Abstract
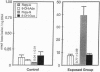
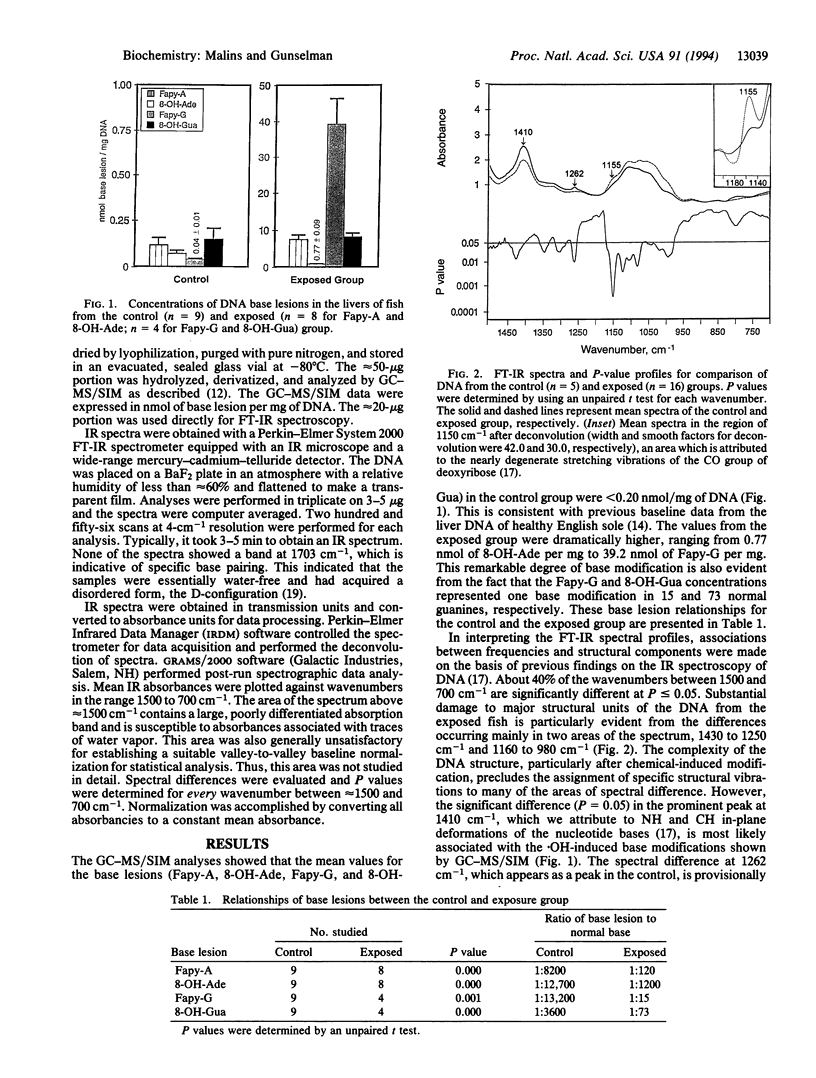
The use of gas chromatography-mass spectrometry with selected ion monitoring (GC-MS/SIM) and Fourier-transform infrared (FT-IR) spectroscopy revealed a remarkable degree of damage in the hepatic DNA of fish exposed to toxic environmental chemicals, compared with controls. The exposed fish, which were neoplasm-free, were part of a population with a high incidence of liver cancer. GC-MS/SIM showed markedly high concentrations of hydroxyl radical-induced ring-opening products (e.g., 2,6-diamino-4-hydroxy-5-formamidopyrimidine) and 8-hydroxy adducts of adenine and guanine (e.g., 8-hydroxyguanine) in the DNA. FT-IR spectroscopy revealed substantial changes in spectral areas, such as those assigned to NH vibrations of nucleotide bases and CO vibrations of deoxyribose. This diverse and extensive damage to DNA provides a perspective of premalignant changes resulting from xenobiotic exposure and a promising basis for predicting cancer risk in animals and humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Klein J. C., Bleeker M. J., Saris C. P., Roelen H. C., Brugghe H. F., van den Elst H., van der Marel G. A., van Boom J. H., Westra J. G., Kriek E. Repair and replication of plasmids with site-specific 8-oxodG and 8-AAFdG residues in normal and repair-deficient human cells. Nucleic Acids Res. 1992 Sep 11;20(17):4437–4443. doi: 10.1093/nar/20.17.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. S., Fletcher R. G., Grond M. P., Inyang A. I., Lu X. P., Brenner D. A., Leffert H. L. Inactivation of plasmid reporter gene expression by one benzo(a)pyrene diol-epoxide DNA adduct in adult rat hepatocytes. Cancer Res. 1993 May 15;53(10 Suppl):2279–2286. [PubMed] [Google Scholar]
- Malins D. C., Haimanot R. 4,6-Diamino-5-formamidopyrimidine, 8-hydroxyguanine and 8-hydroxyadenine in DNA from neoplastic liver of English sole exposed to carcinogens. Biochem Biophys Res Commun. 1990 Dec 14;173(2):614–619. doi: 10.1016/s0006-291x(05)80079-8. [DOI] [PubMed] [Google Scholar]
- Malins D. C., Haimanot R. Major alterations in the nucleotide structure of DNA in cancer of the female breast. Cancer Res. 1991 Oct 1;51(19):5430–5432. [PubMed] [Google Scholar]
- Malins D. C., Holmes E. H., Polissar N. L., Gunselman S. J. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer. 1993 May 15;71(10):3036–3043. doi: 10.1002/1097-0142(19930515)71:10<3036::aid-cncr2820711025>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Malins D. C. Identification of hydroxyl radical-induced lesions in DNA base structure: biomarkers with a putative link to cancer development. J Toxicol Environ Health. 1993 Oct-Nov;40(2-3):247–261. doi: 10.1080/15287399309531792. [DOI] [PubMed] [Google Scholar]
- Malins D. C., Ostrander G. K., Haimanot R., Williams P. A novel DNA lesion in neoplastic livers of feral fish: 2,6-diamino-4-hydroxy-5-formamidopyrimidine. Carcinogenesis. 1990 Jun;11(6):1045–1047. doi: 10.1093/carcin/11.6.1045. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Postlabelling methods--an historical review. IARC Sci Publ. 1993;(124):3–9. [PubMed] [Google Scholar]
- Roy D., Floyd R. A., Liehr J. G. Elevated 8-hydroxydeoxyguanosine levels in DNA of diethylstilbestrol-treated Syrian hamsters: covalent DNA damage by free radicals generated by redox cycling of diethylstilbestrol. Cancer Res. 1991 Aug 1;51(15):3882–3885. [PubMed] [Google Scholar]
- Weitzman S. A., Turk P. W., Milkowski D. H., Kozlowski K. Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1261–1264. doi: 10.1073/pnas.91.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T., Wong R. K., Caputo T. A., Godwin T. A., Rigas B. Infrared spectroscopy of exfoliated human cervical cells: evidence of extensive structural changes during carcinogenesis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):10988–10992. doi: 10.1073/pnas.88.24.10988. [DOI] [PMC free article] [PubMed] [Google Scholar]