Abstract:
Perioperative hyperoxia has been claimed to have a number of therapeutic advantages. However, in the setting of cardiac surgery and cardiopulmonary bypass (CPB), enthusiasm for its use has been tempered by concerns regarding the effect of high partial pressures of oxygen on cardiac, vascular, and respiratory function and the potential for exacerbation of ischemia–reperfusion injury. There is encouraging evidence from animal studies that hyperoxia is effective in myocardial preconditioning, at least in nondiseased hearts. There is also evidence that hyperoxia reduces gas microemboli production and longevity during CPB, although it is unclear whether this translates into a clinical benefit in terms of a reduction in postoperative neurological morbidity. Hyperoxia leads to changes in cardiovascular function. However, the effects of these changes remain unclear. At a tissue level, there is evidence that hyperoxia does not lead to improvement in partial pressure of oxygen. Indeed, the opposite may be the case with reductions in capillary density leading to areas of reduced tissue oxygenation. The risks of hyperoxia in association with CPB include lung injury, increased systemic reactive oxygen species generation, and exacerbation of reactive oxygen species-mediated myocardial injury at the time of reperfusion. Again, it is difficult to know whether the changes demonstrated are temporary or if they translate into a worsening of clinical outcomes. In conclusion, perhaps the key to the use of hyperoxia is in the timing. In the period pre-CPB, hyperoxia may precondition the myocardium and, paradoxically, confer a degree of protection against reactive oxygen species-induced injury at the time of reperfusion. Hyperoxia during CPB is probably harmful and should be avoided unless the risk from gas microemboli is thought to be significant, in which case the risks and benefits to the individual patient must be weighed.
Keywords: cardiopulmonary bypass, reactive oxygen species, reperfusion injury
Perioperative hyperoxia has been claimed to have a number of therapeutic advantages. These include preconditioning of the myocardium to better tolerate ischemia, a reduction in postoperative wound infection rates, and a reduction in gas microemboli generated during cardiopulmonary bypass. However, in the setting of cardiac surgery and cardiopulmonary bypass, enthusiasm for its use has been tempered by concerns regarding the effect of high partial pressures of oxygen on cardiac, vascular, and respiratory function and the potential for exacerbation of ischemia–reperfusion injury. Opinions remain divided and practice differs widely.
HYPEROXIA AND CARDIOVASCULAR FUNCTION
It is common, if not nearly universal, practice to administer high-flow oxygen as part of the treatment for acute cardiac dysfunction. This is done with the assumption that the increase in the partial pressure of oxygen in arterial blood (PaO2) will compensate for the effects of a reduction in cardiac output on tissue oxygenation. However, oxygen is a vasoactive substance.
Oxygen administered at 15 L/min through a nonrebreather mask for 1 hour has been shown to reduce heart rate and cardiac index (CI) while increasing systemic vascular resistance (SVR) and mean arterial pressure in healthy volunteers (1). A later study, which controlled for the potential confounding factor of changes in arterial partial pressure of carbon dioxide, found similar changes together with a reduction in stroke volume (SV) (2). These changes persisted for an hour after the subjects had reverted to breathing air. Haque and colleagues examined the effects of hyperoxia on cardiac function in patients with New York Heart Association Class 3 and 4 heart failure. Measurements were taken before and immediately after breathing 100% oxygen for just 20 minutes (3). Investigators found a significant fall in cardiac output and SV. Both pulmonary artery wedge pressure and SVR were significantly elevated. Mak and colleagues (4) investigated the effects of hyperoxia on left ventricular function in both healthy subjects and those with congestive cardiac failure. In both groups, a 20-minute period breathing 100% oxygen led to impairment of cardiac relaxation and an increase in left ventricular end diastolic pressure. Left ventricular contractility was not affected.
Hyperoxia has been shown to affect coronary vascular blood flow in several studies (4–8). In all studies, coronary blood flow was reduced. The mean reduction in healthy subjects was 17.1%, whereas in those with coronary disease, it ranged between 7.9% and 28.9%. Coronary blood flow remained depressed for up to 30 minutes after termination of high concentration oxygen therapy. In studies in which coronary vascular resistance was measured (5–7), it was shown to be elevated with hyperoxia. Mean changes in coronary vascular resistance ranged from 21.5% to 40.9%.
There is good evidence for reduced coronary blood flow in response to hyperoxia, but what is not clear is whether this is a form of physiological autoregulation or a potentially harmful pathological process leading to tissue ischemia. Ganz and colleagues (5) demonstrated an increase in myocardial lactate extraction with hyperoxia in both normal and diseased coronary arteries. Mak and colleagues (4) found no difference in lactate extraction in response to hyperoxia. These studies have not demonstrated myocardial ischemia despite coronary vasoconstriction in response to hyperoxia.
Animal studies have identified hyperoxia-induced changes at the microvascular level (9,10). Tsai and colleagues (11), using a guinea pig model, demonstrated a reduction in microvascular blood flow of 17% ( p < .05) and a reduction in functional capillary density of 26% leading to increased heterogeneity of tissue oxygenation. The investigators calculated that overall tissue oxygenation was unchanged.
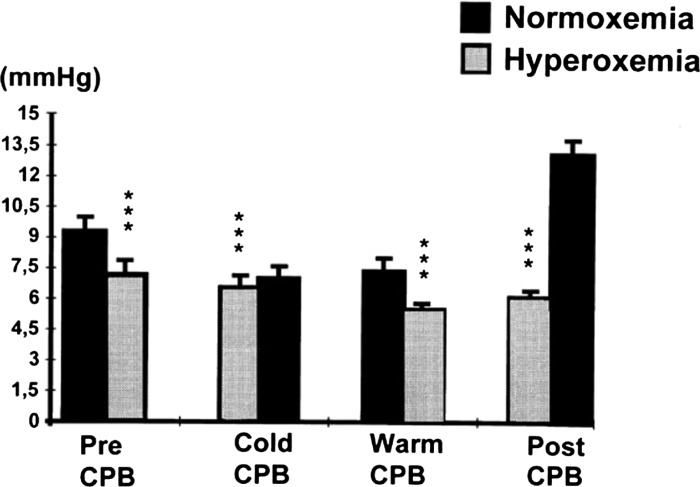
Joachimsson and colleagues (12) undertook a study into the effects of hyperoxemia in 10 patients undergoing cardiac surgery with cardiopulmonary bypass (CPB). One element of the study was the measurement of tissue oxygenation by the placement of an oxygen microelectrode in skeletal muscle (anterior tibial muscle of the leg). The electrode was composed of eight separate platinum wires. At each measurement the electrode was rotated a total of 15 times at 10-second intervals, giving a total of 120 readings per measurement. In each patient, measurements were taken pre-CPB, during CPB, after aortic clamp removal, and with a stable spontaneous cardiac output post-CPB. At each of these stages, patients were rendered both normoxemic (PaO2 75–115 mmHg) and hyperoxemic (PaO2 > 185 mmHg) with a period of 10 minutes after each transition to allow equilibration. Muscle oxygenation was significantly reduced at each stage of the surgery during periods of hyperoxemia as compared with periods of normoxemia with the most marked difference occurring in the post-CPB period. Furthermore, there was an increase in the heterogeneity of distribution of tissue oxygen with hyperoxemia (Figure 1).
Figure 1.
The effects of normoxia and hyperoxia on mean skeletal muscle surface oxygen tensions during hypothermic as well as normothermic CPB, and before and after CPB. Mean ± standard deviations are given. Data were analysed by analysis of variance. *** p < .001. From Joachimsson et al., J Thoracic Cardiovasc Surg 1996;112:812–19, with permission.
There is now substantial evidence for the wide-ranging effects of hyperoxia on the cardiovascular system with impaired ventricular lusitropy, generalized vasoconstriction, reduced coronary blood flow, altered capillary density, and distribution of tissue oxygen. Overall tissue partial pressures of oxygen appear little changed or reduced. The clinical implications of these changes are difficult to determine, particularly in the setting of cardiac surgery. It is, however, difficult to point to any definite advantage to hyperoxia in terms of myocardial and generalized tissue oxygen delivery based on these data.
OXYGEN AND THE GENERATION OF REACTIVE OXYGEN SPECIES
Oxygen exists in nature as a diatomic molecule. In its ground or triplet state, it has two unpaired electrons, that is to say electrons that are the sole occupants of an atomic orbital. Each atomic orbital can contain a pair of electrons, each with a spin in the opposite direction to the other. Orbitals that contain only one electron are unstable. Any species with one or more unpaired electrons will become stable by gaining electrons to fully populate these orbitals. Such reactions are known as oxidation reduction or Redox reactions, and any species with one or more unpaired electrons is said to be a free radical. All free radicals are highly reactive. Thus, oxygen is ideal as the final acceptor of electrons in the energy-generating electron transfer chain within the cellular mitochondria.
Reduction of oxygen by the transfer of electrons, if complete, leads to the formation of water. However, incomplete reduction leads to the formation of a superoxide radical (O2.−) with one unpaired electron remaining. Up to 4% of oxygen within the mitochondria is incompletely reduced (13). The superoxide ion is converted to hydrogen peroxide (H2O2) by one of a family of enzymes, the superoxide dismutases (SOD). H2O2, although not a free radical, remains highly reactive. Most H2O2 is further broken down to oxygen and water by the enzymes catalase and glutathione peroxidase. However, the hydroxyl radical (OH·) may be formed by reaction with either O2.− or with metal cations, usually Fe2+ (the Fenton reaction). The free radicals that derive from oxygen and other reactive compounds containing oxygen such as H2O2 are collectively known as reactive oxygen species (ROS).
The detrimental effects of ROS are well recognized. Being highly reactive, ROS will bind to many cellular components such as proteins, DNA, and membrane lipids leading to dysfunction or loss of structural integrity. Under normal conditions, endogenous antioxidants react with ROS and protect the cell from damage. These antioxidants include SOD, catalase, glutathione peroxidase, coenzyme Q10, and vitamins C and E. ROS play a number of important physiological roles. Their injurious effects can be advantageous. ROS are generated by activated neutrophils and other phagocytic cells and play a role in host defense. There is evidence for ROS production in response to activation of various cell surface receptors and a role in apoptosis (14) and cellular hypertrophy (15). ROS have been identified as important mediators in control of ventilation by the carotid bodies (16) and in erythropoietin generation in the liver and kidney (17).
REACTIVE OXYGEN SPECIES AND CARDIOPULMONARY BYPASS
CPB leads to a significant increase in ROS and the overwhelming of antioxidant capability. Ischemia leads to mitochondrial dysfunction and may increase production of superoxide ions from residual oxygen and leakage into the myocyte. More significantly, reintroduction of oxygen at reperfusion leads to an increase in superoxide production by the dysfunctional mitochondria. In addition, CPB leads to activation of neutrophils and degradation of the protective endothelial glycocalyx (18). Superoxide dismutase, bound to glycoproteins that constitute the glycocalyx, is lost and the endothelial cells exposed, allowing binding of activated neutrophils and release and accumulation of ROS (19).
Elevated levels of ROS cause cellular damage and dysfunction leading to myocyte contractile dysfunction (myocardial stunning) (20,21), necrosis, increased levels of apoptosis, vascular dysfunction, and possibly arrhythmias. ROS-mediated myocyte damage together with mitochondrial toxicity resulting from elevated intramyocyte calcium levels is the main cause of cardiac ischemia–reperfusion injury.
Reactive oxygen species are not just generated in the myocardium during CPB and at the time of reperfusion. Clermont and colleagues (22) undertook a study of patients undergoing cardiac surgery with CPB. Serial blood samples were taken during CPB from the coronary sinus and a systemic artery. Electron spin resonance spectroscopy was used to determine ROS levels. A rapid increase in systemic ROS levels was demonstrated at the commencement of CPB and this persisted until the final measurement at 25 minutes post-removal of the aortic cross-clamp. Interestingly, although coronary sinus sampling showed increased levels at 3 minutes postaortic declamping, consistent with previous studies (23), there was no significant difference from systemic levels, suggesting that much of the ROS burden is generated peripherally before declamping.
HYPEROXIA AND REPERFUSION INJURY
Reperfusion of ischemic myocardium leads to increased ROS production. There is also evidence for systemic ROS production during CPB. ROS production in this setting would be expected to be greater in the presence of hyperoxia. Indeed, there are a number of studies that have shown a link between hyperoxia and increased production of ROS in a number of settings (24,25).
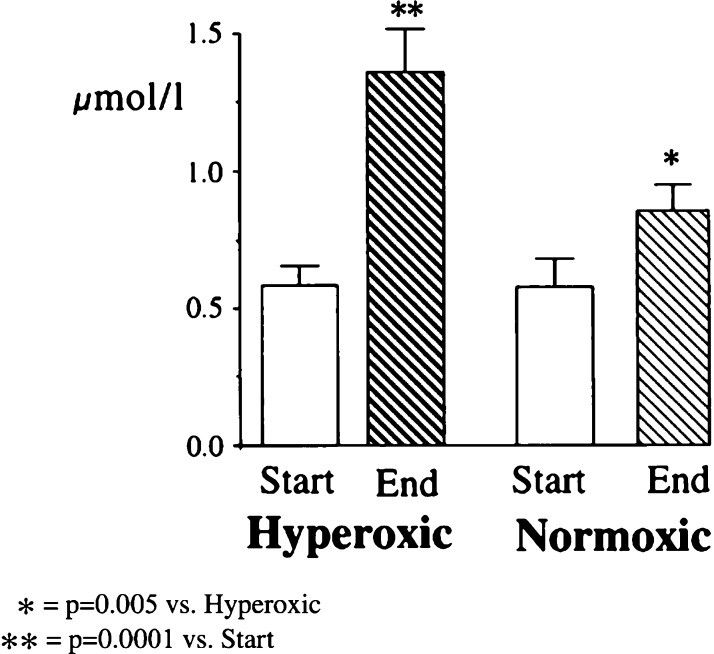
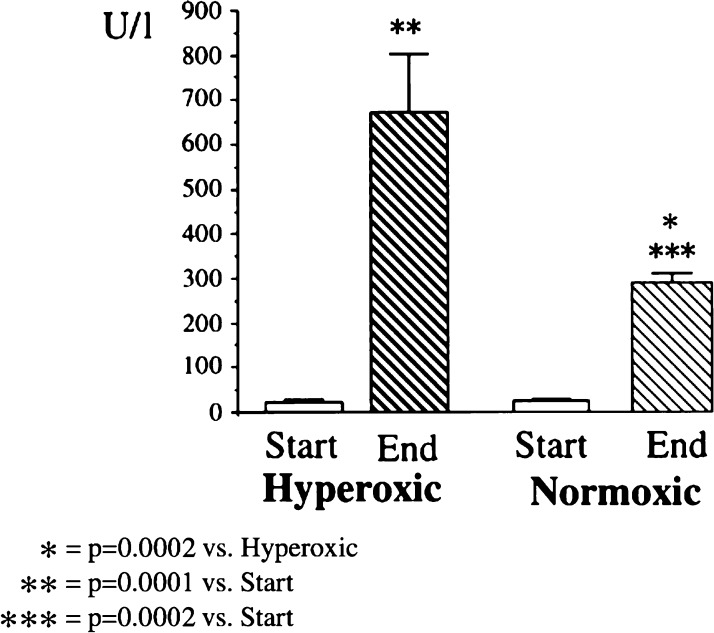
Inoue and colleagues (26) undertook a study to test the hypothesis that oxidative damage at the time of reperfusion was exacerbated by an elevated PaO2. Eight patients undergoing cardiac surgery received hyperoxic CPB (PaO2 = 450–550 mmHg) after declamping, whereas a second group of eight patients received oxygen to a target PaO2 of 200–250 mmHg. They found the CI measured at 30 minutes and 3 hours postaortic declamping to be significantly lower in the hyperoxic group. This difference was lost at 6 hours. Plasma levels of malondialdehyde, a biomarker of oxidative stress, were significantly higher in the hyperoxic group at 30 minutes postdeclamping, but this difference was lost at 3 hours. In a similar study, Ihnken and colleagues randomized 40 patients undergoing elective cardiac surgery to either normoxic or hyperoxic CPB. Various markers of oxidative stress and myocardial damage were assayed both before and after CPB. There was a significantly greater rise in these markers in the hyperoxic group (27). Cardiac index post-CPB did not differ significantly between the two groups (Figures 2 and 3).
Figure 2.
Malondialdehyde in coronary sinus blood at the start and end of CPB in comparison of patients undergoing hyperoxic (PO2 = 400 mmHg) or normoxic (PO2 = 140 mmHg) CPB. From Ihnken et al., with permission.
Figure 3.
Creatine kinase in coronary sinus blood at the start and end of CPB in comparison of patients undergoing hyperoxic (PO2 = 400 mmHg) or normoxic (PO2 = 140 mmHg) CPB. From Ihnken et al., with permission.
HYPEROXIA AND PRECONDITIONING OF THE MYOCARDIUM
Preconditioning is the exposure of the myocardium to some form of stimulus that leads to an alteration in intracellular signaling and subsequent increased tolerance to an ischemic insult such as CPB. The preconditioning stimulus can be a brief period of ischemia of the myocardium itself (28) or of a tissue remote to the myocardium. Halogenated volatile anesthetic agents have been shown to precondition the myocardium (29). More recently, animal studies have also shown hyperoxia to be effective.
In one trial, the hearts of rats were harvested and Langendorff-perfused before being subjected to 30 minutes of ischemia followed by 120 minutes of reperfusion. Those rats subjected to 60 minutes of hyperoxia before harvesting showed a 23% reduction in infarct size as compared with controls (30). Other groups of investigators, again using rats, have demonstrated improved functional recovery as well as reduced necrosis (31) and a reduction in reperfusion-induced arrhythmias (32) after hyperoxic preconditioning. Interestingly, a recent study in rats rendered acutely diabetic showed that although these animals had a reduced infarct size after myocardial ischemia when compared with nondiabetic controls, hyperoxic preconditioning did not confer any additional benefit (33).
HYPEROXIA AND LUNG FUNCTION
There is a large body of evidence to support the adverse effects of high inspired fractions of oxygen on lung function. These effects include direct cellular toxicity resulting from ROS leading to impaired gas exchange and mechanical changes resulting from alveolar collapse, so-called absorption atelectasis. Studies of the effects of 100% inspired oxygen in healthy volunteers have shown that it takes many hours before symptoms are apparent or changes in lung function are identified (34,35). The adverse effects of hyperoxia on already damaged lung, however, evolve far more rapidly (36,37).
CPB is injurious to the lungs. Bronchial blood flow may be reduced (38), and the inflammatory response induced by exposure of blood to the bypass circuit leads to increased pulmonary endothelial permeability (39). Exposure of lung parenchyma to high concentrations of oxygen at this stage could lead to further significant impairment of lung function post-CPB.
Pizov and colleagues (40) investigated the effects of high oxygen concentration on CPB-induced lung injury. Thirty patients were randomized to receive either 100% or 50% oxygen throughout the course of their surgery. While on bypass, the lungs of patients in the 100% oxygen group were flushed with oxygen at 4 L per minute, whereas those in the 50% oxygen group had air insufflated at 4 L per minute. Patients in both groups received 50% oxygen once they arrived in the intensive care unit (ICU). Blood samples were taken for measurement of PaO2 before CPB and at 40 minutes, 2 hours, and 6 hours post-CPB. Assays of two proinflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin 8 (IL-8), were performed on blood and fluid samples obtained from bronchoalveolar lavage (BAL) before CPB and at 40 minutes post-CPB.
Efficiency of gas exchange was assessed using the ratio of arterial oxygen partial pressure to inspired fraction of oxygen (PaO2:FiO2). There was no significant difference between the two groups pre-CPB but a significant fall in values post-CPB. The mean values for each group were at their lowest values at 40 minutes post-CPB but did not differ significantly from each other. However, by 6 hours, the values in the 50% oxygen group had returned to prebypass levels, whereas values in the 100% oxygen group remained significantly reduced.
Serum TNF-α levels were significantly elevated in both groups after CPB. Levels in broncoalveolar lavage (BAL) fluid were significantly elevated in the 100% oxygen group but not in the 50% oxygen group. Plasma IL-8 levels were similarly elevated in both groups post-CPB. No significant increase in IL-8 levels from BAL samples was detected in either group post-CPB.
The investigators concluded that patients treated with 100% oxygen had a delayed recovery of lung function post-CPB and an increase in BAL cytokine levels indicating that high alveolar oxygen concentrations during surgery with CPB have a detrimental effect on the lungs.
Reber and colleagues (41) examined the effects of administering 100% oxygen in the period immediately after CPB and before admission to the ICU as compared with 35% oxygen. The mean duration of treatment was 70 minutes for the 100%oxygen group and 76 minutes for the 35%oxygen group. Those receiving 100%oxygen demonstrated a significant decrease in gas exchange as measured by PaO2 to FiO2 ratio compared with pre-CPB levels. Those receiving 35% oxygen showed no significant deterioration. The difference between the two groups was nonsignificant by the first postoperative day. It is worth noting that the level of oxygenation during CPB was not reported.
Ihnken and colleagues (27) undertook a study into the effects of hyperoxia during cardiopulmonary bypass. Forty consecutive patients were randomized to receive hyperoxic treatment (PaO2 = 400 mmHg) or normoxic treatment (PaO2 = 140 mmHg) during CPB. Included in the many measurements taken (see previously) were lung function tests before and 5 days post-surgery. Those patients receiving hyperoxic CPB demonstrated a significantly greater deterioration in both vital capacity and forced expiratory volume over 1 second. No mention is made of the oxygenation strategies pre- and post-CPB during this study.
GAS MICROEMBOLISM
Gas microemboli (GME) are well recognized as a cause of tissue infarction after cardiac surgery and CPB. Clinically apparent neurological damage occurs in between 1.5% and 10% of cases of open procedures such as valve replacements with a further 18% suffering radiologically apparent but clinically silent infarcts (42). Causes include both particulate and gaseous embolization as well as hypoperfusion.
Gas microemboli are generated in the extracorporeal bypass circuit by cavitation in areas of turbulent flow with the total burden being heavily influenced by the various components used (43). They may also be introduced at the time of circuit priming or the injection of solutions into the circuit (44). The nature of the surgery and surgical technique are important with the potential for the entrainment of gas from both cannulation sites the surgical site during open procedures. Changes in temperature affecting gas solubility will also have an effect (45).
The extent of the problem has been better quantified since the advent of ultrasound imaging of the circulation. There has been much progress in the reduction of the total burden of GME in recent years, predominantly by improvements in the design of various components of the bypass circuit. However, while GME generation has been greatly reduced, it has not been eliminated. Gas emboli are easily deformable and able to divide and recoalesce. Arterial filters are not absolute barriers to their onward passage to the patient’s circulation.
Once in the circulation, GMEs will deform as they pass along the vessels of the arterial tree, eventually coming to rest in capillaries and microvessels. Here they act both as a physical barrier preventing onward flow of blood and as a nidus for the triggering of both the inflammatory and coagulation cascades. Tissues distal and immediately proximal to the site of embolization are rendered ischemic and will eventually infarct.
The gaseous composition of GMEs is important both in the ease of generation and in their duration once formed. The dissolution of gas bubbles within solution depends on a number of factors including both the gas–liquid diffusion constant and partition constant of the given gas (46). Emboli containing an air mix with 80% nitrogen, a relatively insoluble gas, have a lifespan approximately 10 times that of an embolus containing solely oxygen.
Nollert and colleagues (47) undertook a study to investigate the effect of various factors on the generation of GMEs in piglets undergoing CPB. They found that the emboli count, as measured by carotid Doppler ultrasound, was significantly greater in the animals receiving an FiO2 of between 20% and 40% as compared with those receiving an FiO2 of 100%.
Georgiadis and colleagues (48) investigated the effects of oxygen inhalation on the formation of gaseous microemboli in patients with artificial heart valves. They found that patients inhaling 100% oxygen for at least 20 minutes had a significant fall in the number of measured GMEs as compared with those breathing room air.
HYPEROXIA AND CEREBRAL PROTECTION
The effects of oxygen on ischemic cerebral tissue are complex and not yet fully understood. A number of different effects have been demonstrated.
A large body of work has been completed in the area of stroke medicine. Numerous animal trials have been undertaken to investigate the effects of both normobaric and hyperbaric hyperoxia on transient and permanent focal cerebral ischemia (49,50). These have demonstrated a beneficial effect in terms of reduction in infarct size when compared with normoxia. Unfortunately, clinical trials of hyperoxia for the treatment of ischemic stroke have been mixed. There is evidence of transient improvement in clinical features and a reduction in the volume of ischemic tissue on magnetic resonance imaging with hyperoxia (51). However, there is also evidence that hyperoxia at the time of presentation to the ICU after major stroke is associated with increased mortality (52).
In the setting of cardiac surgery with CPB and particularly with deep hypothermic circulatory arrest (DHCA), interest lies in the effects of hyperoxia on transient global, rather than focal, ischemia. Prolonged preconditioning with hyperbaric oxygen has been shown to reduce functional deficit and neuronal cell death in rats after transient global ischemia (53). Furthermore, there is the suggestion by some investigators that hyperoxia before DHCA leads to the generation of a greatly enhanced reservoir of oxygen within the tissues, thus prolonging the time of ongoing aerobic metabolism after the cessation of oxygen delivery (54). There is a lack of clinical trials. Of note, a study by Alex and colleagues (55) demonstrated a reduction in neuropsychometric dysfunction after CPB (without DHCA) after pretreatment with hyperbaric oxygen.
However, as discussed previously, hyperoxia leads to an increased production of ROS. ROS have been demonstrated to play a significant role in reperfusion injury of the brain after DHCA in dogs (56). Hyperoxia after a brief period of global cerebral ischemia, again in dogs, has been shown to increase oxidative stress and lead to hippocampal neuronal cell death (57).
Nollert and colleagues (58), after their investigation of the effects of hyperoxia on the formation of GME (47), undertook a study of the overall effects of hyperoxia on neurological damage after a period of prolonged DHCA in pigs. Five animals were rendered normoxic during CPB (PaO2 64–181 mmHg) and five were hyperoxic (PaO2 400–900 mmHg). Each animal was cooled over a period of 30 minutes before a period of DHCA lasting 120 minutes. After rewarming, each animal was weaned from CPB. After 6 hours of reperfusion, brain tissue was fixed. Histological examination revealed a significant increase in brain damage in the normoxic group as compared with the hyperoxic group although there was a trend toward increased lipid peroxidation in the hyperoxic group suggesting greater oxygen-free radical damage. The authors concluded that in the setting of prolonged DHCA, the advantages of hyperoxia in protecting against ischemic injury outweighed the deleterious effect of increased free radical damage.
SURGICAL SITE INFECTION
Another possible benefit of hyperoxia is the reduction of surgical site infection. There is good evidence that hypoxia limits oxidative killing of bacteria by neutrophils (59). The benefits of hyperoxia are less clear. In a recent article, Qadan and colleagues (60) set out to establish the effects of 80% oxygen on various recognized markers of innate immune function in vitro. Lipopolysaccharide was added to venous blood samples taken from healthy volunteers. Samples were incubated for 2 hours in 21% or 80% oxygen. In the latter group, there was increased ROS generation, although other measurements such as neutrophil phagocytic activity and cytokine release remained unchanged. Does this translate to a clinical benefit? Data from large clinical trials are conflicting.
Recent interest in FiO2 as a method for reducing surgical site infection was first sparked by a study by Greif et al. published in 2000 (61). In this trial, 500 patients undergoing colorectal resection were randomized to receive 30% or 80% oxygen during the operation and for 2 hours afterward. Wounds were evaluated daily until discharge and at follow-up 2 weeks later. Wounds were infected if pus was present and culture-positive. Wound infections were found in 5.2% of patients receiving 80% oxygen compared with 11.2% of the control group, a statistically significant difference. This positive result was soon repeated by other investigators in the setting of both colorectal surgery (62) and appendectomy (63). However, there have also been other trials that have failed to demonstrate a benefit in colorectal and obstetric surgery (64,65). Indeed, in one trial, the incidence of infection was significantly higher in the group receiving 80% oxygen and this resulted in a longer hospital stay (66), although the group receiving an FiO2 of .8 in this study had a significantly greater body mass index, increased length of surgery, larger volumes of intravenous fluid, and greater volumes of blood transfused. All of these differences are potential confounding factors.
The perioperative oxygen fraction-effects on surgical site infection and pulmonary complications after abdominal surgery, a randomized controlled trial (PROXI) (67), published in 2009, investigated the effect of high perioperative oxygen fraction on surgical site infection in 1400 patients undergoing laparotomy. In this, the largest trial to date, there was no reduction in surgical site infection demonstrated with increased oxygenation.
A large trial published by the evaluation of nitrous oxide in the gas mixture for anesthesia (ENIGMA) trial group (68) examined the effect of inhaled nitrous oxide on over 2000 patients receiving general anesthesia. Patients undergoing cardiac surgery were excluded. Patients in the nitrous oxide arm received 70% nitrous oxide with 30% oxygen. The patients randomized to the group not receiving nitrous oxide had a variable inspired oxygen concentration with a mean value across the group of 73%. There was a significantly greater incidence of wound infection in the group receiving nitrous oxide (10% compared with 7.7%, p = .034). The authors pointed out that this difference may have been the result of the absence of nitrous oxide rather than the increase in inspired oxygen. When an analysis of patients in the non-nitrous oxide group was undertaken to determine whether there was an indiependent effect of supplemental oxygen, no measurable effect on wound infection rates was detected.
The conflicting results of studies to date are perhaps not surprising. The heterogeneity of the studies is well demonstrated by the fourfold difference in infection rates in the control groups across the various trials. A metaanalysis of seven trials was published in 2012 (69). Analysis of the pooled data led to the conclusion that high inspired oxygen was not beneficial in the prevention of surgical site infection, although a subgroup analysis of trials in colorectal surgery did show a reduction in the incidence of infection. The investigators pointed out that, although most of the trials compared an FiO2 of .8 with one of .3 or .35 intraoperatively, the duration of postoperative treatments varied. Definitions of surgical site infection also varied.
The lack of a definitive answer at the present time is the result of a number of factors. The partial pressure of oxygen in the tissues at the surgical site is a key factor. Oxygen delivery is crucial. This will be disrupted by both the surgical trauma and inflammatory response. Injudicious use of intravenous fluids will lead to an increase in the interstitial fluid volume with further compression of blood vessels and an increase in the diffusion distance of oxygen to the target tissues.
Greif et al. (61) measured subcutaneous tissue oxygenation (PsqO2) in their subjects at a site distal to the surgical incision. With an FiO2 of .3, mean PsqO2 levels were 59 mmHg rising to 109 mmHg with an FiO2 of .8.
Bakri and colleagues (70) investigated the potential benefit of transdermal oxygen delivery after cardiac surgery. Oxygen was delivered at a rate of 3 mL/hour through catheters placed on the skin surface over the surgical wound and covered by a surgical dressing. Tissue oxygenation was measured using probes placed into the sternal wound at a depth of 5 mm before wound closure. Measurements were taken postoperatively in extubated patients receiving approximately 30% oxygen (mean PaO2, 99 mmHg) or 50% oxygen (mean PaO2, 149 mmHg). There was no difference in tissue oxygenation levels between the group receiving the intervention and the control group. However, some interesting data were generated. Mean PsqO2 levels in those receiving 30% oxygen were 23 mmHg, much lower than those levels measured by Grief in healthy, nontraumatized tissues. The increase in inspired oxygen to 50% led to a significant increase in mean PsqO2 to 27 mmHg, but still a level well below that for nontraumatized tissue.
It may well be that supplementing inspired oxygen can only have a limited effect on tissue hypoxia at the surgical site if delivery is optimized by avoidance of interstitial edema and other factors such as hypothermia and hyperglycemia. Hyperoxia in this setting is certainly not a panacea.
POSTOPERATIVE NAUSEA AND VOMITING
There has been interest in intraoperative hyperoxia as a prophylactic maneuver in the prevention of postoperative nausea and vomiting. Two promising trials found a benefit associated with the use of 80% oxygen intraoperatively during colon surgery (71) and laparoscopic gynecological surgery (72). However, similar studies in the setting of pediatric strabismus surgery (73) and thyroidectomy (74) failed to show a benefit. A recent meta-analysis of 10 trials with a total number of 1729 patients found no overall benefit and concluded that the use of an FiO2 of .8 was ineffective in the reduction of the incidence of postoperative nausea and vomiting (75).
PATIENT SAFETY
Anesthetists may choose to use high inspired fractions of oxygen for reasons other than the putative therapeutic advantages of hyperoxia discussed thus far. One issue is safety. The sudden inadvertent loss of oxygen delivery to the lungs is, clearly, an emergency situation requiring rapid resolution. Causes are many and include displacement or blockage of the endotracheal tube, ventilator failure, circuit blockage or leak, loss of oxygen supply, and acute respiratory pathologies such as pneumothorax and severe bronchospasm. The time available to restore oxygen supply is short and dependent on the inspired oxygen fraction at the time of the event. The oxygen requirement for a resting adult is 3.5 mL/kg/min, approximately 250 mL/min in a 70-kg man. The oxygen reservoir available within the lungs after the sudden cessation of delivery is equal to the functional residual capacity of the lungs multiplied by the fractional concentration within the alveoli. Broadly, in a 70-kg adult breathing 30% oxygen, this will equate to approximately 600 mL. This increases to 2000 mL if the subject is breathing 100% oxygen. The extra time afforded to the anesthetist by this increase in FiO2 before the onset of irreversible cerebral damage resulting from hypoxia may be crucial.
SUMMARY
The potential benefits of hyperoxia have been studied widely. There is encouraging evidence from animal studies that hyperoxia is effective in myocardial preconditioning, at least in nondiseased hearts. There is also substantial evidence that hyperoxia reduces gas microembolism production and longevity during CPB, although it is unclear whether this translates into a clinical benefit in terms of a reduction of postoperative neurological morbidity. Promise of benefit from early studies with regard to reduction in surgical site infection and postoperative nausea and vomiting has not been confirmed by more recent work.
Hyperoxia leads to changes in cardiovascular function. However, the effects of these changes remains unclear. At a tissue level, there is evidence that hyperoxia does not lead to improvement in partial pressure of oxygen. Indeed, the opposite may be the case with reductions in capillary density leading to areas of reduced tissue oxygenation.
The risks of hyperoxia in association with CPB include lung injury, increased systemic ROS generation, and exacerbation of ROS-mediated myocardial injury at the time of reperfusion. Again, it is difficult to know whether the changes demonstrated are temporary or if they translate into a worsening of clinical outcomes.
In conclusion, perhaps the key to the use of hyperoxia is in the timing. Hyperoxia in the period pre-CPB may precondition the myocardium and, paradoxically, confer a degree of protection against ROS-induced injury at the time of reperfusion. However, a number of studies have demonstrated that hyperoxia during CPB and particularly at the time of reperfusion adversely affects myocardial function. Thus, hyperoxia during these stages of cardiac surgery should probably be avoided unless the risk fromgasmicroembolism is thought to be significant, in which case the risks and benefits to the individual patient must be weighed.
REFERENCES
- 1.Waring W, Thomson A, Adwani S, et al. . Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol. 2003;42:245–250. [DOI] [PubMed] [Google Scholar]
- 2.Thomson A, Drummond G, Waring W, Webb D, Maxwell S.. Effects of short-term isocapnic hyperoxia and hypoxia on cardiovascular function. J Appl Physiol. 2006;101:809–816. [DOI] [PubMed] [Google Scholar]
- 3.Haque W, Boehmer J, Clemson B, et al. . Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol. 1996;27:353–357. [DOI] [PubMed] [Google Scholar]
- 4.Mak S, Azevedo E, Liu P, Newton G.. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120:467–473. [DOI] [PubMed] [Google Scholar]
- 5.Ganz W, Donoso R, Marcus H, Swan H.. Coronary hemodynamics and myocardial oxygen metabolism during oxygen breathing in patients with and without coronary artery disease. Circulation. 1972;45:763–768. [DOI] [PubMed] [Google Scholar]
- 6.McNulty P, King N, Scott S, et al. . Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288:H1057–H1062. [DOI] [PubMed] [Google Scholar]
- 7.McNulty P, Robertson B, Tulli M, et al. . Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischaemic heart disease. J Appl Physiol. 2007;102:2040–2045. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar H, Weatherall M, Wijesinghe M, et al. . Systemic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158:371–377. [DOI] [PubMed] [Google Scholar]
- 9.Thorborg P, Gustafsson U, Sjoberg F, Harrison D, Lewis D.. The effect of hyperoxemia and ritanserin on skeletal muscle microflow. J Appl Physiol. 1990;68:1494–1500. [DOI] [PubMed] [Google Scholar]
- 10.Sjoberg F, Gustafsson U, Eintrei C.. Specific blood flow reducing effects of hyperoxaemia on high flow capillaries in the pig brain. Acta Physiol Scand. 1999;165:33–38. [DOI] [PubMed] [Google Scholar]
- 11.Tsai A, Cabrales P, Winslow R, Intaglietta M.. Microvascular oxygen distribution in the awake hamster window chamber model during hyperoxia. Am J Physiol Heart Circ Physiol. 2003;285:H1537–H1545. [DOI] [PubMed] [Google Scholar]
- 12.Joachimsson P, Sjoberg F, Forsman M, et al. . Adverse effects of hyperoxemia during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:812–819. [DOI] [PubMed] [Google Scholar]
- 13.Han D, Williams E, Cadenas E.. Mitochondrial respiratory chaindependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Circu M, Aw T.. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adiga I, Nair R.. Multiple signaling pathways coordinately mediate reactive oxygen species dependent cardiomyocyte hypertrophy. Cell Biochem Funct. 2008;26:346–351. [DOI] [PubMed] [Google Scholar]
- 16.Huang LE, Gu J, Schau M, Bunn HF.. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin–proteosome pathway. Proc Natl Acad Sci USA. 1990;95:7987–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumcke I, Schneider B, Fandrey J, Pagel H.. Effects of pro- and antioxidative compounds on renal production of erythropoietin. Endocrinology. 1999;140:641–645. [DOI] [PubMed] [Google Scholar]
- 18.Rehm M, Bruegger D, Christ F, et al. . Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischaemia. Circulation. 2007;116:1896–1906. [DOI] [PubMed] [Google Scholar]
- 19.Jordan J, Zhao Z, Vinten-Johansen J.. The role of neutrophils in myocardial ischaemia–reperfusion injury. Cardiovasc Res. 1999;43:860–878. [DOI] [PubMed] [Google Scholar]
- 20.Bolli R, Patel B, Jeroudi M, Lai E, McCay P.. Demonstration of free radical generation in stunned myocardium of intact dogs with the use of spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988;82:476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolli R, Jeroudi M, Patel B, et al. . Direct evidence that oxygen-derived free radicals contribute to post-ischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci USA. 1989;86:4695–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clermont G, Vergely C, Jazayeri S, et al. . Systemic free radical activation is a major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology. 2002;96:80–87. [DOI] [PubMed] [Google Scholar]
- 23.Tortolani A, Powell S, Misik V, et al. . Detection of alkolyl and carbon centered free radicals in coronary sinus blood from patients undergoing elective cardioplegia. Free Radic Biol Med. 1993;14:421–426. [DOI] [PubMed] [Google Scholar]
- 24.Brueckl C, Kaestle S, Kerem A, et al. . Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–463. [DOI] [PubMed] [Google Scholar]
- 25.Qadan M, Battista C, Gardner S, et al. . Oxygen and surgical site infection. Anesthesiology. 2010;113:369–377. [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Ku K, Kaneda T, et al. . Cardioprotective effects of lowering oxygen tension after aortic unclamping on cardiopulmonary bypass during coronary artery bypass grafting. Circ J. 2002;65:213–218. [DOI] [PubMed] [Google Scholar]
- 27.Ihnken K, Winkler A, Schlensak C, et al. . Normoxic cardiopulmonary bypass reduces oxidative myocardial damage and nitric oxide. J Thorac Cardiovasc Surg. 1998;116:327–334. [DOI] [PubMed] [Google Scholar]
- 28.Murry C, Jennings R, Reimer K.. Preconditioning with ischaemia: a delay of lethal cell injury in ischaemic myocardium. Circulation. 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 29.Kersten J, Schmeling T, Pagel P, Gross G, Warltier D.. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. [DOI] [PubMed] [Google Scholar]
- 30.Kaljusto M, Stenslokken K, Mori T, et al. . Preconditioning effects of steroids and hyperoxia on cardiac ischemia–reperfusion injury and vascular reactivity. Eur J Cardiothorac Surg. 2008;33:353–363. [DOI] [PubMed] [Google Scholar]
- 31.Petrosillo G, Di Venosa N, Moro N, et al. . In vivo hyperoxic preconditioning protects against rat heart ischaemia/reperfusion injury by inhibiting mitochondrial permeability transition pore opening and cytochrome c release. Free Radic Biol Med. 2011;50:477–483. [DOI] [PubMed] [Google Scholar]
- 32.Pourkhalili K, Hajizadeh S, Tiraihi T, et al. . Ischaemia and reperfusioninduced arrhythmias: role of hyperoxic preconditioning. J Cardiovasc Med. 2009;10:635–642. [DOI] [PubMed] [Google Scholar]
- 33.Pourkhalili K, Hajizadeh S, Akbari Z, et al. . Hyperoxic preconditioning fails to confer additional protection against ischaemia–reperfusion injury in acute diabetic rat heart. EXCLI Journal. 2012;11:263–273. [PMC free article] [PubMed] [Google Scholar]
- 34.Van de Water J, Kagey K, Miller I, et al. . Response of the lung to six to 12 hours of 100 percent oxygen inhalation in normal man. N Engl J Med. 1970;283:621–626. [DOI] [PubMed] [Google Scholar]
- 35.Comroe J, Dripps R, Dumke P, Deming M.. Oxygen toxicity. The effect of inhalation of high concentrations of oxygen for twenty-four hours on normal men at sea level and at a simulated altitude of 18,000 feet. JAMA. 1945;128:710–717. [Google Scholar]
- 36.Nader-Djabal N, Knight P, Davidson B, Johnson K.. Hyperoxia exacerbates microvascular lung injury following acid aspiration. Chest. 1997;112:1607–1614. [DOI] [PubMed] [Google Scholar]
- 37.Haniuda M, Dresler C, Mizuta T, Cooper J, Patterson G.. Free radical-mediated vascular injury in lungs preserved at moderate hypothermia. Ann Thorac Surg. 1995;60:1376–1381. [DOI] [PubMed] [Google Scholar]
- 38.Schelensak C, Doenst T, Preusser S, et al. . Cardiopulmonary bypass reduction of bronchial blood flow: A potential mechanism for lung injury in a neonatal pig model. J Thorac Cardiovasc Surg. 2002;123:1199–1205. [DOI] [PubMed] [Google Scholar]
- 39.Sinclair D, Haslam P, Quinlan G, Pepper J, Evans T.. The effect of cardiopulmonary bypass on intestinal and pulmonary endothelial permeability. Chest. 1995;108:718–724. [DOI] [PubMed] [Google Scholar]
- 40.Pizov R, Weiss Y, Oppenheim-Eden A, et al. . High oxygen concentration exacerbates cardiopulmonary bypass-induced lung injury. J Cardiothorac Vasc Anesth. 2000;14:519–523. [DOI] [PubMed] [Google Scholar]
- 41.Reber A, Budmiger B, Wenk M, et al. . Inspired oxygen concentration after cardiopulmonary bypass: Effects on pulmonary function with regard to endothelin-1 concentrations and venous admixture. Br J Anaesth. 2000;84:565–570. [DOI] [PubMed] [Google Scholar]
- 42.Floyd T, Shah P, Price C, et al. . Clinically silent cerebral ischaemic events after cardiac surgery: Their incidence, regional vascular occurrence and procedural dependence. Ann Thorac Surg. 2006;81:2160–2166. [DOI] [PubMed] [Google Scholar]
- 43.Groom R, Reed D, Lennon P, et al. . Detection and elimination of microemboli related to cardiopulmonary bypass.. Circ Cardiovasc Qual Outcomes. 2009;2:191–198. [DOI] [PubMed] [Google Scholar]
- 44.Taylor R, Borger M, Weisel R, Fedorko L, Feindel C.. Cerebral microemboli during cardiopulmonary bypass: increased emboli during perfusionist interventions. Ann Thorac Surg. 1999;68:89–93. [DOI] [PubMed] [Google Scholar]
- 45.Donald D, Fellows J.. Relation of temperature, gas tension and hydrostatic pressure to the formation of gas bubbles in extracorporeally oxygenated blood. Surg Forum. 1959;10:589–592. [PubMed] [Google Scholar]
- 46.Epstein P, Plesset M.. On the stability of gas bubbles in liquid–gas solutions. J Chem Phys. 1950;18:1505–1509. [Google Scholar]
- 47.Nollert G, Nagashima M, Bucerius J, Shin’oka T, Jonas R.. Oxygenation strategy and neurological damage after deep hypothermic circulatory arrest. 1. Gaseous microemboli. J Thorac Cardiovasc Surg. 1999;117:1166–1171. [DOI] [PubMed] [Google Scholar]
- 48.Georgiadis D, Wenzel A, Lehmann D, et al. . Influence of oxygen ventilation on Doppler microemboli signals in patients with artificial heart valves. Stroke. 1997;28:2189–2194. [DOI] [PubMed] [Google Scholar]
- 49.Bigdeli M.. Preconditioning with prolonged normobaric hyperoxia induces ischaemic tolerance partly by upregulation of antioxidant enzymes in rat brain tissue. Brain Res. 2009;1260:47–54. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Shi H, Liu K, et al. . Interstitial pO2 in ischaemic penumbra and core are differentially affected following transient focal cerebral ischaemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. [DOI] [PubMed] [Google Scholar]
- 51.Sighal A, Benner T, Roccatagliata L, et al. . A pilot study of normobaric oxygen therapy in acute ischaemic stroke. Stroke. 2005;36:797–802. [DOI] [PubMed] [Google Scholar]
- 52.Kang J, Maltenfort M, Vibbert M, et al. . Significance of arterial hyperoxia in critically ill stroke patients. Neurology. 2012;78 Meeting abstract PO2.222. [Google Scholar]
- 53.Ostrowski R, Graupner G, Titova E, et al. . The hyperbaric oxygen preconditioning-induced protection is mediated by a reduction of early apoptosis after transient global cerebral ischaemia. Neurobiol Dis. 2008;29:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearl J, Thomas D, Grist G, Duffy J, Manning P.. Hyperoxia for management of acid-base status during deep hypothermia with circulatory arrest. Ann Thorac Surg. 2000;70:751–755. [DOI] [PubMed] [Google Scholar]
- 55.Alex J, Laden G, Cale A, et al. . Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: A prospective randomized double blind trial. J Thorac Cardiovasc Surg. 2005;130:1623–1629. [DOI] [PubMed] [Google Scholar]
- 56.Zhen R, Wenxiang D, Zhaokang S, et al. . Mechanisms of brain injury with deep hypothermic circulatory arrest and protective effects of coenzyme Q10. J Thorac Cardiovasc Surg. 1994;108:126–133. [PubMed] [Google Scholar]
- 57.Vereczki V, Martin E, Rosenthal R, et al. . Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal cell death. J Cereb Blood Flow Metab. 2006;26:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nollert G, Nagashima M, Bucerius J, et al. . Oxygenation strategy and neurological damage after deep hypothermic circulatory arrest. II. Hypoxic versus free radical injury. J Thorac Cardiovasc Surg. 1999;117:1172–1179. [DOI] [PubMed] [Google Scholar]
- 59.Allen D, Maguire J, Mahdavian M, et al. . Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–996. [DOI] [PubMed] [Google Scholar]
- 60.Qadan M, Battista C, Gardner S, et al. . Oxygen and surgical site infection. Anesthesiology. 2010;113:369–377. [DOI] [PubMed] [Google Scholar]
- 61.Greif R, Akca O, Horn E, Kurz A, Sessler D.. Supplemental perioperative oxygen to reduce the incidence surgical wound infection. N Engl J Med. 2000;342:161–167. [DOI] [PubMed] [Google Scholar]
- 62.Belda F, Aguilera L, Garcia de la Asuncion J, et al. . Supplemental perioperative oxygen and the risk of surgical wound infection. A randomised controlled trial. JAMA. 2005;294:2035–2042. [DOI] [PubMed] [Google Scholar]
- 63.Bickel A, Gurevits M, Vamos R, Ivry S, Eitan A.. Perioperative hyperoxygenation and wound site infection following surgery for acute appendicitis. Arch Surg. 2011;146:464–470. [DOI] [PubMed] [Google Scholar]
- 64.Mayzler O, Weksler N, Domchik S, et al. . Does supplemental perioperative oxygen administration reduce the incidence of wound infection in elective colorectal surgery? Minerva Anestesiol. 2005;71:21–25. [PubMed] [Google Scholar]
- 65.Gardella C, Goltra L, Laschansky E, et al. . High concentration supplemental perioperative oxygen to reduce the incidence of postcesarian surgical site infection. Obstet Gynecol. 2008;112:545–552. [DOI] [PubMed] [Google Scholar]
- 66.Pryor K, Fahey T, Lien C, Goldstein P.. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population: A randomised controlled trial. JAMA. 2004;291:79–87. [DOI] [PubMed] [Google Scholar]
- 67.Meyhoff C, Wetterslev J, Jorgensen L, et al. ; for the PROXI Trial Group. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery. The PROXI randomized clinical trial. JAMA. 2009;302:1543–1550. [DOI] [PubMed] [Google Scholar]
- 68.Myles P, Leslie K, Chan M, et al. ; ENIGMA Trial Group. Avoidance of nitrous oxide for patients undergoing major surgery. A randomized controlled trial. Anesthesiology. 2007;107:221–231. [DOI] [PubMed] [Google Scholar]
- 69.Togioka B, Galvagno S, Sumida S, et al. . The role of perioperative oxygen therapy in reducing surgical site infection: A meta-analysis. Anesth Analg. 2012;114:334–342. [DOI] [PubMed] [Google Scholar]
- 70.Bakri M, Nagem H, Sessler D, et al. . Transdermal oxygen does not improve sternal wound oxygenation in patients recovering from cardiac surgery. Anesth Analg. 2008;106:1619–1626. [DOI] [PubMed] [Google Scholar]
- 71.Greif R, Lacyni S, Rapf B, Hickle R, Sessler D.. Supplemental oxygen reduces the incidence of postoperative nausea and vomiting. Anesthesiology. 1999;91:1246–1252. [DOI] [PubMed] [Google Scholar]
- 72.Goll V, Akca O, Grief R, et al. . Ondansetron is no more effective than supplemental intraoperative oxygen for prevention of postoperative nausea and vomiting. Anesth Analg. 2001;92:284–287. [DOI] [PubMed] [Google Scholar]
- 73.Treschan T, Zimmer C, Nass C, et al. . Inspired oxygen fraction of 0.8 does not attenuate postoperative nausea and vomiting after strabismus surgery. Anesthesiology. 2005;1034:6–10. [DOI] [PubMed] [Google Scholar]
- 74.Joris J, Poth N, Djamadar A, et al. . Supplemental oxygen does not reduce postoperative nausea and vomiting after thyroidectomy. Br J Anaesth. 2003;91:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orhan-Sungur M, Kranke P, Apfel C.. Does supplemental oxygen reduce postoperative nausea and vomiting? A meta-analysis of randomised trials. Anesth Analg. 2008;106:1733–1738. [DOI] [PubMed] [Google Scholar]