Abstract:
An important mechanism for postoperative cognitive impairment after cardiac surgery using cardiopulmonary bypass (CPB) is microemboli. One component of the CPB circuit—the cardiotomy—is a major source of gaseous microemboli because it aspirates significant volumes of air with blood from the operative field and intracardiac chambers. Cardiotomies are either integrated within an open hardshell venous reservoir (IC-HSVR) or are a separate canister attached to a softshell collapsible venous reservoir bag (SC-SSVR). The purpose of this study was to compare the Medtronic IC-HSVR (Affinity NT CVR) with Medtronic’s SC-SSVR (CB 1351, CBMVR 1600) in terms of relative microbubble transmission during cardiotomy infusion. A recirculating in vitro circuit primed with blood was used to compare the two cardiotomy-reservoir systems with the venous reservoir in the SC-SSVR further assessed in a fully closed or partially open state (SC-SSVR-closed; SC-SSVR-open). Microbubbles were detected using a GAMPT BC100 Doppler system in the outflow line of the venous reservoir. Measurements were taken before (baseline) and after aerated prime was pumped into the cardiotomy while altering pump flow rates (3 L/min; 5 L/min) and reservoir prime volumes (400 mL; 900 mL). Infusing cardiotomy blood into the venous reservoir was associated with an increase in microbubbles and bubble volume transmitted by both cardiotomy-reservoir systems with the magnitude rising with reduced prime volumes. The effect was markedly greater with the IC-HSVR. The IC-HSVR also transmitted larger bubbles, particularly with reduced prime volumes. There was no significant difference in microbubble transmission seen between the SC-SSVR-closed and SC-SSVR-open. The SC-SSVR transmits fewer microbubbles than the IC-HSVR during cardiotomy infusion and should be considered as the preferential system. Because both cardiotomy-reservoir systems transmitted microbubbles during cardiotomy infusion, particularly at the lower venous reservoir volume, it is important to use strategies to minimize cardiotomy microbubble infusion.
Keywords: cardiotomy, hardshell venous reservoir, softshell venous reservoir, microbubbles
Cardiopulmonary bypass (CPB) remains a popular solution to the challenge of operating on the heart. Such is the success of CPB that cardiac operations are being performed on older and sicker patients (1,2). However, elderly patients are more predisposed to neurological complications after cardiac operations (3). An important cause of postoperative cognitive impairment after cardiac surgery is microemboli (4–6).
A significant source of emboli during CPB is the extracorporeal circuit (ECC) itself (7–9). Most microemboli occurring during CPB are gaseous (10). One component of the ECC—the cardiotomy—is potentially a major contributor of microbubbles because it typically aspirates significant volumes of air with the blood; this aerated-frothy blood may not be completely debubbled before being infused into the venous reservoir (11–13).
Two kinds of cardiotomy systems are currently used: the more popular cardiotomy integrated within a hardshell venous reservoir (IC-HSVR) and a separated cardiotomy canister that is attached to a softshell collapsible venous reservoir bag (SC-SSVR) (14). A recent in vitro investigation examined five models of IC-HSVR showing that they were associated with significantly increased microbubble transmission in the reservoir outlets when aerated blood prime was pumped into their respective cardiotomies (15).
No known study has been published specifically examining the microbubble handling properties of the SC-SSVR system or compared the SC-SSVR with the IC-HSVR. Ranking the relative microbubble handling capabilities of both cardiotomy-reservoir designs at baseline, and when emulating clinical cardiotomy suctioning by pumping aerated prime into the cardiotomy, would give perfusionists a better understanding of the performance of either system. More specifically, we wanted to determine if these two systems transmit microbubbles when aerated prime is introduced into their respective cardiotomies and, if they do, which of the cardiotomy-reservoir systems transmits fewer microbubbles. This was investigated by comparing a hardshell venous reservoir and integrated cardiotomy (Trillium Affinity NT 541T Integrated CVR; Medtronic, Australasia) with a softshell venous reservoir (CBMVR 1600; Medtronic, Australasia) and separated cardiotomy (Intersept CB1351; Medtronic, Australasia) in terms of relative microbubble transmission at baseline and during aerated cardiotomy prime infusion, in vitro, over a range of priming volumes and flow rates.
MATERIALS AND METHODS
Test Circuit
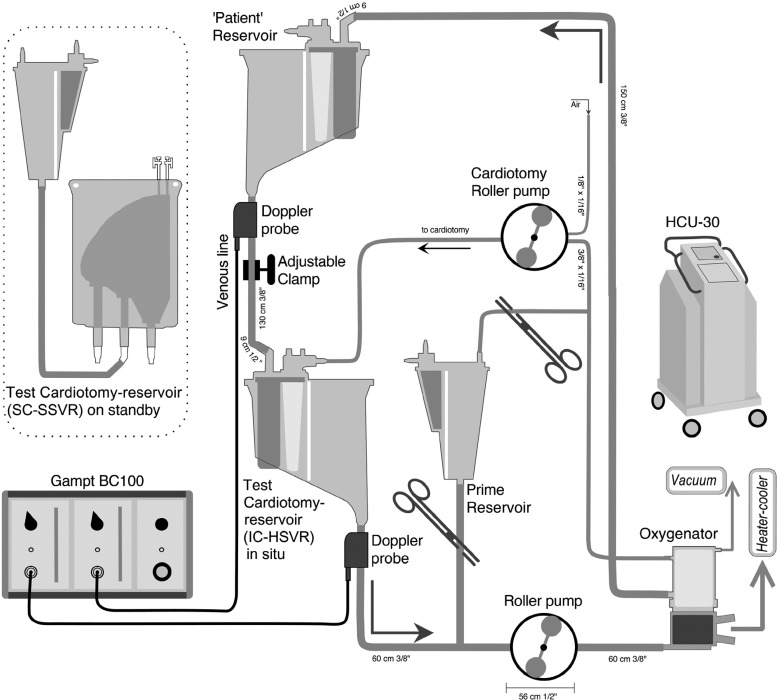
A recirculating test circuit was constructed; all components were new (Figure 1). The patient was simulated by a HSVR (Trillium Affinity NT 541T Integrated CVR; Medtronic). This “patient” reservoir was filled to the 1000-mL level marker to facilitate deairing the recirculating prime. To provide a constant siphonage drainage pressure, the “patient” reservoir fluid level was kept at 100 cm above the outlet of the test reservoir; this was accomplished by adjusting a variable clamp positioned on the “venous line” connecting the “patient” reservoir to the test reservoir.
Figure 1.
In vitro circuit. See text for details. Note: not to scale.
The fluid that drained into the test reservoir was pumped out into a hollow fiber membrane oxygenator (Trillium Affinity NT 541T; Medtronic, Australasia) by a calibrated and properly occluded roller pump (Sarns 8000; Terumo, Australasia). This oxygenator helped remove microbubbles that had been introduced into the circuit, a process that was facilitated by connecting the oxygenator’s gas inlet port to suction (−250–300 mmHg) while sealing all other openings. From the oxygenator, the prime returned to the “patient” reservoir.
Changes in test venous reservoir volumes were made by adding or draining prime through a volume-calibrated cardiotomy reservoir (Intersept CB1351; Medtronic) positioned between the test reservoir and the roller pump. To set a baseline, the test reservoir was bypassed. Then, with a confirmed 900 mL in this “priming” reservoir and 1000 mL in the “patient” reservoir, the test reservoir was installed. The test reservoir was then primed by draining 900 mL or 400 mL as needed from the “priming” reservoir. No priming volume adjustment was made to account for hold-up volumes (dynamic prime volumes) at the two flow rates used.
Prime
To simulate the non-Newtonian rheological properties of blood, the prime consisted of human red blood cells suspended in crystalloid. The circuit was initially primed with 3000 mL Plasma-Lyte 148 (Baxter Healthcare, Australia) to which expired packed red blood cells were added. By hemofiltering, 3150 mL prime with an hematocrit 25–26% was created. A heater-cooler unit (HCU-30; Macquet, Australia Pty. Ltd.) attached to the in situ oxygenator kept this prime at approximately 34°C.
Test Venous Reservoirs and Cardiotomies
Two reservoir designs with accompanying cardiotomies were assessed for their air-handling performance: the softshell collapsible venous reservoir bag (CBMVR 1600; Medtronic) attached to a separated cardiotomy (Intersept CB1351; Medtronic) and the hardshell open venous reservoir with integrated cardiotomy (Trillium Affinity NT 541T Integrated CVR; Medtronic). The SSVR is housed in a cage that can be opened or closed to, respectively, increase or decrease reservoir volumes. The SSVR was tested in a fully closed (minimal volume) or in a one-step out-of-latch position on the reservoir bag holder back plate. The operation of the SSVR at both the fully closed (SSVRclosed) and one latch open position (SSVR-open) was investigated to understand any changes in air-handling abilities between these two, commonly used modes. Three copies of each cardiotomy-reservoir system were used. Investigating the SSVRs as fully closed or one latch open—in addition to the IC-HSVR—created three cardiotomy-reservoir types (SC-SSVR-closed, SC-SSVR-open, IC-HSVR).
The SSVR has a 1600-mL volume capacity and incorporates an internal screen filter of 105 μm to aid in trapping air and other debris that may enter this reservoir from its venous and cardiotomy inlets. No minimum operating volume is noted in its instructions for use. Similar to our clinical use, the cardiotomy reservoir was attached to the SSVR with 65 cm of 3/8-inch × 3/32-inch polyvinyl chloride tubing. Each SSVR had its own cardiotomy reservoir, i.e., three cardiotomy reservoirs were permanently attached to the three SSVRs. The cardiotomy outlet was positioned at the same height as the top of its accompanying SSVR. This cardiotomy has a 20-μm filtering capability whereby entering blood is initially processed by an open-cell polyurethane defoamer and then a 20-μm micro-aggregate filter covered with a polyester sleeve.
The HSVR is a top-entry model consisting of a combined venous and cardiotomy reservoir. Venous blood passes a 200-μm inlet screen before passing through a final reservoir screen filter of 150 μm. It has a recommended minimal operating level of 200 mL. The integrated cardiotomy consists of a 30-μm depth filter and defoamer; cardiotomy blood mixes with the venous blood through the 150-μm reservoir screen filter.
Cardiotomy Infusion
To simulate cardiotomy function during CPB, aerated prime was pumped into the cardiotomy. A separate roller pump was used to aspirate prime from the circuit while simultaneously entraining air (1.8 parts prime to one part air) using dual tubing (1/4-inch × 1/16-inch and 3/16-inch × 1/16-inch polyvinyl chloride) that converged before being pumped into the cardiotomy. The frothy prime was pumped at a rate of 500:280 mL/min (prime: air) into the cardiotomy. Once reaching a steady state—and after an additional minute—60 seconds of recording were made to determine venous reservoir outlet microbubble transmission.
Microbubble Detection
A GAMPT BC100 pulsed ultrasound Doppler system (GAMPT mbH, Merseburg, Germany) was used to detect microbubbles in the outflow line of the test venous reservoirs. The GAMPT Doppler was configured for microbubble detection in the 10 to 500-μm range. If a >500-μm bolus volume occurred (“overflow”), the instrument records the volume but cannot discriminate the count. In addition to a factory calibration, during measurement, the instrument automatically self-calibrates to account for the influences of fluid and tubing variations and flow rates (16). A transluminal 3/8-inch ID microbubble detection probe was attached to the tubing approximately 8 cm distal to the outflow of the test venous reservoir. The detection probe was connected to the bubble counter BC100, itself connected to a laptop computer running BCView Version 3.4.4 data acquisition software. To maximize the reproducibility of all readings, the same probe was used thereby avoiding issues of subtle differences in probe sensitivities. Ultrasonic gel was used to couple the probe to the tubing to exclude any air. The probe was reapplied for every trial. A second microbubble detection probe was positioned immediately distal to the “patient” reservoir to confirm that the circuit was effective at removing any introduced microbubbles by observing that the recirculation of microbubbles was minimal (<15 counts/min or <3%).
Test Procedure
The nine test cardiotomy-reservoirs (three IC-HSVR, three SC-SSVR-closed, three SC-SSVR-open) were randomly subjected to two pump flows (3 L/min; 5 L/min) and two static reservoir volumes (400 mL; 900 mL). Tolerances of approximately 2.5% are given for flows and volumes measured. Both flow rates and primes were seen as being representative of adult pump flows and operating levels. The sequencing of the reservoirs was also randomized. Thus, 36 test runs were performed in one trial; three trials were run for a total of 108 tests.
Each of the test runs consisted of a first part—“baseline”—whereby any de novo microbubble generation of a test reservoir was examined by a baseline measurement of 60 seconds, and a second part–“infusion”—whereby the test reservoir’s microbubble transmission was determined during cardiotomy infusion as described previously.
Statistical Analysis
Microbubble counts, volumes, and sizes in tables are presented as medians and interquartile range. Microbubble size was derived from the median bubble size transmitted during each test. No analysis of bubble size was made for the baseline phase as a result of the rarity of bubbles counted creating many missing data fields. A null hypothesis of no difference in median microbubble generated during “baseline” (counts and volume) or transmitted during “infusion” (counts, volume, and size) among the three reservoir types or within each reservoir type—at varying flows or prime volumes-was rejected if p < .05.
To compare the three cardiotomy-reservoir types’ baseline versus cardiotomy infusion microbubble count and volume transmission, the paired nonparametric test (Wilcoxon signed rank test for paired groups) was used. Effect sizes (r) of significant differences were calculated with r = .1 as small, r = .3 as medium, and r = .5 as having a large effect. To compare the differences among the three cardiotomy-reservoir types’ microbubble generation during baseline (counts and volume) and microbubble transmission (counts, volume, and size) during cardiotomy infusion at the varying flow rates and volumes, nonparametric comparisons (using Kruskal-Wallis test for more than two groups) were computed. If a significant difference was found, post hoc testing using Mann-Whitney tests with Bonferroni correction identified differences between reservoirs. Effect sizes of significant differences were also calculated. To compare each of the cardiotomy-reservoir types’ (within) difference in microbubble generation during baseline (counts and volumes) or transmission during cardiotomy infusion (counts, volume, and size) at varying flow rates and volumes, the nonparametric Friedman test was used. Post hoc analysis with Wilcoxon signed rank tests was conducted with a Bonferroni correction applied. Additionally, effect sizes (r) of significant differences were calculated.
Statistical analyses were performed using StatView (StatView; Abacus Concepts, Berkeley, CA). Bolus air—“overflow”—was analyzed as one count per bolus with the volume added to the total.
RESULTS
Microbubble Counts
Microbubble Transmission (counts) during Cardiotomy Infusion versus Baseline for Each Cardiotomy-Reservoir Type: At both pump flows and either reservoir volume, the three cardiotomy-reservoir types transmitted significantly more microbubbles during cardiotomy infusion than during baseline. The effect sizes were large (r = .64, except SC-SSVR-closed at 900 mL and 5 L/min where r = .58) (Table 1).
Table 1.
Microbubble transmission (counts): median microbubbles (counts/min) in the outflow of the three cardiotomy-reservoir types during baseline and cardiotomy infusion (500 mL/min blood + 280 mL/min air) at reservoir volumes of 400 mL or 900 mL and pump flows of 3 L/min or 5 L/min.
| 400 mL |
900 mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3 L/min |
5 L/min |
3 L/min |
5 L/min |
|||||
| Reservoir Type | Baseline | Infusion | Baseline | Infusion | Baseline | Infusion | Baseline | Infusion |
| IC-HSVR | 21† | 5743†§¶ | 66* | 6250†§ | 4‡ | 2541†§ | 3 | 3916†§ |
| (44.8) | (764) | (299) | (511) | (6.3) | (638.3) | (6.5) | (1139.3) | |
| SC-SSVR-closed | 0 | 361§ | 0 | 499§ | 0 | 10§ | 0 | 33‖ |
| (.3) | (273.3) | (.8) | (256.8) | (0) | (31.5) | (1.0) | (44.3) | |
| SC-SSVR-open | 0 | 252§ | 0 | 447§ | 0 | 35§ | 0 | 80§ |
| (0) | (238.3) | (2) | (383.8) | (.8) | (20.3) | (1.5) | (40.5) | |
Values are medians and (interquartile range).
p < .01 IC-HSVR versus SC-SSVR-closed and SC-SSVR-open.
p < .001 IC-HSVR versus SC-SSVR-closed and SC-SSVR-open.
p < .05 IC-HSVR versus SC-SSVR-closed.
p < .01 versus baseline.
p < .05 versus baseline.
Includes one count of “overflow.”
IC-HSVR, cardiotomy integrated within a hardshell venous reservoir; SC-SSVR, separated cardiotomy attached to a softshell venous reservoir.
Microbubble Transmission (counts) between Cardiotomy-Reservoir Types during Baseline and Cardiotomy Infusion: At baseline, both the SC-SSVR-open and SC-SSVR-closed generated negligible microbubbles; however, the IC-HSVR was a significant microbubble generator, especially at the lower 400 mL prime (IC-HSVR versus either SC-SSVRs at 400 mL and both flow rates: r = .73–.87; IC-HSVR versus SSVR-closed at 900 mL and 3 L/min: r = .68) (Table 1).
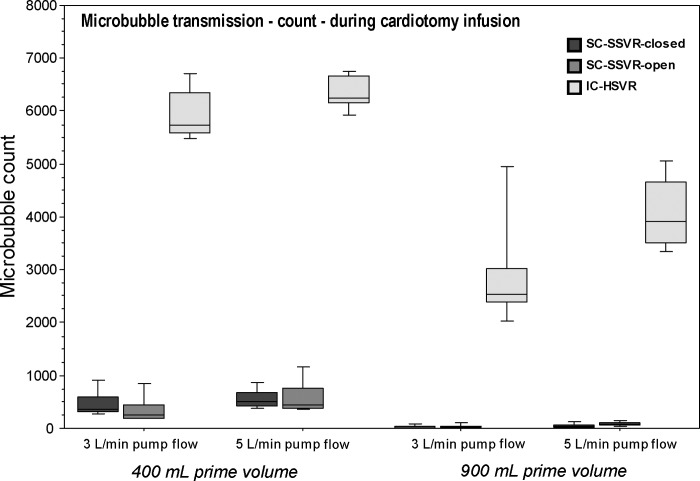
The quantity of microbubbles transmitted through the IC-HSVR during cardiotomy infusion was markedly higher than both the SC-SSVRs, being over 10–100 times more (r = .84) (Table 1; Figure 2).
Figure 2.
Boxplots of microbubble transmission (counts/min) in the outflow of the three cardiotomy-reservoir types during cardiotomy infusion at pump flows of 3 L/min or 5 L/min and reservoir volumes of 400 mL or 900 mL.
Microbubble Transmission (counts) within Each Cardiotomy-Reservoir Type during Baseline and Cardiotomy Infusion: Only the IC-HSVR showed a significant effect of the four reservoir volume–pump flow states on baseline microbubbles generated (p = .002); however, the conservative post hoc analysis failed to identify a statistically significantly different volume–flow comparison (Table 1).
During cardiotomy infusion, all cardiotomy-reservoir types revealed a significant effect of the four reservoir volume–pump flow scenarios on microbubble counts (p < .0001). Post hoc testing, in all reservoirs types, showed significantly higher microbubble counts associated with the lower 400-mL reservoir volume at both flow rates versus 900 mL at either 3 or 5 L/min (p < .05; r = .64). Furthermore, the SC-SSVR-open showed significant differences among all the reservoir volume–pump flow groups (p < .05; r = .64) (Table 1; Figure 2).
Microbubble Volume
Microbubble Transmission (volume) during Cardiotomy Infusion versus Baseline for Each Cardiotomy-Reservoir Type: During cardiotomy infusion-with the exceptions of the SC-SSVR-closed at 900 mL and 5 L/min and the SC-SSVR-open at 900 mL and 3 L/min—all cardiotomy-reservoir types transmitted significantly more air irrespective of reservoir volume or pump flow rate (r = .64) (Table 2).
Table 2. Microbubble transmission (volume): median microbubbles volume (nL/min) in the outflow of the three cardiotomy-reservoir types during baseline and cardiotomy infusion (500 mL/min blood + 280 mL/min air) at reservoir volumes of 400 mL or 900 mL and pump flows of 3 L/min or 5 L/min.
| 400 mL |
900 mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3 L/min |
5 L/min |
3 L/min |
5 L/min |
|||||
| Reservoir Type | Baseline | Infusion | Baseline | Infusion | Baseline | Infusion | Baseline | Infusion |
| IC-HSVR | 10.30† | 2938.7†§‖ | 63.13* | 3949.6†§ | 4.8‡ | 307.3†§ | 1.18 | 624.7†§ |
| (34.14) | (3292.7) | (160.1) | (2190.8) | (10.61) | (98.3) | (6.56) | (488.2) | |
| SC-SSVR-closed | 0 | 21.23§ | 0 | 34.51§ | 0 | .53§ | 0 | 2.51 |
| (.01) | (11.95) | (.11) | (15.49) | (0) | (1.76) | (.91) | (2.32) | |
| SC-SSVR-open | 0 | 14.60§ | 0 | 28.08§ | 0 | 2.06 | 0 | 5.12§ |
| (0) | (14.89) | (4.06) | (37.41) | (.7) | (2.31) | (.13) | (5.30) | |
Values are medians and (interquartile range).
p < .01 IC-HSVR versus SC-SSVR-closed and SC-SSVR-open.
p < .001 IC-HSVR versus SC-SSVR-closed and SC-SSVR-open.
p < .05 IC-HSVR versus SC-SSVR-closed.
p < .01 versus baseline.
Includes one count of “overflow.”
IC-HSVR, cardiotomy integrated within a hardshell venous reservoir; SC-SSVR, separated cardiotomy attached to a softshell venous reservoir.
Microbubble Transmission (volume) between Cardiotomy-Reservoir Types during Baseline and Cardiotomy Infusion: Microbubble air volume generated by the IC-HSVR versus either of the SC-SSVRs during baseline was significantly larger when at 400 mL with flows of 3 or 5 L/min (r = .71–.84, respectively). There was also significantly more air generated by the IC-HSVR than the SC-SSVR-closed at 900 mL flowing at 3 L/min (r = .68). All cardiotomy-reservoir types generated similarly low air volumes at 900 mL and 5 L/min during baseline (Table 2).
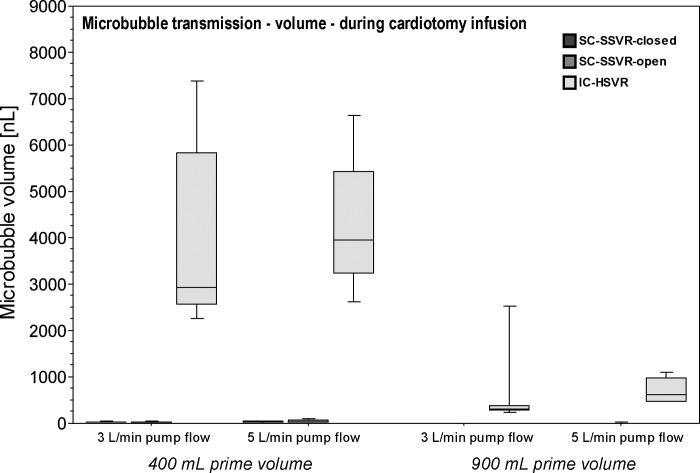
Cardiotomy infusion noticeably increased the air transmitted by the IC-HSVR over 100-fold compared with either of the SC-SSVRs (r = .84) (Table 2; Figure 3).
Figure 3.
Boxplots of microbubble transmission (nL/min) in the outflow of the three cardiotomy-reservoir types during cardiotomy infusion at pump flows of 3 L/min or 5 L/min and reservoir volumes of 400 mL or 900 mL.
Microbubble Transmission (volume) within Each Cardiotomy-Reservoir Type during Baseline and Cardiotomy Infusion: During baseline, only the IC-HSVR revealed a significant effect of the four flow-volume states on microbubble volume generated (p = .002). Although most air was generated by the IC-HSVR when at 400-mL prime and flowing at 5 L/min, the conservative post hoc test failed to identify which pump flow–reservoir volume pair was significantly different (Table 2).
There were statistically significant differences in air transmitted among the four reservoir volume–flow rate combinations during cardiotomy infusion in all three cardiotomy-reservoir types (p < .0001). Post hoc testing of the IC-HSVR and SC-SSVR-closed showed significantly more air transmitted with reservoir volumes of 400 mL versus 900 mL irrespective of pump flow rate (p < .05; r = .64). The SC-SSVR-open yielded significantly larger transmitted bubble volumes at 400 mL flowing at 5 L/min versus the three other scenarios and between 400 mL at 3 L/min and 900 mL at 5 L/min (p < .05; r = .64) (Table 2; Figure 3).
Microbubble Size
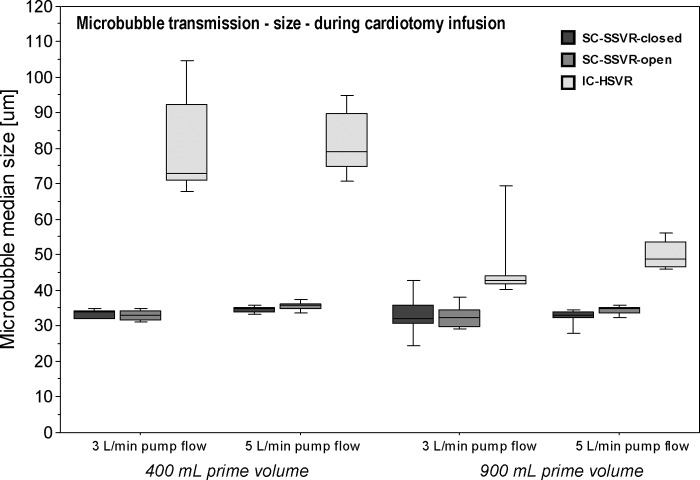
Microbubble Transmission (size) between Cardiotomy-Reservoir Types during Cardiotomy Infusion: Median microbubble size transmitted by the IC-HSVR was consistently larger than either the SC-SSVR-open or SC-SSVR-closed during each of the reservoir volume–flow rate combinations. The greatest discrepancy in bubble size was seen at the lower reservoir volume of 400 mL in which IC-HSVR median bubble diameters were more than twice the size of those transmitted by either of the SC-SSVR types (r = .84) (Table 3; Figure 4).
Table 3.
Microbubble transmission (size): median microbubbles size (m) in the outflow of the three cardiotomy-reservoir types during cardiotomy infusion (500 mL/min blood + 280 mL/min air) at reservoir volumes of 400 mL or 900 mL and pump flows of 3 L/min or 5 L/min.
| 400 mL |
900 mL |
|||
|---|---|---|---|---|
| Reservoir Type | 3 L/min | 5 L/min | 3 L/min | 5 L/min |
| IC-HSVR | 73.0† | 79.0† | 43.0‡§ | 49.0* |
| (21.5) | (15.0) | (2.5) | (6.8) | |
| SC-SSVR-closed | 34.0 | 35.0 | 32.0 | 33.0 |
| (2.3) | (1.3) | (5.1) | (2.4) | |
| SC-SSVR-open | 33.0 | 36.0 | 32.5 | 35.0 |
| (2.4) | (1.3) | (4.9) | (1.5) | |
Values are medians and (interquartile range).
p < .01 IC-HSVR versus SC-SSVR-closed and SC-SSVR-open.
p < .001 IC-HSVR versus SC-SSVR-closed and SC-SSVR-open.
p < .001 IC-HSVR versus SC-SSVR-open.
p < .05 IC-HSVR versus SC-SSVR-closed.
IC-HSVR, cardiotomy integrated within a hardshell venous reservoir; SC-SSVR, separated cardiotomy attached to a softshell venous reservoir.
Figure 4.
Boxplots of median microbubble size (mm) in the outflow of the three cardiotomy-reservoir types during cardiotomy infusion at pump flows of 3 L/min or 5 L/min and reservoir volumes of 400 mL or 900 mL.
Microbubble Transmission (size) within Each Cardiotomy-Reservoir Type during Cardiotomy Infusion: Both the SC-SSVR-open and SC-SSVR-closed transmitted consistently similarly sized bubbles despite varying the reservoir volume or pump flow rates (p = nonsignificant). Conversely, the IC-HSVR showed significant differences in bubble sizes associated with the four reservoir volume– pump flow states (p = .0001). Post hoc testing identified significant differences between 400 mL at 5 L/min versus 900 mL at both flow rates, and between 400 mL at 3 L/min and 900 mL at 5 L/min ( p < .05; r = .64); the lower reservoir volumes being associated with larger bubbles being transmitted (Table 3; Figure 4).
DISCUSSION
This in vitro study showed that both the Medtronic softshell venous reservoir with separated cardiotomy and Medtronic’s hardshell venous reservoir with integrated cardiotomy transmit more microbubbles during cardiotomy infusion of aerated cardiotomy prime than during baseline. Moreover, the transmission of microbubbles by the venous reservoir during cardiotomy infusion is less with the softshell venous reservoir with separated cardiotomy than the hardshell venous reservoir with integrated cardiotomy.
Infusing aerated cardiotomy blood into the venous reservoir was associated with an increase in microbubbles and bubble volume transmitted by both cardiotomy-reservoir systems. Dramatic increases in microbubbles exiting the venous reservoir when pumping aerated prime into the cardiotomy was also demonstrated by Myers and colleagues (15) using the same Medtronic hardshell venous reservoir with integrated cardiotomy. They also demonstrated this phenomenon with four other contemporary integrated cardiotomy HSVR designs. Thus, although both the cardiotomy and venous reservoir are designed to remove introduced air, we have shown that some of this introduced air manages to elude their bubble removal mechanisms.
A lower prime exacerbated the increase in microbubble transmission during cardiotomy infusion in both cardiotomy-reservoir systems. We speculate that a smaller prime lowers the fluid height within the venous reservoir (and adjoining conduit to the cardiotomy with the SSVR), resulting in a shorter distance for any cardiotomy-infused bubble to travel to the venous reservoir outlet and a decreased transit time for any entrained bubbles to be eliminated.
Increasing the pump flow rate also directly shortens the blood transit time within the venous reservoir. However, this effect on increasing microbubble transmission, although seen, was not as noticeable as decreasing the prime volume. Conversely, in our previous study, in which bubbles were directly introduced into the venous line, there were more transmitted microbubbles seen in both venous reservoir systems when flows were increased, irrespective of reservoir volume (17). Thus, the debubbling process within the cardiotomy seems to be less affected by the pump flow rate through the venous reservoir; it appears to function as a separate system infusing microbubbles into the venous reservoir at a rate more determined by the cardiotomy flow. We would anticipate less effective cardiotomy debubbling at higher suctioning rates. Here the buoyancy force on a bubble tending to allow it to rise and be dissipated would be opposed by drag force associated with the blood flow, which tends to drive bubbles forward into the venous reservoir (18). Further studies investigating different cardiotomy pumping rates could confirm this proposition.
Although both cardiotomy-reservoir systems transmitted more microbubbles during cardiotomy infusion, the magnitude of the effect was markedly greater with the IC-HSVR. During cardiotomy infusion, microbubbles transmitted by the IC-HSVR system are more numerous and of a larger total volume than the SC-SSVR. This is the first known study demonstrating the superiority of the SC-SSVR system over the IC-HSVR system in cardiotomy air handling. When investigating the bubble-handling properties of the reservoirs alone during venous air infusion, our previous study showed no superiority of either venous reservoir system (despite the 150-mm screen filter in the HSVR versus the 105-mm screen filter in the SSVR). Thus, the significant increase in microbubbles transmitted by the IC-HSVR during cardiotomy use implicates its cardiotomy (17). One explanation is that the IC-HSVR’s cardiotomy filters at 30 μm, whereas the SC-SSVR cardiotomy filters down to 20 μm.
The air-handling discrepancy between the two systems is exacerbated at lower prime volumes. We suggest that in the SC-SSVR system, the fluid column created between the cardiotomy and the reservoir further aids in deairing and causes less turbulent cardiotomy fluid entry into the venous reservoir bag. In contrast, a lower reservoir volume in the IC-HSVR increases the distance for the integrated cardiotomy blood to trickle down to the reservoir prime, potentially causing turbulence-induced microbubble generation.
The median size of transmitted bubbles during cardiotomy infusion of the SC-SSVR system remained consistent regardless of flow rate or prime volume. Conversely, the IC-HSVR transmitted larger bubbles, particularly with the reduced prime volume. The formation of a fluid column between the separated cardiotomy and the SSVR may facilitate the retention and elimination of larger and more buoyant microbubbles (19). A clinical issue is that larger bubbles may not be removed by other components of the ECC but are disintegrated into additional smaller bubbles that subsequently enter the patient (20).
The IC-HSVR transmitted significantly more air than the SC-SSVR system during baseline (i.e., without the cardiotomy pump turned on) at the lower 400-mL prime indicating that de novo microbubble generation had occurred. This was seen despite the dynamic prime volume being at or slightly above the manufacturer’s recommended 200 mL. Our previous study showed the predilection of the HSVR to generate microbubbles at a lower prime volume (17). Hardshell venous reservoirs are associated with increased microbubble generation when running at low prime volumes or higher pump flows (21–23). Both lower reservoir volumes and higher flow rates increase any turbulent waterfall effect. Within the IC-HSVR design, venous return blood tumbles down from the higher cardiotomy tier and splashes into the venous reservoir compartment inducing turbulence and subsequent microbubble generation. The SC-SSVR system was relatively free from de novo bubble generation possibly because the draining venous blood directly enters and mixes with the blood in the venous reservoir.
There was no significant difference in microbubble transmission seen between the SC-SSVR-closed and SC-SSVR-open. Improved cardiotomy microbubble handling properties could be anticipated when closing the cage housing because the compressed venous reservoir bag displaces prime back up into the cardiotomy thereby raising the fluid column’s meniscus. Not only is the fall of cardiotomy fluid into this meniscus reduced (minimizing splashing), but also the distance and time for infused bubbles to be eliminated by buoyancy forces are increased. Although there was a trend seen in improved air handling with the SC-SSVR-closed, it did not reach statistical significance. Thus, no advantage was seen by operating the SSVR in either the open or closed position under the studies’ parameters of flow and volumes.
Importantly, because both cardiotomy-reservoir systems transmitted microemboli during cardiotomy infusion, particularly at lower reservoir volumes, it is prudent to consider ways to minimize cardiotomy microbubble infusion. Perfusion strategies relevant to all cardiotomy-reservoir systems include reducing the volume of air mixed with aspirated blood by avoiding excessive suctioning, effectively running the vent and suckers at the minimal rate as the surgeon will tolerate (24). Also, the suction tip of the cardiotomy, vent cannulae, or sump should be below the blood level. Another approach is to flood the operative field with carbon dioxide. The more soluble and denser carbon dioxide displaces air. Thus, CO2 and not air is aspirated from the operative field into the cardiotomy. Any bubbles infused by the cardiotomy would be relatively harmless because they should rapidly resorb by the time they reach the brain (25). Further blood-handling benefits may be achieved by isolating the cardiotomy blood in closed systems. This ability for the perfusionist to hold blood in the cardiotomy allows more time for entrained bubbles to dissipate. Intermittently releasing the—now better debubbled—cardiotomy blood into the venous reservoir could reduce the microbubble content of the blood exiting the venous reservoir (24), a strategy that is worthy of further investigations. This approach may also be applied in the HSVR system to minimize microbubble transmission by adding a secondary cardiotomy when high suction is anticipated (26).
The Gampt bubble counter may underestimate microbubble counts and overestimate bubble size at higher flow rates (27). Nevertheless, the findings are still valid because the purpose of the study was to quantitatively rank the cardiotomy-reservoir systems in terms of microbubble counts, volumes, and size transmitted.
In summary, we investigated the relative microbubble-handling capabilities of two kinds of adult cardiotomy-reservoir systems: Medtronic’s softshell venous reservoir with separated cardiotomy and Medtronic’s hardshell venous reservoir with integrated cardiotomy. These cardiotomy-reservoir systems’ relative microbubble-handling capabilities were examined under equal in vitro conditions of baseline and cardiotomy microbubble infusion while varying the prime volumes and pump flow rates. We conclude that the SC-SSVR transmits less microbubbles than the IC-HSVR and should be considered as the preferential system. Importantly, because both cardiotomy-reservoir systems transmitted microbubbles during cardiotomy infusion, particularly at the lower reservoir volume, it is important to use strategies to minimize cardiotomy microbubble infusion.
REFERENCES
- 1.Lee R, Li S, Rankin S, et al. Fifteen-year outcome trends for valve surgery in North America. Ann Thorac Surg. 2011;91:677–684. [DOI] [PubMed] [Google Scholar]
- 2.Hickey GL, Grant SW, Murphy GJ, et al. Dynamic trends in cardiac surgery: Why the logistic EuroSCORE is no longer suitable for contemporary cardiac surgery and implications for future risk models. Eur J Cardiothorac Surg. 2012. November 14 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann GM, Grega MA, Borowicz LM, Baumgartner WA, Selnes OA.. Stroke and encephalopathy after cardiac surgery an update. Stroke. 2006;37:562–571. [DOI] [PubMed] [Google Scholar]
- 4.Fearn SJ, Pole R, Wesnes K, Faragher EB, Hooper TL, McCollum CN.. Cerebral injury during cardiopulmonary bypass: Emboli impair memory. J Thorac Cardiovasc Surg. 2001;121:1150–1160. [DOI] [PubMed] [Google Scholar]
- 5.Likosky DS, Roth RM, Saykin AJ, Eskey CJ, Ross CS, O’Connor GT.. Neurological injury associated with CABG surgery: Outcomes, mechanisms, and opportunities for improvement. Heart Surg Forum. 2004;7:650–661. [DOI] [PubMed] [Google Scholar]
- 6.Motallebzadeh R, Bland JM, Markus HS, Kaski JC, Jahangiri M.. Neurocognitive function and cerebral emboli: Randomized study of on-pump versus off-pump coronary artery bypass surgery. Ann Thorac Surg. 2007;83:475–482. [DOI] [PubMed] [Google Scholar]
- 7.Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S.. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–1399. [DOI] [PubMed] [Google Scholar]
- 8.Groom C, Quinn RD, Lennon P, et al. Microemboli from cardiopulmonary bypass are associated with a serum marker of brain injury. J Extra Corpor Technol. 2010;42:40–44. [PMC free article] [PubMed] [Google Scholar]
- 9.Groom RC, Quinn RD, Lennon P, et al. Detection and elimination of microemboli related to cardiopulmonary bypass. Circ Cardiovasc Qual Outcomes. 2009;2:191–198. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Omar Y, Balacumaraswami L, Pigott DW, Matthews PM, Taggart DP.. Solid and gaseous cerebral microembolisation during off-pump, on-pump, and open cardiac surgery procedures. J Thorac Cardiovasc Surg. 2004;127:1759–1765. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher G, Pearson DT.. Ultrasonic identification of sources of gaseous microemboli during open heart surgery. Thorax. 1973;28:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers GJ.. Preventing gaseous microemboli during blood sampling and drug administration: An in vitro investigation. J Extra Corpor Technol. 2007;39:192–198. [PMC free article] [PubMed] [Google Scholar]
- 13.Svitek V, Lonsky V, Anjum F.. Pathophysiological aspects of cardiotomy suction usage. Perfusion. 2010;25:147–152. [DOI] [PubMed] [Google Scholar]
- 14.Baker RA, Wilcox TW.. Australian and New Zealand perfusion survey: Equipment and monitoring. J Extra Corpor Technol. 2006; 38:220–229. [PMC free article] [PubMed] [Google Scholar]
- 15.Myers GJ, Voorhees C, Haynes R, Eke B.. Post-arterial filter gaseous microemboli activity of five integral cardiotomy reservoirs during venting: An in vitro study. J Extra Corpor Technol. 2009;41:20–27. [PMC free article] [PubMed] [Google Scholar]
- 16.Gesellschaft fur Angewandte Medizinische Physik und Technik mbH. Bubble Counter BC100: A device for the detection of micro bubbles in streaming fluids (user manual). Merseburg: GAMPT mbH; April 2010. [Google Scholar]
- 17.Potger KC, McMillan D, Ambrose M.. Microbubble generation and transmission of Medtronic’s affinity hardshell venous reservoir and collapsible venous reservoir bag: An in-vitro comparison. J Extra Corpor Technol. 2011;43:115–122. [PMC free article] [PubMed] [Google Scholar]
- 18.Barak M, Yeshayahu K.. Microbubbles: Pathophysiology and clinical implications. Chest. 2005;128:2918–2932. [DOI] [PubMed] [Google Scholar]
- 19.Dexter F, Hindman BJ, Marshall JS.. Estimate of the maximum absorption rate of microscopic arterial air emboli after entry into the arterial circulation during cardiac surgery. Perfusion. 1996;11:445–450. [DOI] [PubMed] [Google Scholar]
- 20.De Somer F.. Evidence-based used, yet still controversial: The arterial filter. J Extra Corpor Technol. 2012;44:27–30. [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell SJ, Willcox T.. Bubble generation and venous air filtration by hard-shell venous reservoirs: A comparative study. Perfusion. 1997;12:325–333. [DOI] [PubMed] [Google Scholar]
- 22.Rodriquez RA, Williams KA, Babaev A, Rubens F, Nathan HJ.. Effect of the perfusionists technique on cerebral embolization during cardiopulmonary bypass. Perfusion. 2005;20:3–10. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen PF, Funder JA, Jenson MO, Nygaard H.. Influence of venous reservoir level on microbubbles in cardiopulmonary bypass. Perfusion. 2008;23:347–353. [DOI] [PubMed] [Google Scholar]
- 24.Pearson DT, Watson BG, Waterhouse PS.. An ultrasonic analysis of the comparative efficiency of various cardiotomy reservoirs and micropore blood filters. Thorax. 1978;33:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb WR, Harrison LH, Helmcke FR, et al. Carbon dioxide field flooding minimizes residual intracardiac air after open heart operations. Ann Thorac Surg. 1997;64:1489–1491. [DOI] [PubMed] [Google Scholar]
- 26.Ündar A, Palanzo D, Wang S.. Using a secondary reservoir for pump suckers to avoid the generation of foam during CPB procedures in pediatric patients. Perfusion. 2012;27:556–558. [DOI] [PubMed] [Google Scholar]
- 27.De Somer FM, Vetrano MR, Van Beeck JP, Van Nooten GJ.. Extracorporeal bubbles: A word of caution. Interact Cardiovasc Thorac Surg. 2010;10:995–1001. [DOI] [PubMed] [Google Scholar]