Abstract:
The goal of this cardiopulmonary bypass (CPB) quality improvement initiative was to maximize hemoglobin nadir concentration by minimizing hemodilution and, in turn, eliminating allogeneic blood product transfusion. The effects of transitioning from “one-size-fits-all” to “right-sized” oxygenators, reservoirs, and arterial–venous tubing loops were evaluated through a 2-year retrospective review of 3852 patient perfusion records. Using a sizing algorithm, derived from manufacturers’ recommendations, we were able to create individualized “right-sized” extracorporeal circuits based on patient body surface area, cardiac index, and target blood flows. Use of this algorithm led to an increase in the percent of algorithm-recommended smaller oxygenators being used from 39% to 63% (p < .01) and an increase in average hemoglobin nadir from 8.38 to 8.76 g/dL (p < .01). Decreased priming volumes led to increased hemoglobin nadir and decreases in allogeneic blood transfusion (p = .048). Patients with similar body surface areas who previously were exposed to larger oxygenators, reservoirs, and arterial–venous loops were now supported with smaller circuits as a result of the use of the right-sized algorithm. Adjustments to the algorithm were made for unique patients and procedural situations including age, gender, and length and type of procedure. Larger heat exchanger surface area oxygenators were used for circulatory arrest procedures as a result of the need for increased heat exchange capability. Despite the generally higher costs of smaller circuits, reduced transfusion-related expenditures and decreased exposure risks justify the use of smaller circuit components. This quality improvement initiative demonstrated that as an integral part of a multidisciplinary, multimodal blood conservation effort, the use of the “right-sized” circuit algorithm can help to elevate hemoglobin nadir during CPB and eliminate allogeneic blood transfusions to patients undergoing CPB.
Keywords: cardiopulmonary bypass, oxygenator, perfusion index, extracorporeal circuit, hemodilution
Dilutional anemia and coagulopathy have long been negatively linked to the conduct of cardiopulmonary bypass (CPB). Patients with small body surface areas (BSA) and female patients are subject to greater hemodilution during CPB because of their lower circulating blood volume and the use of relatively constant extracorporeal circuit prime volumes (1). Larger prime volumes result in lower hemoglobin (Hgb) nadir levels, which have been associated with increased in-hospital stay and long-term mortality, increased troponin release, longer mechanical ventilation times, and decreased estimated glomerular filtration rates (2–4). Additionally, a strong inverse relationship has been shown between Hgb nadir and massive blood transfusions (MBT), indicating reductions in hemodilution may reduce the risk for MBT (5). Transfusion of stored blood has been linked to an increased risk of complications and mortality in both the postoperative and long-term (>5-year) periods (6,7).
Perfusion techniques such as retrograde autologous priming, vacuum-assisted venous autologous priming, and circuit miniaturization have been developed to reduce crystalloid prime volumes, thereby raising Hgb nadir levels on bypass (1,8). Furthermore, minimization of CPB circuits has been indicated as a “Class 1, Level of Evidence A” guideline to reduce hemodilution and avoid allogeneic blood transfusions (9,10). Minimizing circuits proved to increase the Hgb nadir and reduce transfusion requirements by McCusker and associates (11). In an attempt to apply the results of these techniques in our institution, a quality improvement initiative was undertaken to “right-size” and minimize adult patient circuits.
Our quality improvement project was designed to decrease the prime volume of the CPB circuit by matching oxygenators, reservoirs, and arterial–venous (A–V) loops to patients based on patient blood flow requirements during CPB. It was hypothesized that by reducing the prime volumes for smaller BSA patients, Hgb nadir would increase and, in turn, help to reduce allogeneic red blood cell and blood product transfusions. This study used an observational analysis of 3852 adult cardiac surgical patients who underwent CPB in our institution between January 1, 2011 and March 31, 2013.
METHODS
Extracorporeal Circuit
The heart–lung machine (HLM) used in the bypass procedures was the Sorin S5® with an arterial 1/2-inch ID tubing roller pump (Sorin, Arvada, CO). The S5 HLM was connected to the Sorin Data Management System® (DMS). The adult CPB extracorporeal circuit was composed of Capiox® RX or FX oxygenators (Terumo Cardiovascular Systems, Ann Arbor, MI) with R30 (3000 mL) or R40 (4000 mL) reservoirs and X-Coating® tubing packs with either 1/2 × 3/8-inch or 3/8 × 3/8-inch A-V loops. CPB cardiotomy suction blood was returned to the reservoir.
Patients were cooled to 18–34°C depending on the procedure. An alpha-stat ventilation regimen was used above 32°C. Below 32°C, pH-stat was used during rapid cooling and then alpha-stat was initiated at the target cold temperature and during rapid warming. Frequently, 10% of the patient’s blood volume was collected for acute normovolemic hemodilution before heparinization. Patient heparin loading dose varied between 350 and 450 U/kg to achieve an activated clotting time > 500 seconds measured by the iSTAT® Celite® Activated Clotting Time test (Abbott Point of Care, Princeton, NJ). A protamine dose equivalent to the heparin loading dose was used for heparin reversal.
Step One
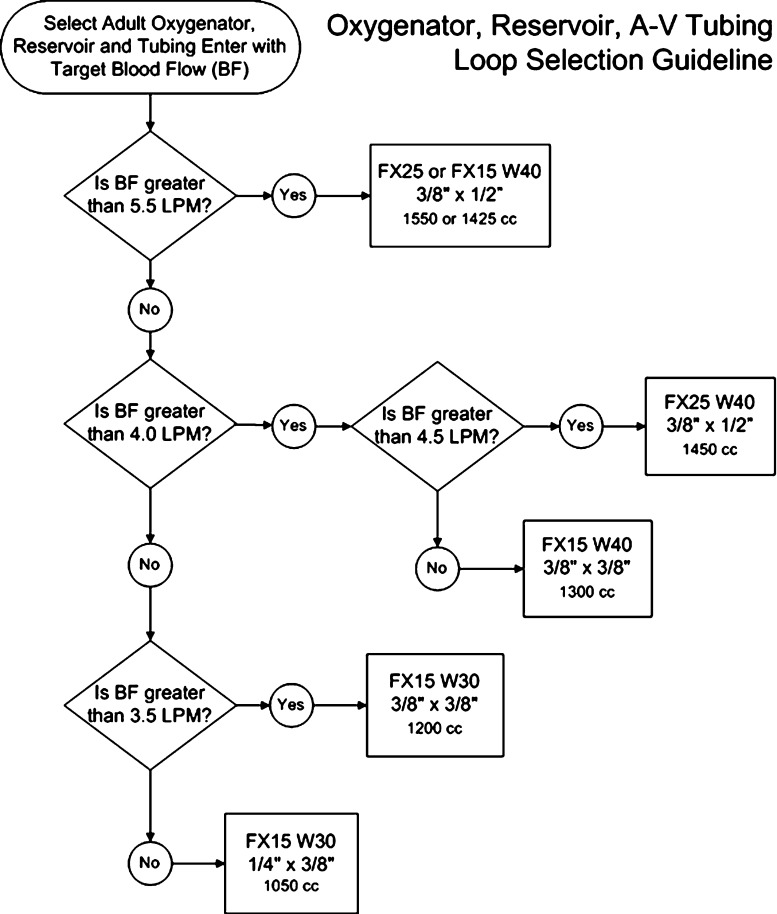
Perfusionists were provided the algorithm in Figure 1 with the aim to “right-size” circuits and eliminate transfusions. The algorithm was posted in the perfusion work area where extracorporeal circuits (ECCs) are tailored to each patient and assembled just before the procedure. The algorithm was updated over the study period as we improved our practice.
Figure 1.
Flowchart depicts the selection process for the right-sized oxygenator, reservoir, and tubing arterial–venous loop. FX25 W40, FX15 W40, and FX15 W30 are Terumo trademark names for oxygenators and reservoirs. The volumes are minimal crystalloid circuit prime volumes before autologous priming.
Step Two
The next improvement effort of the study was made in December 2011. Our team discontinued using the Terumo Capiox® RX25 R40 and RX15 R40 oxygenators, with external arterial filters, and began to use the Capiox® FX25 R40, FX15 R40, and FX15 R30 (Terumo Cardiovascular Systems) oxygenators with integrated arterial filters as a means of reducing prime volumes. Vacuum-assisted venous drainage was available on all cases and recommended for use with the 3/8-inch–3/8-inch A-V loop.
Prime volumes varied based on oxygenator, reservoir, and A–V loop selections. The FX15 R30 and FX15 R40 oxygenator and roller pump tubing setups required 750 mL of fluid, whereas the FX25 R40 was primed with 870 mL. The 3/8-inch–3/8-inch A-V loop added 350 mL of prime, whereas the 3/8-inch–1/2-inch loop added 500 mL. The oxygenators and A–V loops could be selected separately, allowing for a more prescriptive circuit and prime volume control (Figure 1). One challenge to volume minimization with our circuit is that our A–V loop is 16 feet in length to allow adequate tubing length for femoral cannulation. Lines were shortened at the surgical field depending on cannulation needs.
Patient blood flow requirements were estimated based on each patient’s BSA and a target cardiac index of 2.4 L/min/m2. Each perfusionist had the liberty of tailoring the specific circuit based on additional patient characteristics, creating some variability in ECC selection. For example, patients undergoing procedures requiring deep hypothermic circulatory arrest were generally prescribed the FX25 oxygenator, the R40 reservoir, and the 3/8-inch–1/2-inch loop, to use higher cardiac indices and the higher heat exchanger performance factor to augment cooling and rewarming times.
Step Three
On a quarterly basis, information from the perfusion and hospital electronic medical records of all adult patients undergoing CPB were reviewed to assess the perfusion team’s compliance with the algorithm and evaluate the effect of prescriptive oxygenator sizing on blood product use and the observed Hgb nadir. The Hgb nadir generally occurred in the first 10 minutes after CPB initiation. Institutional patient databases (Amalga Unified Intelligence System; Microsoft Corporation, Redmond, WA) were queried to quantify blood product use for each patient during their operating room and intensive care encounters (12).
The chart review project was qualified for exemption as a quality improvement project by the Mayo Clinic Institutional Review Board. At the end of each quarter, all adult CPB patient pump record information from the DMS was exported to Microsoft Excel® (Microsoft Corporation) files. The Excel® files were imported into a Microsoft Access® database. Hospital blood management database query results were also imported into the Access® database. Access® queries were conducted and the results exported to Excel® for uploading to JMP® Statistical Software (JMP 10.0, Cary, NC) for analysis. Table 1 lists the query input and output parameters created for each quarterly report.
Table 1.
Parameters collected and evaluated in the quality improvement project on a quarterly basis.
| Input parameters | Output parameters |
|---|---|
| Age, gender, BSA | Demographic profiles |
| Target perfusion index and blood flow oxygenator and reservoir model | Percent correct selection of oxygenator model by patient size and target blood flow |
| Pre-CPB Hgb and Hgb nadir in the operating room | Hgb nadir by date, patient size, and oxygenator model |
| Blood product (RBC, platelet packs, FFP units, cryoprecipitate units) usage during surgery and in the ICU | Blood product use by date, patient size, and oxygenator reservoir model |
BSA, body surface area; CPB, cardiopulmonary bypass; Hgb, hemoglobin concentration (g/dL); RBC, red blood cell; FFP, fresh-frozen plasma; ICU, intensive care unit.
For the quarterly reports, patients were divided into target blood flow (BF), BSA, and oxygenator model groups for trending and analysis. When data were missing, the patient record was not included in the analysis. Demographic and percent result contingency tables were created and analyzed using χ2 analysis. Statistical process run charts were created and analysis of variance with Tukey comparisons was used to compare Hgb nadir and blood product use by patient groups. Statistical significance was set at p < .05.
RESULTS
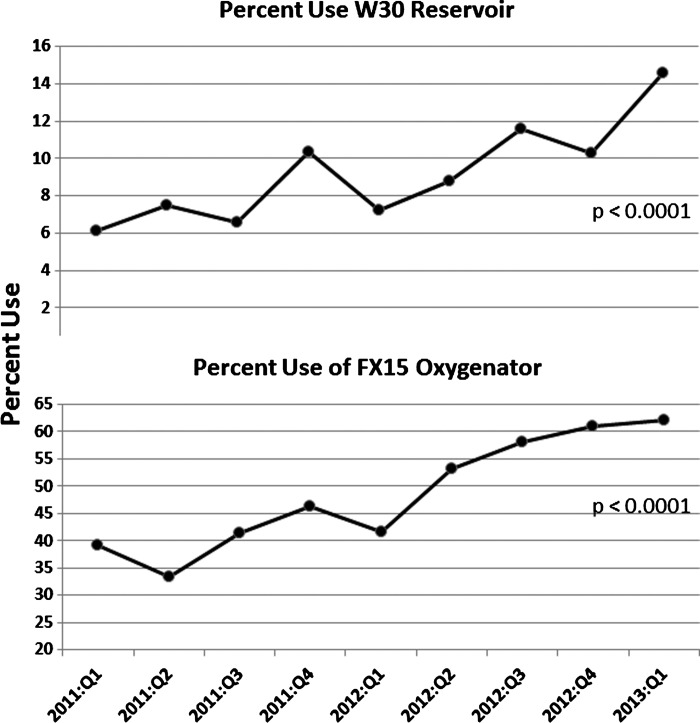
Table 2 presents the distribution of patients undergoing CPB by surgical procedure and gender. Figure 2 plots the percent use of FX15 and R30 devices, indicating an overall significant increase (p < .0001) in adoption. The current use of FX15 oxygenators is approximately 63% and rising. Increased use of the FX15 and R30 circuit components was associated with decreased prime volumes and thus contributed to less hemodilutional anemia as shown by the rise in the average Hgb nadir.
Table 2.
Distribution of patients by surgical procedure groups.*
| Distribution of Patients by Procedure and Gender | ||
|---|---|---|
| Procedure Group (no.) | Includes Procedures | Female |
| Valve (1696) | Mitral, aortic, pulmonary and tricuspid: repairs and/or replacements | 39% |
| CABG (635) | Coronary artery and internal mammary artery bypass and coronary unroofing procedures | 21% |
| Adult congenital (503) | Ebstein repair, tetralogy repair, ASD closure, revision Glenn, Fontan, and septal myectomy procedures | 44% |
| Aorta (331) | Aortic root enlargements and replacements, hemi- and total arch replacement, descending aorta replacement procedures with and without DHCA | 30% |
| Transplant/VAD/other (225) | Heart, lung and heart–lung transplant, placement of left ventricular assist devices | 30% |
| Valve + CABG (462) | Valve procedures with coronary artery bypass procedures | 39% |
| Reoperation (497; 13%) | First to fifth time redo mediastinal incisions | 34% |
A total of 3852 total procedures with 34% of patients being female. Redo procedures are also 34% female.
CABG, coronary artery bypass graft; VAD, ventricular assist device; ASD, atrial septal defect; DHCA, deep hypothermic circulatory arrest.
Figure 2.
The percent use for the FX15 oxygenator and W30 reservoir devices, indicating an overall significant increase in adoption of the smaller devices.
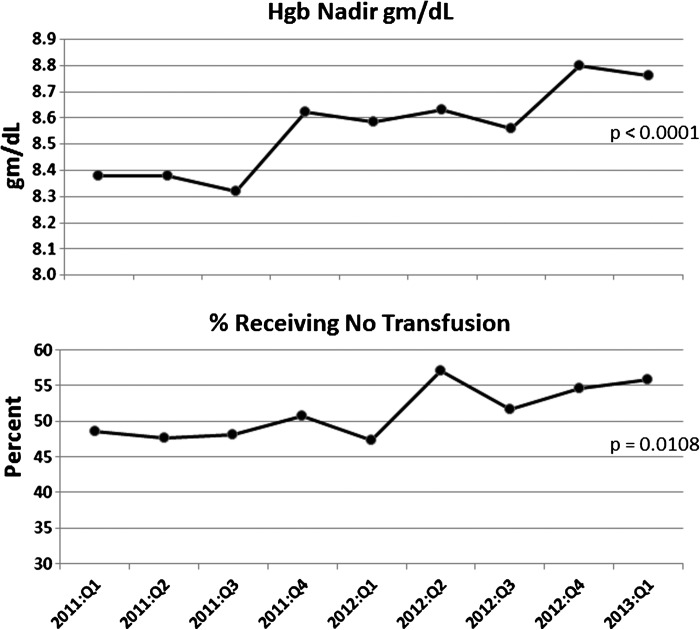
Figure 3 illustrates a significant increase in the average Hgb nadir, which helped to decrease blood use in the operating room (OR) and surgical intensive care unit (ICU). The percent of the patients who did not receive a transfusion increased significantly over the nine quarters.
Figure 3.
The increase in average operating room hemoglobin (Hgb) nadir over the nine quarters. The percent increase in patients receiving no transfusions in the operating room and intensive care unit.
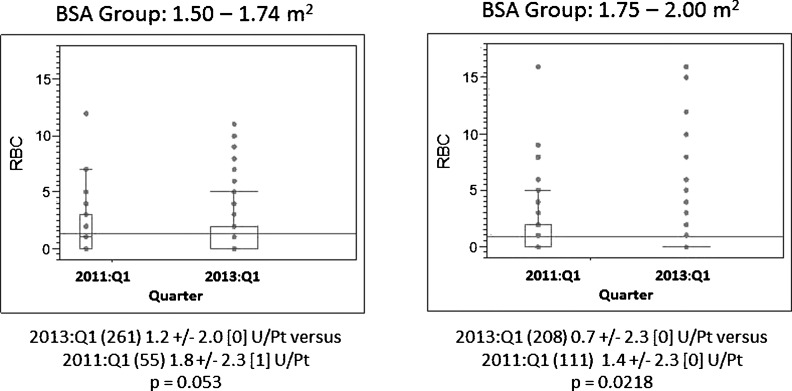
Blood component use and transfusion rates were analyzed by patient BSA, target CPB blood flow groups, and gender. In our institution, the total cost of delivering one unit of allogeneic red blood cells (RBCs) (330 mL) is approximately $1275. Figure 4 includes the distribution of RBC use for two BSA groups. In the 1.5 < BSA < 1.74-m2 group, average RBC use for all patients was reduced from 1.8 to 1.2 units/patient, reducing the average cost per patient from $2295 to $1530 (−$765 per patient). For Quarter 1 of 2013, 262 patients in this BSA group, the savings was approximately $200,430. We estimate savings of approximately $167,076 in the 1.50 < BSA < 1.99-m2 group of 208 patients in Quarter 1 of 2013; these cost savings pay for the higher cost of the FX15R30 devices compared with the FX25R40 components.
Figure 4.
BSA is body surface area for male and female patients within these limits. RBC U is one unit of allogeneic red blood cells (330 mL). 2011:Q1 is year and quarter. RBC use is mean ± 1 standard deviation [median] units RBC per patient. Points above the interquartile range are individual outlier patients.
Patients in the 4.50 < BF < 4.99-L/min blood flow group experienced the greatest reduction in blood use. From Quarter 1 in 2011, average of 1.3 units of blood to Quarter 2 in 2012, RBC transfusion average rate decreased to 0.4 units per patient (Figure 4). This significant decrease in blood use, at a cost of $1275 per unit, translates to an annual savings of approximately $558,450 based on our institution’s caseload.
Comparing the project’s first and last quarter results, the largest decrease in average RBC use for females was in the 4.50 < BF < 4.99-L/min BF group and the 1.50 < BSA < 1.74-m2 BSA groups (p < .08). The largest increase in percent freedom from transfusion for females was in the 4.50 < BF < 4.99-L/min BF group and in the 1.75 < BSA < 1.99-m2 BSA group (p < .025). Males in the 5.00 < BF < 5.49-L/min BF group benefited most from a significant reduction in average RBC usage (p = .047) from the beginning to the end of the project.
DISCUSSION
The recent Institute of Medicine (IOM) report has stated that policies and procedures need to be developed so that patient care is evidence-based. Further evidence indicates that low Hgb and blood transfusions are harmful to patients, inferring that centers should adopt strategies to reduce unwarranted variation in care such as unnecessary hemodilution (13).
In response to the IOM report, our right-sizing of ECCs began as a quality improvement initiative implemented by a team of 21 perfusionists attempting to use prescriptive oxygenator selection to eliminate transfusion rates for patients undergoing CPB. In its infancy, the initiative took on skepticism and criticism; however, a team culture of respect, teamwork, and open communication allowed for development of a more refined and evidence-based practice. Perhaps the single most remarkable success point regarding this initiative is the success in leading a large and diverse team to change their ways and adopt a new strategy.
In the beginning of the project, resistance to down-sizing patient circuits was indicated in the low use of the FX15 oxygenator (Figure 2). As more trust and understanding of the smaller circuits developed, a trend toward increased use of the FX15 oxygenator resulted in lower prime volumes, less hemodilutional anemia, and increased Hgb nadir. The increase in Hgb nadir correlated to a decrease in the use of allogeneic blood products in the OR and ICU in accordance with Loor and associates’ findings (2).
In the future, improvement in compliance to the right-sizing algorithm and ECC selection by our staff may further improve these outcomes. Opportunities to reduce blood exposures, especially for female patients, continue to exist. The largest decrease in RBC use for females was in the 4.00 < BF < 4.49-L/min BF group; however, the greatest statistically significant reductions in RBC use, within the BF groups, were observed in the male population subgroups. This suggests that it is important to not focus only on female patients, but to “right-size” each patient, each case. Every patient can benefit from a “right-sized” circuit selection.
In an effort to increase staff compliance with the “right-sizing” protocol, a data wall, consistent with Stammers et al., will be constructed to track each perfusionist’s selection of oxygenator, reservoir, and A-V loop (14). These data will then be crossreferenced to the right-sizing algorithm, creating a percent of recommended circuits used, with a goal of achieving the highest possible percentage both individually and as a group (15).
Barriers to reaching 100% compliance with the guideline are defined in the three “Ps” of prescriptive ECC sizing: the patient, procedure, and people. Patients have varying metabolic needs, red cell masses, pathologies, and native physiologies that may alter a clinician’s ECC selection. Procedural protocols such as cannulation sites, cannula sizes, depth of hypothermia, use of circulatory arrest, and predicted blood loss can restrict or augment the use of smaller ECCs. Finally, people, including surgeons, anesthesiologists, and perfusionists, are often autonomous in their practices and can alter the choice of circuit based on their personal preferences, comfort level, and clinical judgment for specific patient needs.
Although personal preference can induce resistance to change, adversely affecting overall results, more progressive practitioners can downsize ECCs through cooperation and communication with the cardiovascular multidisciplinary team members, leading to improved patient outcomes. In the future, implementation of more complete autologous circuit priming on many patients may supplement current efforts to reduce prime volumes.
In the future, flow requirement predictions based on lean body mass (LBM) and oxygen delivery (DO2, mLO2/min/m2) need to be made. The DO2 index, used to assess a patient’s oxygen requirements, is the product of perfusion flow index and arterial blood oxygen content. A DO2 index of 272 mL O2/min/m2 is considered adequate under normothermic conditions. These DO2 requirements have been shown to decrease under hypothermia, as metabolic activity decreases, allowing for lower blood flow (16).
Body surface area is widely used to predict blood flow requirements for patients; however, evidence shows LBM to be a better predictor of metabolically active tissues and thus flow requirements (17). The use of LBM, in conjunction with gender, in our right-sizing algorithm might allow for the creation of a more accurate flow prediction and perhaps lower target BF requirements allowing for more effective sizing of ECCs, especially for patients with a large BSA and a high body mass index (18).
The transfusion rate for coronary artery bypass graft operations reported in a 2010 survey reported wide variability between 2% and 98% with a median rate of approximately 38% receiving no RBC allogeneic transfusions (12). We reviewed 3852 adult perfusion surgical procedures, including redo sternotomy, of varied risk for transfusion using several blood conservation techniques, including prescriptive ECC sizing. In our quality improvement project we were able to demonstrate a significant increase in percent patients with no transfusions from 49% to 56% (Table 3).
Table 3.
Cost savings per BSA group.
| BSA Group | ||
|---|---|---|
| Quarter | 1.50 < BSA < 1.74 | 1.75 < BSA < 1.99 |
| 2013:Q1 | 1.2 U RBC/patient (p < .0001) | 0.7 U RBC/patient (p = .0404) |
| $1530/patient (−$765/patient) | $931/patient (−$803/patient) | |
| 2011:Q1 | 1.8 U RBC/patient | 1.4 U RBC/patient |
| $2295/patient | $1734/patient | |
| Saved in 2013:Q1 | −$200.430 (262 patients) | −$167,076 (208 patients) |
BSA, body surface area; Q, quarter; RBC, red blood cell.
We have demonstrated the ability to reduce Hgb nadir, blood product transfusion rates, and autologous donor exposures through a multimodal, multidisciplinary blood management program that includes ECC patient “right-sizing.” We have shown significant reductions in donor exposures in midrange patient BSA sizes and BF limits for both female and male patients. To discern the exact role of prescriptive patient oxygenator and reservoir sizing, a prospective trial, in which different size ECCs are assigned to the same size patients, will need to be completed.
The main limitation of our quality improvement project was the observational retrospective design. Additional limitations include missing data points that occurred with manipulation of information between databases and information lost during patient-side charting. Each quarter the missing patient and missing data rate was approximately 1–3%. Reporting average blood use and transfusion rates compared with median rates was affected by the presence of outliers. The results observed here were not singularly the result of prescriptive patient circuit sizing, but rather contributed to in part by many other blood conservation efforts and transfusion reduction working concurrently.
In conclusion, by “right-sizing” patient extracorporeal circuits and using other methods to eliminate allogeneic blood use, dilutional anemia is reduced, thereby increasing Hgb nadir. These techniques contribute to the elimination of allogeneic blood product transfusion and transfusion-related complications and expenditures.
ACKNOWLEDGMENT
We sincerely thank the talented perfusion team, anesthesia team, operating room nursing team, and cardiac surgeons at the Mayo Clinic. Without the cooperation of the entire team, the implementation of this project and completion of this article would not have been possible. We appreciate the technical assistance of Andrew A. Higgins, RN, and Lance A. Trewhella, RN, to provide the blood use and Hgb nadir information.
REFERENCES
- 1.Ranucci M, Pazzaglia A, Bianchini C, Bozzetti G, Isgro G.. Body size, gender, and transfusions as determinants of outcome after coronary operations. Ann Thorac Surg. 2008;85:481–486. [DOI] [PubMed] [Google Scholar]
- 2.Loor G, Li L, Sabik JF 3rd, Rajeswaran J, Blackstone EH, Koch CG.. Nadir hematocrit during cardiopulmonary bypass: End-organ dysfunction and mortality. J Thorac Cardiovasc Surg. 2012;144:654–662. [DOI] [PubMed] [Google Scholar]
- 3.DeFoe GR, Ross CS, Olmstead EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 2001;71:769–776. [DOI] [PubMed] [Google Scholar]
- 4.Fang WC, Helm RE, Krieger KH, et al. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation. 1997;96:II-194–II-199. [PubMed] [Google Scholar]
- 5.Karkouti K, O’Farrell R, Yau TM, Beattie WS.. Prediction of massive blood transfusion in cardiac surgery. Can J Anaesth. 2006;53:781–794. [DOI] [PubMed] [Google Scholar]
- 6.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. [DOI] [PubMed] [Google Scholar]
- 7.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ.. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–1186. [DOI] [PubMed] [Google Scholar]
- 8.Zangrillo A, Garozzo FA, Biondi-Zoccai G, et al. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: A meta-analysis of randomized controlled studies. J Thorac Cardiovasc Surg. 2010;139:1162–1169. [DOI] [PubMed] [Google Scholar]
- 9.Shann KG, Likosky DS, Murkin JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–290. [DOI] [PubMed] [Google Scholar]
- 10.Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. [DOI] [PubMed] [Google Scholar]
- 11.McCusker K, Vijay V, DeBois W, Helm R, Sisto D.. MAST system: A new condensed cardiopulmonary bypass circuit for adult cardiac surgery. Perfusion. 2001;16:447–452. [DOI] [PubMed] [Google Scholar]
- 12.Ereth MH.. Decision to transfuse comfort zones: Move out or get pushed out. Transfusion. 2012;52:2–3. [DOI] [PubMed] [Google Scholar]
- 13.Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 14.Stammers AH, Trowbridge CC, Pezzuto J, Casale A.. Perfusion quality improvement and the reduction of clinical variability. J Extra Corpor Technol. 2009;41:48–58. [PMC free article] [PubMed] [Google Scholar]
- 15.Bonk R, Trowbridge C, Stammers A, et al. Soluble fibrin monomer complex and cardiopulmonary bypass. J Extra Corpor Technol. 2009;41:157–160. [PMC free article] [PubMed] [Google Scholar]
- 16.Ranucci M, Romitti F, Isgro G, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–2220. [DOI] [PubMed] [Google Scholar]
- 17.Abramov D, Tamariz MG, Sever JY, et al. The influence of gender on the outcome of coronary artery bypass surgery. Ann Thorac Surg. 2000;70:800–805. [DOI] [PubMed] [Google Scholar]
- 18.Alston RP, Anderson A, Sanger K.. Is body surface area still the best way to determine pump flow rate during cardiopulmonary bypass? Perfusion. 2006;21:139–147. [DOI] [PubMed] [Google Scholar]