preservation solution. J Heart Transplant. 1988;7:456–467. [PubMed] [Google Scholar]
- 21.O’Blenes SB, Friesen CH, Ali A, Howlett S.. Protecting the aged heart during cardiac surgery: The potential benefits of del Nido cardioplegia. J Thorac Cardiovasc Surg. 2011;141:762–769. [DOI] [PubMed] [Google Scholar]
- 22.Braunwald E, Kloner RA.. Myocardial reperfusion: A double-edged sword? J Clin Invest 1985;76:1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell WJ, DiBona DR, Flores J.. The protective effect of hyperosmotic mannitol in myocardial ischemia and necrosis. Circulation. 1976;54:603–615. [DOI] [PubMed] [Google Scholar]
- 24.Larach DR, Solina AR.. Cardiovascular drugs. In: Hensley FA, Martin DE, eds. A Practical Approach to Cardiac Anesthesia. 2nd ed. Boston: Little, Brown and Company; 1995:32–95. [Google Scholar]
- 25.Iseri LT, French JH.. Magnesium: Nature’s physiologic calcium blocker. Am Heart J. 1984;108:188–193. [DOI] [PubMed] [Google Scholar]
- 26.Brown PS, Holland FW, Parenteau GL, Clark RE.. Magnesium ion is beneficial in hypothermic crystalloid cardioplegia. Ann Thorac Surg. 1991;51:359–367. [DOI] [PubMed] [Google Scholar]
- 27.del Nido PJ, Wilson GJ, Mickle DAG, et al. The role of cardioplegic solution buffering in myocardial protection. J Thorac Cardiovasc Surg. 1985;89:689–699. [PubMed] [Google Scholar]
- 28.Rovetto MJ, Lamberton WF, Neely JR.. Mechanisms of glycolytic inhibition in ischemic rat hearts. Circ Res. 1975;37:742–751. [DOI] [PubMed] [Google Scholar]
- 29.Guyton RA.. The myocardium: Physiology and protection during cardiac surgery and cardiopulmonary bypass. In: Mora CT, ed. Cardiopulmonary Bypass. New York: Springer-Verlag; 1995:21–39. [Google Scholar]
- 30.Dobson GP, Jones MW.. Adenosine and lidocaine: A new concept in nondepolarizing surgical myocardial arrest, protection, and preservation. J Thorac Cardiovasc Surg. 2004;127:794–805. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien JD, Howlett SE, Burton HJ, O’Blenes SB, Litz DS, Friesen CLH.. Pediatric cardioplegia strategy results in enhanced calcium metabolism and lower serum troponin T. Ann Thorac Surg. 2009;87:1517–1524. [DOI] [PubMed] [Google Scholar]
- 32.Suaudeau J, Shaffer B, Dagget WM, Austen WG, Erdman AJ.. Role of procaine and washed red cells in the isolated dog heart perfused at 5 degrees C. J Thorac Cardiovasc Surg. 1982;84:886–896. [PubMed] [Google Scholar]
- 33.Amark K, Berggren H, Bjork K, et al. Blood cardioplegia provides superior protection in infant cardiac surgery. Ann Thorac Surg. 2005;80:989–994. [DOI] [PubMed] [Google Scholar]
- 34.Caputo M, Modi P, Imura H, et al. Cold blood versus cold crystalloid cardioplegia for repair of ventricular septal defects in pediatric heart surgery: a randomized controlled trial. Ann Thorac Surg. 2002;74:530–534. [DOI] [PubMed] [Google Scholar]
- 35.Chambers DJ.. Polarization and myocardial protection. Curr Opin Cardiol. 1999;14:495–500. [DOI] [PubMed] [Google Scholar]
- 36.Charette K, Gerrah R, Quaegebeur J, et al. Single dose myocardial protection utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion. 2012;27:98–103. [DOI] [PubMed] [Google Scholar]