Abstract:
Three scenarios are presented, based on real patients, illustrating various clinical dilemmas involving critical illness and extracorporeal membrane oxygenation (ECMO). The scenarios are outlined in the form of questions and answers. In most cases there is no single correct answer to the dilemmas presented. The pros and cons of particular interventions are discussed along with the actual treatment provided. In all cases, the ECMO circuit consisted of a polymethylpentene oxygenator (Quadrox PLS; MAQUET Cardiovascular, Hirlingen, Germany) and a centrifugal pump (Rotaflow; MAQUET Cardiovascular). Case 2 has been previously published as a letter to the editor (1).
Keywords: extracorporeal membrane oxygenation, ECMO, complications, critical care, extracorporeal life support, respiratory failure, cardiac failure
CASE 1: ACUTE ‘ASTHMA’
A 44-year-old Indian woman presented to the hospital with acute shortness of breath (SOB). She had developed increasing SOB over the preceding 2 weeks but had become acutely unwell on the day of presentation. She had a history of asthma treated with inhaled corticosteroids and β2 agonists. Several years previously she had experienced an episode of acute SOB after the birth of a child, the cause of which was unknown.
In the emergency room she was treated for acute asthma with intravenous (IV) bolus doses of salbutamol (albuterol) and dexamethasone (8 mg). The total dose of salbutamol given by IV bolus was 5 mg. A salbutamol infusion was started at .2 μg/kg/min. Despite this therapy, she rapidly deteriorated with increasing dyspnea and falling peripheral oxygen saturation (SpO2). Her SpO2 fell from 95% to 85% while breathing oxygen through a rebreathing facemask. A decision was made to intubate and ventilate her after which her SpO2 was 90% (FiO2 1.0). Heart rate (HR) was 145 beats/min and mean arterial blood pressure (MAP) was 85 mmHg. A chest radiograph was performed (Figure 1). Blood gas analysis revealed a PaO2 of 7.1 kPa (53 mmHg), PaCO2 of 9.2 kPa (69 mmHg), a pH of 7.01, a base excess of −11, and lactate 7.3 mmol/L.
Figure 1.
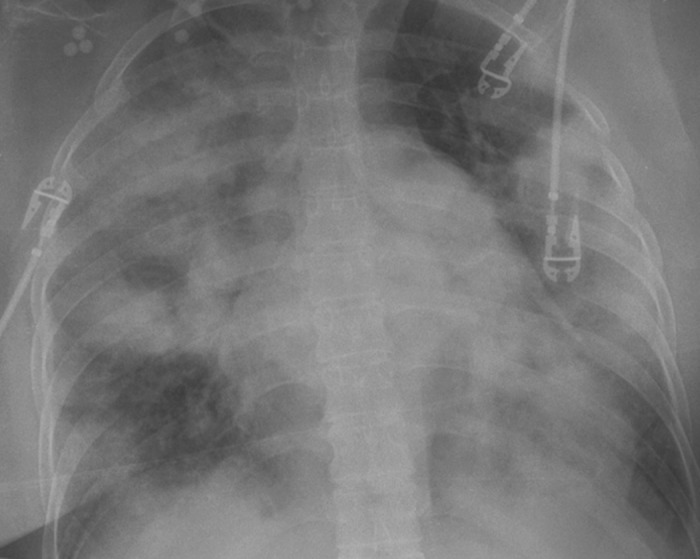
Chest radiograph showing widespread alveolar shadowing.
Question: What is the differential diagnosis?
Answer: The chest radiograph demonstrates widespread alveolar shadowing consistent with pulmonary edema or pneumonia (not asthma).
The patient was transferred to the cardiovascular intensive care unit (CVICU), Auckland City Hospital, Auckland, New Zealand. Initial treatment in the CVICU consisted of positive pressure ventilation (PPV) with positive endexpiratory pressure (PEEP) of 15 cm H2O, 40 mg IV furosemide, and IV antibiotics (meropenem and moxifloxacin).
Question: What is the next appropriate investigation?
Answer: An echocardiogram would help determine the cause of the chest radiographic abnormalities.
A transesophageal echocardiogram (TEE) was performed. The results are shown in Figures 2–4.
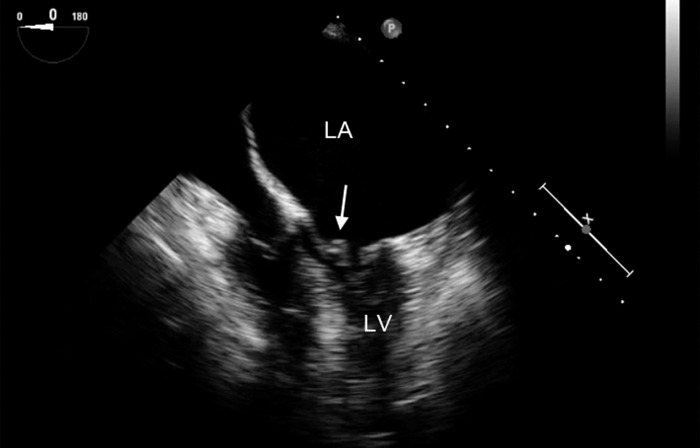
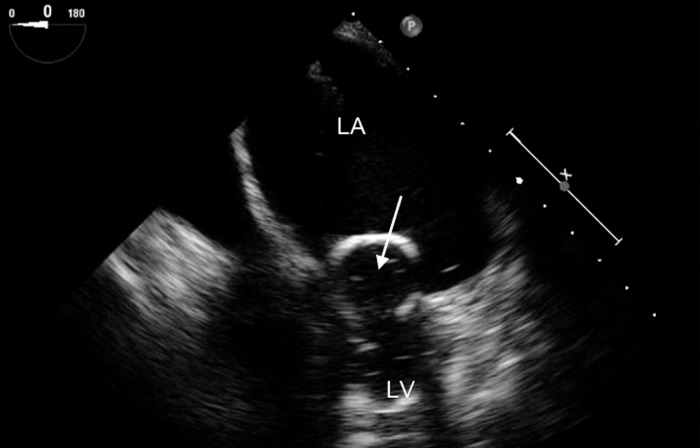
Figure 2.
Midesophageal four-chamber view showing a large left atrium (LA) and small left ventricle (LV), typical of mitral stenosis. The arrow points to the thickened anterior mitral leaflet. The frame is obtained during diastole and shows limited opening of the mitral leaflets.
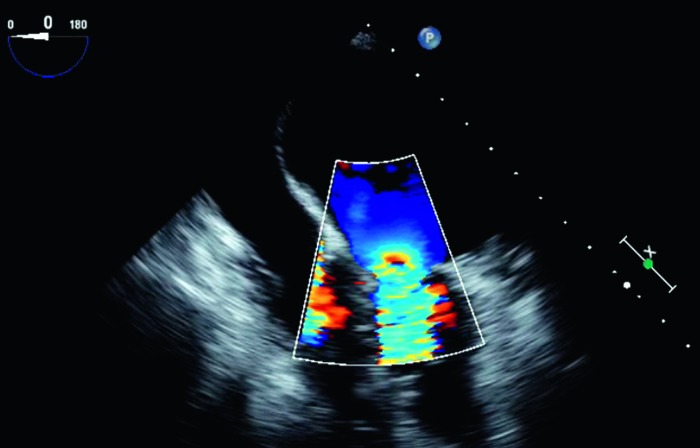
Figure 3.
Midesophageal four-chamber view with color flow Doppler imaging demonstrating flow acceleration toward the mitral valve in diastole, suggesting narrowing of the mitral orifice.
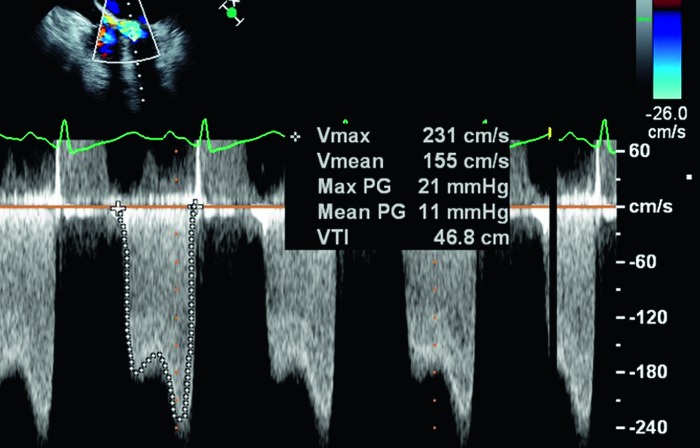
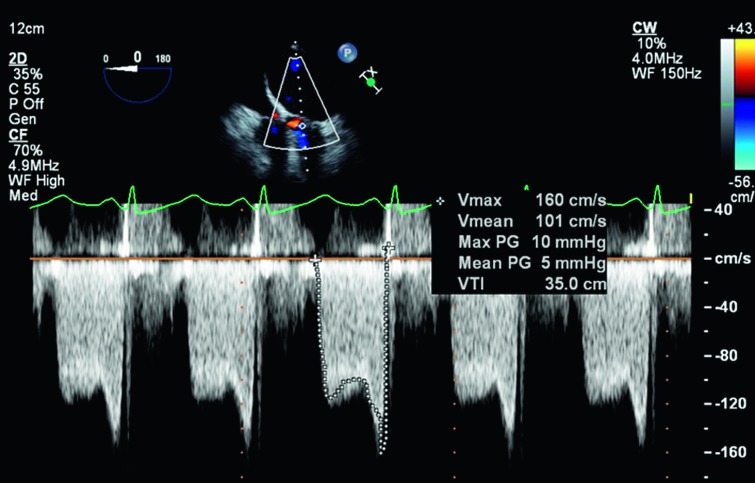
Figure 4.
Continuous-wave Doppler waveform through the mitral valve demonstrating a mean diastolic gradient of 11 mmHg, consistent with severe mitral stenosis.
Question: What is pathology is demonstrated in Figures 2–4?
Answer: Rheumatic mitral stenosis.
In the CVICU, the patient’s respiratory status further deteriorated. Frothy pulmonary edema fluid appeared in the endotracheal tube despite 15 cm H2O PEEP. PPV was not associated with any significant tidal ventilation despite a peak inspiratory pressure (PIP) of 45 cm H2O. SpO2 fell to 70% despite hand ventilation through a manual resuscitator. The patient’s MAP fell from 75 mmHg to 45 mmHg with manual ventilation.
Question: Why did the patient’s respiratory status deteriorate in the emergency room?
Answer: The most likely reason is that salbutamol exacerbated the effects of mitral stenosis.
Salbutamol-induced tachycardia shortens diastole, which, in a patient with mitral stenosis (who is critically dependent on an adequate diastolic filling time to maintain cardiac output), will increase left atrial (LA) pressure and worsen pulmonary edema. Also, as a nonspecific pulmonary vasodilator, salbutamol inhibits hypoxic pulmonary vasoconstriction, worsening ventilation/perfusion matching in the lung and therefore reducing arterial oxygen saturation (SaO2).
Question: Why did the patient’s MAP fall after initiation of manual ventilation?
Answer: High intrathoracic pressure reduced right ventricular (RV) preload and increased RV afterload. Additionally, salbutamol-induced lactic acidosis may have contributed to myocardial depression.
Hand ventilation with a manual resuscitator can generate very high intrathoracic pressures (>50 cm H2O). High intrathoracic pressure reduces systemic venous return (reduced RV preload) and increases RV afterload, which, in a patient with marginal cardiac output (as a result of the combination of mitral stenosis and tachycardia) and salbutamol-induced systemic vasodilation, would likely cause severe hypotension.
Question: What are the options for acute cardiorespiratory support in this patient?
Answer: In the first instance, stopping the salbutamol infusion and administering a vasopressor such as norepinephrine may be beneficial. More definitive options for cardiorespiratory support include 1) cardiopulmonary bypass (CPB) with surgical correction of the mitral stenosis; 2) extracorporeal membrane oxygenation (ECMO); 3) high-frequency oscillation (HFO) ventilation; and 4) an intraaortic balloon pump (IABP).
The salbutamol infusion was stopped and a norepinephrine infusion was started at .3 μg/kg/min. Despite this, MAP remained low (40–50 mmHg), tachycardia persisted (HR 130 beats/min), and SpO2 was 60–70% despite manual ventilation. In view of the inability to provide adequate oxygenation despite manual ventilation at high pressure, HFO was ruled out as unlikely to ameliorate the situation. Although an IABP may have provided some circulatory support and helped reduced LA pressure, it is unlikely to have corrected the critical state, particularly in view of the persisting tachycardia. CPB and ECMO were both considered possible solutions. In view of the institutional experience with rapid deployment of ECMO, a decision was made to proceed to urgent ECMO.
Question: Should venoarterial (VA) or venovenous (VV) ECMO be used in this patient?
Answer: This is not a straightforward choice. VA EMCO directly supports the lungs and the circulation but may be associated with upper body hypoxemia in this patient. VV ECMO potentially affords easier cannulation in an emergency, avoids the problem of upper body hypoxemia, and provides indirect circulatory support.
Despite having underlying cardiac pathology (mitral stenosis), the predominant problem in this patient was respiratory failure. Although VV EMCO does not provide direct circulatory support, reduction of ventilation to rest settings after institution of VV ECMO often results in a dramatic improvement in the hemodynamic state.
A particular concern with peripheral VA EMCO (in which the arterial return cannula is placed in the femoral artery as opposed centrally in the aortic root) is the potential for upper body hypoxemia. Upper body hypoxemia occurs when cardiac function is vigorous but pulmonary function is impaired. Deoxygenated blood, having passed through poorly functioning lungs, is ejected from the left ventricle (LV), preferentially perfusing the upper body (coronary and cerebral circulations). This patient would have been at high risk for this problem.
Our preferred method of cannulation for all forms of peripheral ECMO is intensivist-led cannulation in the CVICU through a Seldinger technique (2,3). Surface ultrasound is used to confirm placement of the Seldinger wire in the femoral or jugular vessels and TEE is used to confirm placement of the wires in the aorta and vena cavae. Arterial cannulation is technically more difficult and more prone to complications than venous cannulation.
For the reasons outlined here, we elected to place the patient on VV ECMO.
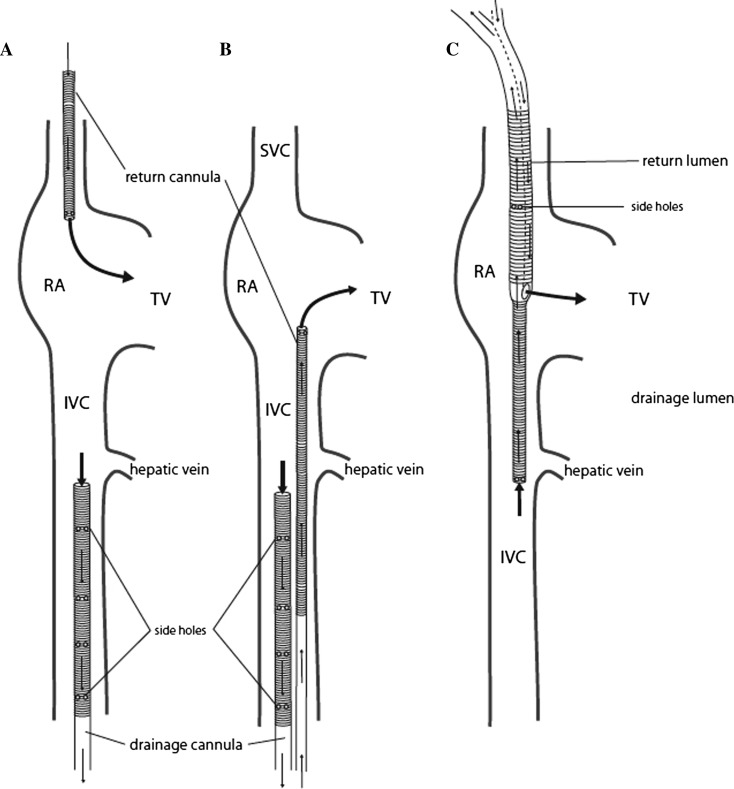
Possible cannulae configurations for VV ECMO are shown in Figure 5. (2). Our preferred technique is configuration A: For return, a 19-French (Fr) cannula (HLS Cannula System; MAQUET Cardiovascular, Hirlingen, Germany) was inserted in the right internal jugular vein and advanced so the tip lay at the junction of the superior vena cava and right atrium. For drainage, a 25-Fr multilumen cannula (HLS Cannula System; MAQUET Cardiovascular) was inserted in a femoral vein and advanced into the inferior vena cava (IVC) so the tip lay just below the origin of the hepatic vein.
Figure 5.
Cannula configurations for venovenous extracorporeal membrane oxygenation (VV ECMO). With configuration A, the return cannula is placed in the right internal jugular vein and advanced so the tip lies at the junction of the superior vena cava (SVC) and right atrium (RA); the drainage cannula is placed in a femoral vein and advanced into the inferior vena cava (IVC) so the tip lies at the level of the level of the hepatic vein. With configuration B, the return cannula is advanced into the RA from a femoral vein and the drainage cannula is advanced into the inferior vena cava (IVC) to the level of the hepatic vein through the other femoral vein. With configuration C, a double-lumen ECMO cannula is placed in the right internal jugular vein. The return lumen exits the cannula in the RA and the tip of the drainage lumen lies in the IVC at the level of the hepatic vein. From Sidebotham D, Allen SJ, McGeorge A, Ibbott N, Willcox T. Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anaesth. 2012;26: 893–909, Image 7. Used with permission.
With VV ECMO, ideally all of the oxygenated blood from the return cannula passes through the tricuspid valve and passes into the pulmonary circulation. If oxygenated blood from the return cannula passes into the IVC, recirculation through the ECMO circuit occurs and results in high oxygen saturation in the drainage limb of the circuit and a low (patient) SaO2. If circuit flow is at least 70% of cardiac output, and recirculation is minimal, a SaO2 above 88% can be achieved in most adults, even in the absence of any pulmonary function.
After institution of VV ECMO at 5 L/min, SaO2 increased to 93% on rest ventilator settings (PIP 25 cm H2O, PEEP 10 cm H2O, respiratory rate 10 breaths/min). MAP increased to 80 mmHg and the norepinephrine infusion was rapidly weaned off.
Question: Now that the patient is stable on VV ECMO, what are the options for further management?
Answer: The options are 1) continue ECMO with no further acute interventions; 2) urgent mitral valve surgery; or 3) urgent balloon mitral valvotomy (BMV).
Conservative management would allow time for the effects of salbutamol to wear off and for the pulmonary edema to resolve. If the patient’s deterioration were secondary to a chest infection, a period of antimicrobial therapy would be beneficial. It is possible that a conservative strategy would allow the patient to be weaned from ECMO and return to their premorbid state. The mean transmitral pressure gradient of 11 mmHg, documented before EMCO, may reduce once the patient’s heart rate normalizes. Countered against this is the possibility of persisting pulmonary edema resulting in failure to wean from ECMO.
Urgent mitral valve surgery or BMV provides definitive management of the mitral stenosis, but both techniques are not without risk in a critically ill patient on ECMO. Acutely, cardiac surgery with CPB may lead to worsening of the patient’s cardiorespiratory function. BMV is contraindicated in the presence of significant mitral regurgitation or heavy valvular calcification, neither of which was present in this patient.
Repeat TEE, once the patient’s HR had fallen to 90 beats/min, demonstrated a mean transmitral gradient of 10 mmHg. Therefore, a decision was made to proceed to urgent BMV in the cardiac catheterization laboratory. The procedure was performed uneventfully (Figure 6) and resulted in a reduction in the mean transmitral gradient to 5 mmHg (Figure 7).
Figure 6.
Midesophageal four-chamber view obtained during balloon inflation (arrow) during balloon mitral valvotomy. The balloon is inflated across the mitral valve. LA, left atrium; LV, left ventricle.
Figure 7.
Continuous-wave Doppler signal across the mitral valve after balloon valvotomy. The mean diastolic gradient across the valve is now 5 mmHg, indicating mild stenosis.
Over the next 12 hours, the patient’s respiratory function improved dramatically with complete resolution of pulmonary edema on the chest radiograph. ECMO was discontinued after 18 hours and the patient was extubated after 36 hours. At follow-up, 6 months later, the patient remained symptomatically well with mild mixed mitral valve disease.
The case highlights the difficulties in choosing between VV and VA EMCO in patients with combined cardiorespiratory failure. Primary issues to consider are the likelihood that the hemodynamic state will recover once rest ventilator settings are instituted and the risk of upper body hypoxemia with peripheral VA EMCO.
CASE 2: CARDIOGENIC SHOCK
A 61-year-old man presented to a peripheral hospital with a 5-day history of an influenza-like illness. At presentation, his blood pressure was 50/30 mmHg and his HR was 50 beats/min. He had no respiratory distress and normal mentation. His electrocardiogram demonstrated sinus rhythm, right bundle branch block, and 3 mm of ST elevation in leads I, II, and III. His troponin T was greater than 10,000 μg/L (normal < .03 μg/L). Chest radiograph was normal.
Question: What is the differential diagnosis?
Answer: The most likely diagnosis is an inferior ST elevation myocardial infarction. The 5-day history of an influenza-like illness raises the possibility of viral myocarditis.
Initial treatment included 2000 mL of IV crystalloid fluid, IV dobutamine infusion at 10 μg/kg/min, and aspirin and clopidogrel. He was transferred by air ambulance to our tertiary referral hospital for further diagnosis and treatment. On arrival he was hypotensive (blood pressure 70/50 mmHg), bradycardic (HR 40 beats/min), but had normal mentation and no respiratory distress.
Question: What further treatment options are appropriate?
Answer: An epinephrine infusion and an IABP will help support the circulation. A temporary pacing wire may be required to support the HR.
Question: What investigations should be performed?
Answer: Urgent coronary angiography is indicated. If the coronary angiogram does not indicate obstructive coronary lesions, a myocardial biopsy should be obtained looking for evidence of myocarditis. Additionally, an echocardiogram should be performed.
An epinephrine infusion was started at .2 μg/kg/min. Blood pressure increased to 85/45 mmHg and HR increased to 70 beats/min. After coronary angiography, an IAPB was inserted in the cardiac catheterization laboratory. Angiography demonstrated normal coronary arteries; therefore, a myocardial biopsy was obtained. Transthoracic echocardiography demonstrated severe biventricular dysfunction (estimated LV ejection fraction 20%) and mild aortic regurgitation (Figure 8). On the basis of these results, a presumptive diagnosis of viral myocarditis was made, which was subsequently confirmed when the results of the myocardial biopsy became known.
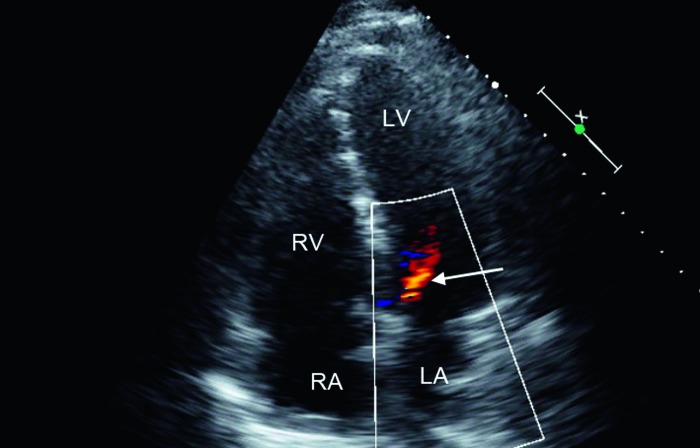
Figure 8.
Apical four-chamber view (transthoracic imaging) demonstrating mild aortic regurgitation (arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Over the next few hours in the CVICU, the patient developed progressive hypotension (despite increasing the epinephrine infusion to .4 μg/kg/min), poor peripheral perfusion, anuria, and he became disorientated. A plan was made to intubate and ventilate him before cannulation for VA ECMO. After induction of anesthesia (3 mg midazolam, 20 mg propofol, 100 mg rocuronium), the patient became profoundly hypotensive necessitating cardiac massage and bolus epinephrine (1 mg + 1 mg). Cannulation for ECMO was performed during external cardiac massage.
A 19-Fr return cannula (HLS Cannula System; MAQUET Cardiovascular) was placed in the left femoral artery, a 12-Fr distal perfusion cannula was placed retrogradely in the femoral artery, and a 25-Fr multilumen drainage cannula (HLS Cannula System; MAQUET Cardiovascular) was placed in a right femoral vein. VA ECMO was initiated at a flow of 5.5 L/min. Unfractionated heparin (3000 international units) was administered. The initial activated clotting time (ACT) was 220 seconds. MAP was 65 mmHg and the arterial waveform was nonpulsatile. However, within 5 minutes of starting ECMO, frothy pulmonary edema fluid appeared in the endotracheal tube.
Question: What is the usual cause of pulmonary edema after initiation of VA ECMO for fulminant myocarditis?
Answer: In patients with fulminant myocarditis, myocardial depression can be so severe that the LV becomes noncontractile. However, there may be ongoing blood return to the LV through the pulmonary, bronchial, and Thebesian circulations. The combination of a noncontractile LV and ongoing left-heart blood return leads to LV distension, high LA pressure, and pulmonary edema.
Performing an echocardiogram and demonstrating a distended, noncontractile LV diagnoses the problem. The aortic valve opens intermittently or not at all, and there is typically severe mitral regurgitation.
Question: How can acute LV distension be treated?
Answer: Increasing ECMO flow and starting (or increasing) inotropic support may ameliorate the problem. If these measures are ineffective, decompressing the LV by performing an atrial septostomy is indicated (4,5).
Increasing ECMO flow increases the proportion of the systemic venous return that passes into the drainage cannula, thereby reducing flow through the pulmonary circulation. Increasing inotropic support may promote some LV ejection, thereby decompressing the LV. An atrial septostomy, which can be performed percutaneously in the cardiac catheter laboratory, decompresses the LV by allowing left-to-right flow across the atrial septum.
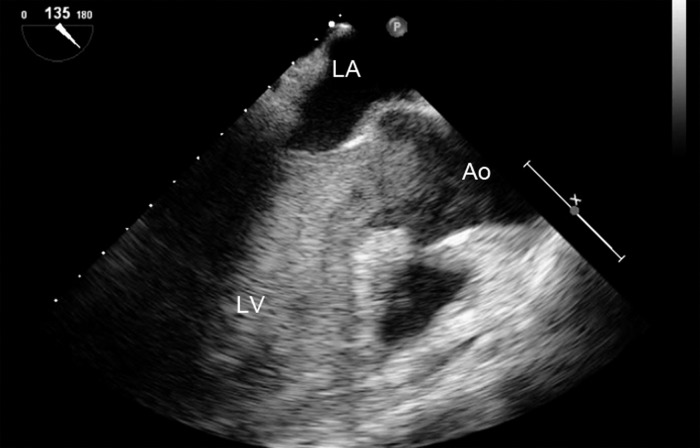
The TEE findings in this patient are shown in Figures 9–11.
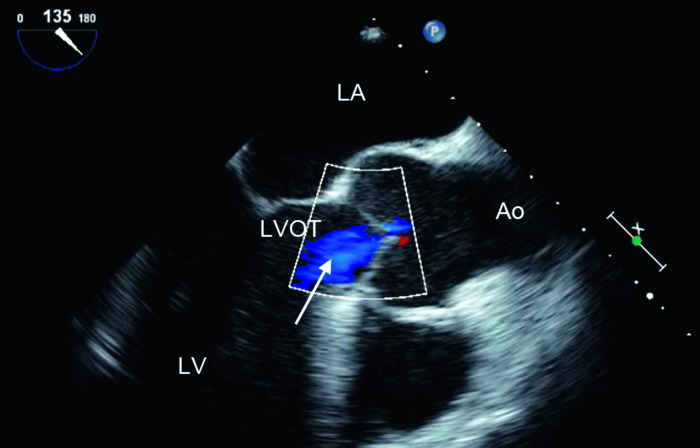
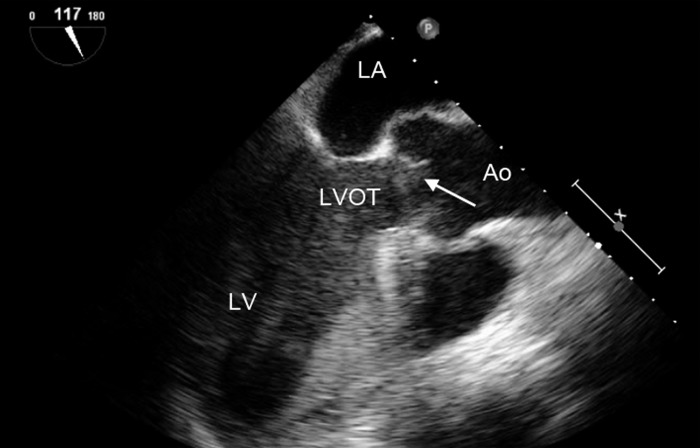
Figure 9.
Midesophageal long axis view, demonstrating low-velocity aortic regurgitation (arrow). LV, left ventricle; LA, left atrium; LVOT, left ventricular outflow tract; Ao, aorta.
Figure 10.
Midesophageal long axis view demonstrating the aortic valve in the open position (arrow). When viewed as a loop (not demonstrated in this still frame), the aortic valve is in the open position throughout the cardiac cycle. LV, left ventricle; LA, left atrium; LVOT, left ventricular outflow tract; Ao, aorta.
Figure 11.
Midesophageal long axis view demonstrating dense echo contrast in the LV, consistent with thrombus. LV, left ventricle; LA, left atrium; Ao, aorta.
Question: What do the TEE images in Figures 9–11 demonstrate?
Answer: In this patient, in addition to the mechanisms outlined previously, mild aortic regurgitation led to distension of the LV. Within a short period of time, LV end-diastolic pressure (LVEDP) equalized with MAP, causing the aortic valve to remain open and resulting in the absence of blood flow in the LV, leading to thrombus formation despite adequate anticoagulation.
In Figure 9, the aortic regurgitation is low velocity because LVEDP is very high (i.e., similar to LVEDP), thus reducing the pressure gradient between the aorta and the LV. Figures 10 and 11 were obtained a few minutes after Figure 9. The aortic valve is in the open position (and on the movie clip was in the open position throughout the cardiac cycle), suggesting that LVEDP and MAP have equalized. Figure 11 demonstrates extensive thrombus in the LV.
Question: Can anything be done to retrieve the situation?
Answer: There are few options in this circumstance.
The epinephrine infusion was increased but this maneuver but did not promote LV contraction. An atrial septostomy was performed in an attempt to decompress the LV but this was not successful. Increasing circuit flows would have increased MAP, exacerbating LV distension, and so was not attempted. After extensive discussion, the situation was felt to be futile. ECMO was discontinued and the patient died.
This case highlights the problem of LV distension in patients supported with VA ECMO who have profound myocardial depression and even mild degrees of aortic regurgitation. Alternative strategies for mechanical cardiovascular support in this circumstance would include placement of a left-ventricular assist device (which provides more complete LV unloading than VA ECMO), direct LV venting (e.g., by a surgically placed vent), and surgical correction of the aortic regurgitation.
CASE 3: PRIMARY GRAFT FAILURE AFTER HEART TRANSPLANTATION
An 18-year-old man underwent cardiac transplantation for dilated cardiomyopathy. After implantation of the graft, the patient was unable to separate from CPB as a result of severe biventricular failure and was therefore placed on central VA ECMO through the same cannulae used for CPB. ECMO flow was 5–6 L/min with a pump speed of 3000–4000 revolutions/min (rpm).
After this procedure, the patient had severe coagulopathy and thrombocytopenia. There was persistent bleeding from the surgical site despite transfusing multiple blood products (packed cells, fresh-frozen plasma, cryoprecipitate, and platelets). The sternum was left open but the skin was closed over the wound and cannulae and the patient brought to the CVICU. In the CVICU there was high output (>100 mL/h) from the chest drains despite normal coagulation tests and platelet count.
Questions: Should the patient be heparinized for VA ECMO and is it appropriate to administer recombinant activated Factor VII (VIIa) to help control postoperative bleeding?
Answer: In the presence of persistent postoperative bleeding, systemic heparinization should be avoided in ECMO-supported patients. There are limited data on the safety of recombinant Factor VIIa in patients receiving ECMO. Although Factor VIIa has been used successfully during ECMO (6), it has been associated with fatal cardiac thrombosis in this circumstance (7,8).
We elected not to administer Factor VIIa. Over the next few hours, drain output decreased (<50 mL/h). Six hours postoperatively, the patient developed low ECMO flow (2.5 L/min) despite high pump speed (4500 rpm).
Question: What is the differential diagnosis for low flow and high pump speed?
Answer: Possible causes in this patient include cannula malposition, thrombus in the circuit or cannulae, cardiac tamponade, or hypovolemia.
TEE examination demonstrated thrombus compressing the heart. Surgical re-exploration was performed in the CVICU with removal of large amounts of blood and clot from the pericardial space. The chest was left open at the end of the procedure. After this surgical re-exploration, ECMO flow and pump speed normalized.
Subsequently, a moderate heparin effect was documented (activated partial thromboplastin time 51 seconds; ACT 160 seconds); therefore, 50 mg protamine was administered.
The findings from a repeat TEE examination, performed 30 minutes later to exclude reaccumulation of pericardial tamponade, are shown in Figures 12 and 13:
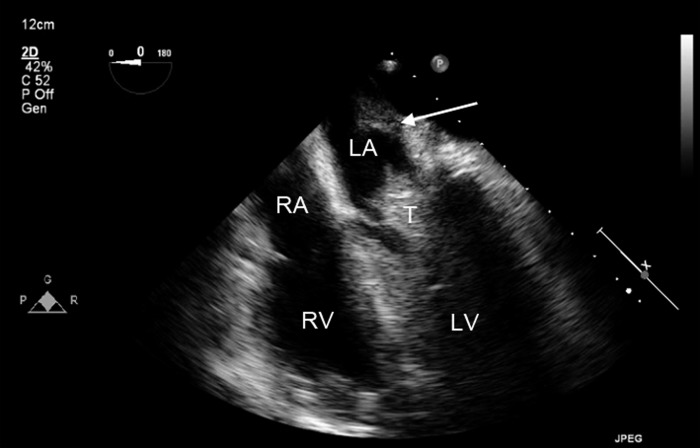
Figure 12.
Midesophageal four-chamber view demonstrating thrombus (T) in the left heart lodged between the open leaflets of the mitral valve. This patient has undergone heart transplantation. The ridge, identified by the arrow, demonstrates the suture line between the native and donor left atrium (LA). LV, left ventricle; RA, right atrium; RV, right ventricle.
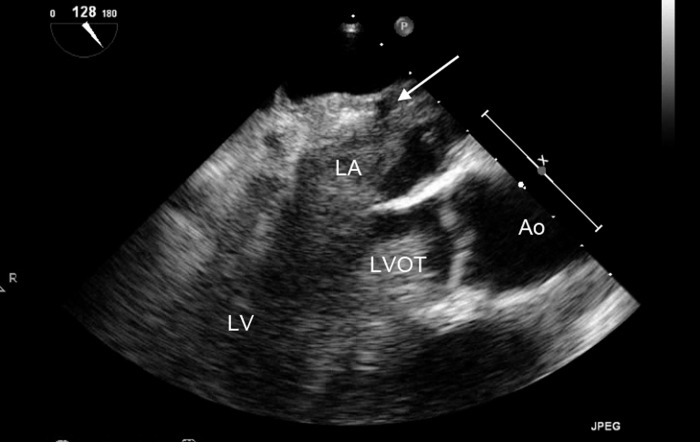
Figure 13.
Midesophageal long axis view demonstrating extensive thrombus throughout the left heart. The suture line between the native left atrium (LA) (no thrombus seen) and the donor LA (thrombus present) is identified by the arrow. LV, left ventricle; LVOT, left ventricular outflow tract; Ao, aorta.
Question: What is demonstrated in Figures 12 and 13?
Answer: There is extensive thrombus throughout left heart.
The combination of very poor LV function, resulting in stasis within the left heart, and no anticoagulation led to extensive thrombus formation. The earlier period of cardiac tamponade, with compression of the heart, may have increased the likelihood of intracavity thrombus forming.
Question: What are the therapeutic options?
Answer: Promoting LV ejection by increasing inotropic therapy would cause systemic embolization of thrombus. Options for thrombus removal (while minimizing the chance of systemic embolization) include administering a thrombolytic agent and surgical thrombectomy. If clot removal were successful, subsequent interventions to promote blood flow in the LV would be required to prevent the situation recurring.
Given the risk of causing further bleeding, thrombolytic therapy was felt to be contraindicated. A decision was made to take the patient to the operating room and remove as much thrombus as possible through aortic and LA incisions. Additionally, an atrial septostomy was performed to help minimize blood stasis in the left heart. After this procedure, the patient was fully anticoagulated with heparin and inotropic therapy was introduced to promote LV ejection.
Subsequently, the patient was weaned from VA ECMO on Day 5 and made a full neurological recovery. He was discharged from the CVICU on Day 21.
This case highlights the risk of intracardiac thrombus forming in patients supported with VA ECMO who are not anticoagulated and have blood stasis as a result of poor LV function and/or cardiac compression from tamponade.
REFERENCES
- 1.Sidebotham D, Allen S, McGeorge A, Beca J.. Catastrophic left heart distension following initiation of venoarterial extracorporeal membrane oxygenation in a patient with mild aortic regurgitation. Anaesth Intensive Care. 2012;40:568–569. [PubMed] [Google Scholar]
- 2.Sidebotham D, Allen SJ, McGeorge A, Ibbott N, Willcox T.. Venovenous extracorporeal membrane oxygenation in adults: Practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth. 2012;26:893–909. [DOI] [PubMed] [Google Scholar]
- 3.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J.. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: Part 2-technical considerations. J Cardiothorac Vasc Anesth. 2010;24:164–172. [DOI] [PubMed] [Google Scholar]
- 4.Koenig PR, Ralston MA, Kimball TR, Meyer RA, Daniels SR, Schwartz DC.. Balloon atrial septostomy for left ventricular decompression in patients receiving extracorporeal membrane oxygenation for myocardial failure. J Pediatr. 1993;122:S95–S99. [DOI] [PubMed] [Google Scholar]
- 5.Seib PM, Faulkner SC, Erickson CC, et al. . Blade and balloon atrial septostomy for left heart decompression in patients with severe ventricular dysfunction on extracorporeal membrane oxygenation. Catheter Cardiovasc Interv. 1999;46:179–186. [DOI] [PubMed] [Google Scholar]
- 6.Dunne B, Xiao P, Andrews D.. Successful use of factor VIIa to control life-threatening post-operative haemorrhage in a patient on extracorporeal membrane oxygenation. Heart Lung Circ. 2012;21:229–230. [DOI] [PubMed] [Google Scholar]
- 7.Syburra T, Lachat M, Genoni M, Wilhelm MJ.. Fatal outcome of recombinant factor VIIa in heart transplantation with extracorporeal membrane oxygenation. Ann Thorac Surg. 2010;89:1643–1645. [DOI] [PubMed] [Google Scholar]
- 8.Chalwin RP, Tiruvoipati R, Peek GJ.. Fatal thrombosis with activated factor VII in a paediatric patient on extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2008;34:685–686. [DOI] [PubMed] [Google Scholar]